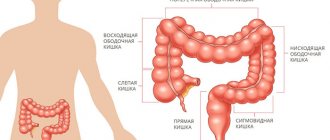
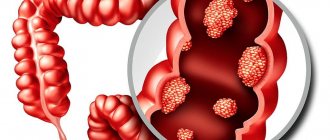
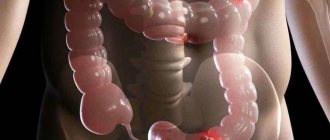
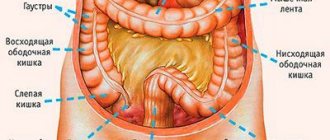
Colorectal cancer is the collective name for all carcinomas of the large intestine, starting from the cecum and continuing to the anus. Malignant processes in the cecum, transverse colon and sigmoid part of the intestine are usually classified as diseases of the colon. Some researchers consider sigmoid colon tumors to be a separate group - rectosigmoid carcinomas.
- Colorectal cancer: what is it?
- Causes of the disease
- Symptoms of colorectal cancer
- Classification of colorectal cancer
- Diagnosis of colorectal cancer
- Metastasis
- Treatment of colorectal cancer
- Surgery
- Chemotherapy for colorectal cancer
- Radiation therapy
- Prognosis and survival of colorectal carcinoma
- Prevention of colorectal cancer
Colorectal cancer: what is it?
Colorectal cancer (CRC) is two different diseases, one is localized in the colon, the other in the rectum, they are united by anatomy, similar causes of development and the coincidence of some aspects of antitumor drug treatment.
For these diseases, diagnosis, clinical manifestations, approaches to surgery and surgical treatment itself, attitudes towards the use of radiation therapy and, of course, prognosis differ significantly.
Combining them into one disease is useful for carrying out preventive measures, but for the utilitarian purposes of oncology, the term CRC does not mean the unity of the treatment and diagnostic approach, because these are two very different malignant processes with their own clinical features and therapeutic difficulties.
In most cases, rectal carcinoma has an aggressive course and, despite the mandatory addition of radiation to surgical treatment, survival rates are much worse than for colon carcinoma.
Causes of the disease
Colorectal adenocarcinomas are thought to develop from long-standing villous polyps, and timely removal of polyps during colonoscopy has been shown to reduce the incidence of carcinomas in the population. There is an opinion that the malignant process may begin not in the polyp, but with dysplasia of the mucous membrane.
An unambiguous etiological opinion has not yet been formed; on the contrary, the occurrence of a malignant process in several areas of the intestinal mucosa at once, often not morphologically similar, cannot be ruled out at all.
Since the majority of neoplasms are localized in places of physiological retention of contents, confidence in the active carcinogenic role of dietary characteristics and patterns is growing:
- the incidence of disease in the African population on a plant diet quickly evacuated through the intestinal tube is insignificant;
- Europeans and Americans, who prefer high-protein foods and fast food, get sick more often.
Hereditary colorectal cancer is very rare - hardly every 20th patient can trace the family incidence, however, a whole list of genetic syndromes with the involvement of colorectal cancer has been compiled. With a hereditary disease, carcinoma occurs tens of times more often and earlier in age.
They assume a negative effect of alcohol, and not a specific drink, but the volume of consumption of ethyl alcohol.
Risk factors for colorectal cancer also include:
- smoking,
- low physical activity,
- obesity,
- diabetes,
- chronic inflammation of the intestinal mucosa in ulcerative colitis.
Knowing the exact causes of malignant diseases is important for finding ways of prevention; when identifying a disease in a particular person, the causes that caused it are unimportant; it is necessary to select adequate treatment, but first carry out a full diagnosis.
Causes and mechanisms of disease development
Colorectal cancer occupies a leading position in morbidity and mortality from malignant tumors in Russia. The development of molecular biology has led to the deciphering of the mechanisms of tumor formation and progression. These processes require the accumulation of genetic and epigenetic changes in the tumor cell. There is an accumulation of mutations in genes that control the growth and differentiation of epithelial cells. This leads to their genetic instability.
One of the variants of genetic changes is microsatellite instability in colorectal cancer. It is characterized by a violation of the repair mechanism (a special function of cells that consists in the ability to correct chemical damage and breaks in unpaired DNA bases). This leads to the fact that mutations in the cell's genome accumulate at a faster rate than in the normal state.
In familial adenomatous polyposis, gene mutations occur that damage the DNA of cells with the formation of microsatellites. In patients with hereditary forms of colorectal cancer, the presence of gene defects in various chromosomes has been established. In hereditary forms of the disease, structural changes in nucleotides have been identified. In sporadic (non-hereditary) colorectal cancer, hereditary predisposition is the cause of the disease in 18% of patients.
A direct transition from a normal cell to adenocarcinoma is possible. More often, this process develops sequentially: first, the differentiation of colonocytes decreases, then benign neoplasms (adenomatous, adenopapillomatous) are formed, then they degenerate into a cancerous tumor.
Exogenous and endogenous factors play a certain role in the development of colorectal cancer, including nutritional and functional disorders of the large intestine (chronic constipation). Nutritional risk factors for colorectal cancer include:
- Eating large amounts of red meat (beef, pork, lamb);
- Excess dietary fats;
- The predominance of refined products devoid of plant fiber;
- Systematic alcohol consumption.
An important risk factor for the development of colorectal cancer is chronic idiopathic inflammatory diseases of the colon: granulomatous and ulcerative colitis. High-grade dysbiosis of the large intestine plays a certain role. Adenomatous polyps almost inevitably transform into colorectal cancer. Malignancy occurs at different times over 13–15 years. The cause of sporadic colorectal cancer may be disturbances in the cells of the gastrointestinal tract, capable of producing and accumulating biogenic amines and peptide hormones.
Symptoms of colorectal cancer
Clinical manifestations of the disease depend on:
- level of tumor localization,
- size and degree of obstruction of the lumen of the intestinal tube,
- on the aggressiveness of cancer cells and the associated rate of development of metastases.
One of the most common symptoms of colorectal cancer is pain, but it is not a sign of early cancer. To initiate pain, there must be a significant disruption of peristaltic function or the tumor extending beyond the intestine with involvement of the richly innervated peritoneum into the cancer conglomerate. Intense progressively increasing pain is characteristic of intestinal obstruction caused by partial or complete blockage of the lumen of the tube. In most cases of colorectal cancer, pain is short-term and unstable in intensity; patients often interpret it as gastric discomfort.
Delayed movement of feces is manifested by feelings of bloating, negatively affects appetite and can cause nausea, but belching is more common. Vomiting with a fecal odor is characteristic of intestinal obstruction.
Changes in stool, usually in the form of an unstable alternation of constipation and liquid, foul-smelling feces, and the appearance of mucus during bowel movements more often occur when the rectum and left half of the colon are affected. With neoplasms of the right parts of the colon, there is a tendency to constipation. When the tumor is localized in the rectum, patients are often dissatisfied with the result of defecation - there is no feeling of complete emptying.
Symptoms of intoxication - weakness and fatigue are caused by stagnation of intestinal contents with increased processes of putrefaction and absorption of endotoxins into the blood through the wall. Destruction of blood vessels by cancer can manifest as anemia due to chronic bleeding.
Symptoms of colorectal cancer in women differ little from those in men, but women listen more carefully to the voice of their body and are much less likely to lead themselves to obstruction.
In most cases, the symptoms of colorectal cancer are so diverse and unstable that they do not allow the process to be detected at an early stage; the disease clearly manifests itself far from the initial stage.
Book a consultation 24 hours a day
+7+7+78
Screening tests
There are 4 main screening tests for colorectal cancer:
- stool occult blood test,
- flexible sigmoidoscopy,
- fibrocolonoscopy (FCS)
- irrigoscopy using double contrast contrast of the colon.
Recently, virtual colonoscopy has been added to these options. A number of authors insist on a combination of two methods, such as: stool occult blood analysis followed by sigmoidoscopy.
The cheapest and most widely used screening method is a fecal occult blood test, based on the detection of hemoglobin with peroxidase activity using the guaiac test, benzidine or pyramidone test. Most tests used in outpatient practice are based on guaiac glue, which can oxidize in the presence of peroxidase and hydrogen peroxide. The procedure for performing the occult blood test varies slightly depending on the type of test and is described in many manuals. Its main disadvantages are the large number of false negatives, when polyps or a tumor of the colon remain undetected, and false positive results, when in order to exclude pathology the patient has to undergo an unpleasant, expensive and risky procedure - fibrocolonoscopy. Colon tumors usually bleed little and are difficult to detect with an occult blood test. Formations larger than 2 cm in diameter bleed more often [6]. The effectiveness of the test depends on the size and location of the tumor; it is also greatly influenced by diet and a number of medications. To reduce the number of false-positive and false-negative results, several days before the test it is recommended to exclude the consumption of meat, non-steroidal anti-inflammatory drugs, iron supplements and antioxidants such as vitamin C. It is advisable to conduct the test with samples taken from several portions of feces (3 consecutive bowel movements). Using this test, it is impossible to determine the amount of blood in the stool; it is also nonspecific, since in benign diseases: hemorrhoids, stomach and duodenal ulcers, inflammatory bowel diseases, it can also be positive. Several versions of this test have been developed (Hemoccult, Hemoccult II), but their sensitivity does not exceed, according to the most optimistic data, 50%, and in early forms of CRC it is only about 5-10% [9]. Lieberman D et al. reports low sensitivity of this test (23.9%) even in diagnosing tumors greater than 10 mm in diameter. In the same study, false positive results occurred in 6.2% of cases [14]. According to Letsou G et al. 59% of patients with polyps and 36% with colon cancer tested negative for occult blood [12]. A screening examination of more than 60 thousand patients aged 47-75 years conducted in Germany using an occult blood test showed a reduction in mortality from colon cancer over 10 years in the screening group by 18% [10]. It is recommended that an occult blood test be performed at least once a year. When comparing two screening methods using an occult blood test—an annual test or every two years—survival in the annual screening group was significantly longer [15].
The next step in examining a patient with a positive occult blood test result should be fibrocolonoscopy or double contrast irrigography (preferably in combination with sigmoidoscopy).
Using a combination of occult blood testing and sigmoidoscopy improves screening performance compared with either method alone. However, even the combination of a single occult blood test with sigmoidoscopy can detect a malignant tumor of the colon in only 75.8% of patients [14].
Sigmoidoscopy can successfully diagnose CRC of the left half of the colon [19]. Based on a study of 2885 patients, Lieberman D et al. reported that flexible sigmoidoscopy can detect intestinal neoplasm in 70.3% of cases [14], according to other authors, this technique can detect up to 95% of adenomas and 80% of cases of invasive CRC [18]. However, the proximal parts of the colon are not accessible for examination by this method. The disadvantage is the need for a repeat study - FCS, if pathology of the left half of the colon is detected. Based on a study of 4411 patients, Muller et al. reported a 60% reduction in the risk of death from colorectal cancer when using screening sigmoidoscopy [16]. Sigmoidoscopy is performed either with a flexible sigmoidoscope - a device about 35-60 cm long, or with a rigid sigmoidoscope 25-30 cm long, although it can also be performed with a standard fiber colonoscope. It must be emphasized that preparation for sigmoidoscopy should be the same as for FCS. Unfortunately, 2-3 enemas in the morning before the study, sometimes recommended for preparation, often do not allow for adequate cleaning of the area of the colon being examined, leading to difficulties in performing the study and certain doubts in its results. The area of the colon that can be examined during sigmoidoscopy varies from the junction of the sigmoid colon to the descending colon to the splenic flexure and sometimes the left half of the transverse colon. The principles of performing sigmoidoscopy are fully consistent with those for FCS and are given below. It is recommended to perform a sigmoidoscopy at least once every 3-5 years. The choice of such a period of time between examinations is due to a number of factors: there is evidence that the screening effectiveness of sigmoidoscopy is the same for an annual examination and for an examination every three years; the development of a malignant tumor from a polyp rarely occurs faster than 3 years [8, 21]. According to a number of authors, the “protective effect” of sigmoidoscopy can last up to 10 years [20].
Patients with colon polyps or tumors identified during sigmoidoscopy must undergo fibrocolonoscopy or irrigography.
Irrigography with double contrast is a fairly sensitive method for detecting colorectal cancer and large adenomas (more than 90%). A number of authors report its higher effectiveness against infiltrative tumor growth [4]. The disadvantages of irrigography are the low percentage of diagnoses of early colorectal cancer, especially its superficially spreading forms, a significant number of false positive results and the impossibility of morphological verification of the diagnosis. This technique allows us to detect 50-80% of polyps less than 1.0 cm, 70-90% of polyps more than 1.0 cm and 55-85% of Duke stage A, B cancer [5, 7]. In any case, when pathology is detected, FCS is necessary to confirm and morphologically verify the diagnosis. Winawer S et al. based on a survey of 973 patients, compared the effectiveness of diagnosing colon polyps in patients who had previously undergone polypectomy by comparing double-contrast barium and FCS bowel studies [23]. Irrigography revealed only 39% of polyps detected during FCS, and this figure varied from 32 to 52% for formations less than 0.5 and more than 0.6 cm, respectively. Those. the degree of detection of polyps during irrigography largely depended on their size. It should be noted that 48% of adenomas larger than 1.0 cm, the most dangerous in terms of malignancy, were not identified. During FCS, 20% of adenomas were not detected (26% and 6% for formations less than 0.5 cm and 0.6 - 1.0 cm, respectively), diagnosed using irrigography, but not one formation more than 1.0 cm in diameter was not missed. The preparation and technique for performing irrigography are perfectly described by L.M. Tailor in the methodological manual [4]. He notes that only good preparation of the intestines for the study makes it highly informative. Oral preparation with Fortrans is, in his opinion, the method of choice. The frequency of double contrast irrigography for colorectal cancer screening is once every 5 years.
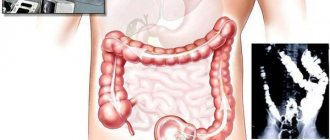
Fibercolonoscopy currently plays a leading role in establishing or excluding colorectal pathology [2,13]. However, FCS should not be considered a method that absolutely and 100% excludes colon pathology. So, according to P.A. Nikiforov, 28 out of 377 patients a year after colonoscopy were diagnosed with stage II or III cancer, and in 34, stage I cancer, which indirectly indicates that this pathology was missed during the initial examination [2]. Rex et al reported that 25% of adenomas smaller than 10 mm and 6% larger than 10 mm were missed with standard FCS [17]. In one study, screening FCS reduced the incidence of colorectal cancer by 3570 cases per 100,000 population over 10 years [16]. We can talk about a successfully performed FCS only if the device reaches the dome of the cecum. FCS is performed in patients after adequate bowel preparation. It must be emphasized that the term “adequate preparation” means complete removal of the contents of the colon along its entire length (only a small amount of clear fluid is allowed). Oral preparation is the method of choice, as it leads to adequate colon cleansing in more than 95% of cases, is easier to tolerate by the patient, and is associated with a lower risk of complications and side effects than a cleansing enema.
The importance of complete cleansing of the colon can be illustrated by the following clinical example: Patient R., 28 years old, was referred to the FCS with a suspected tumor of the right half of the colon. Since the woman had a caesarean section 15 days before the examination, it was decided to conduct the examination under general anesthesia. Preparation for the procedure was carried out according to the method accepted at that time - two enemas of 1.5 liters in the evening on the day preceding the study and two in the morning on the day of the study. Enemas were performed by an experienced nurse. Upon examination, already at the level of the sigmoid colon, a large amount of content was discovered, which, from the level of the splenic flexure of the colon, completely covered all the walls of the intestine, allowing, however, to pass the apparatus through the lumen to the dome of the cecum. Due to difficulties with repeated use of anesthesia, it was decided to continue the procedure. No pathology was found. However, clinical manifestations characteristic of the toxicoanemic form of colon tumor made it possible to doubt the results of FCS. Diagnostic laparoscopy revealed a tumor of the ascending colon with predominantly endophytic growth without stenosis of the intestinal lumen. A right hemicolectomy with anastomosis was performed. Examination of the gross specimen allowed us to conclude that, provided adequate preparation, the tumor would undoubtedly have been detected during an endoscopic examination (FCS).
Our experience allows us to assert that the drug for oral bowel cleansing Fortrans is the optimal means of preparation for FCS, sigmoidoscopy and irrigography. Macrogol 4000, which is the basis of the drug Fortrans, is not metabolized and is not absorbed in the intestine, provides effective lavage without the development of electrolyte disturbances and side effects. To prepare for the study, take 3-4 sachets of the drug, dissolving each in a liter of water. Patients with constipation are recommended to take 4 sachets, and for loose stools - three. Despite the need to drink a fairly large amount of liquid (3-4 liters), the average rate of taking the drug is 1 glass every 15 minutes, in the vast majority of cases this makes it not burdensome for the patient and is easy for them to do. The release of rinsing water begins in most cases 60-80 minutes after the start of taking the drug and continues until 45-60 minutes after the end of taking it. When preparing for an outpatient examination, we do not see an alternative to the oral preparation method, because Only a very small percentage of the population can effectively cleanse the colon using enemas.
In this work we will not dwell in detail on the technical aspects of the implementation of the FCS, limiting ourselves only to the general principles:
- Inflate the intestine with air as little as possible to maintain vision, and aspirate excess air whenever possible.
- Avoid forming extra loops.
- Retreat back and “gather” the gut at every opportunity.
- Assess the distance to which the colonoscope is inserted, focusing on the anatomical features of the intestine (splenic angle 50 cm from the edge of the anus).
- Pay attention to the patient's discomfort, which indicates excessive looping or excessive distention of the intestine.
The use of an intestinal antispasmodic - Dicytel in a dosage of 50 mg 3 times a day for 3 days before the study and 50 mg immediately before performing FCS can reduce discomfort during and after the procedure. The frequency of colonoscopy as a screening method in a group of patients at average risk is once every 5-10 years.
A new method for diagnosing colon pathology is virtual colonoscopy, first proposed in 1994. It allows you to obtain an image of the colon from the inside based on many sections obtained using a computed tomograph and processed with a special program. This method makes it possible to detect formations larger than 10 mm in 90% of cases, and less than 10 mm in 70-80% of cases [11]. However, false-positive results are also possible, most often due to inadequate preparation. A big disadvantage of virtual colonoscopy is the impossibility of morphological verification of the diagnosis. We did not find data on the required frequency of performing virtual colonoscopy for screening purposes in the available literature.
Classification of colorectal cancer
Based on the type of growth of colorectal carcinoma, there are exophytic - a form that grows like a polyp and an endophytic form that spreads inside the intestinal wall and often narrows the lumen of the tube.
Based on the cellular structure, eight out of ten patients have colon adenocarcinoma, one has mucous adenocarcinoma, and signet ring cell and squamous cell carcinoma are even less common.
Regardless of the level of localization of the process, the following distribution by prevalence is assumed:
- Stage 1 - the tumor occupies only the mucous membrane;
- Stage 2 - carcinoma is inside the intestinal wall, but has not gone outside - into the abdominal cavity;
- Stage 3 - cancer conglomerate of any size with metastatic lymph nodes;
- Stage 4 - metastases are found in other organs.
The stage of the malignant process is established before chemotherapy with radiation or after surgery. A correctly established stage is the key to choosing optimal therapy, so it is necessary to have high-precision diagnostic equipment in the clinic, all modern tests must be done, and doctors must be experienced. Euroonco patients have access to everything they need for this.
Diagnosis of colorectal cancer
Diagnosing colorectal cancer is not particularly difficult - colonoscopy is recognized as the “gold standard” for detecting any intestinal pathology and allows taking material for microscopy. The entire intestinal tube is examined - completely. If colonoscopy is not possible, they resort to irrigoscopy - fluoroscopy with the introduction of contrast into the intestine, or to modern CT colonography.
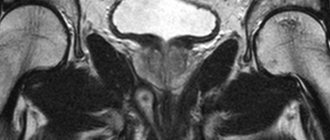
An MRI of the pelvis is mandatory for rectal cancer; an MRI of the abdominal cavity or a similar volume of CT will reveal all extraintestinal manifestations of the disease. A comprehensive examination - endoscopy with simultaneous ultrasound will allow you to understand the extent of damage to the intestinal wall.
If metastases in the abdominal cavity are suspected, laparoscopy is performed.
Tumor markers CEA and CA 19.9. do not help in the early detection of cancer, because they increase for many everyday reasons, but monitoring their level allows us to understand the dynamics of the process and evaluate the effectiveness of treatment.
To select the optimal drug treatment, genetic mutations and microsatellite instability are identified in a piece of the tumor.
Table 1 Sensitivity of the main screening tests
| Screening tests | Sensitivity (%) | |
| Formation less than 10 mm | Formation more than 10 mm | |
| 1. Occult blood test | Around 17 | 23,9 |
| 2. Sigmoidoscopy | About 65* | 70,3* |
| 3. Barium enema | 32-52 | 48-90 |
| 4. Fiber colonoscopy | 75-85 | 94 |
| 5. Virtual colonoscopy | 70-80 | 90 |
*The sensitivity of sigmoidoscopy in detecting formations of the rectum and sigmoid colon is similar to that of FCS.
In patients at high risk for colorectal cancer, screening methods and frequency do not differ from those at average risk, but screening should begin at age 40, or 10 years earlier than the earliest age of onset of colon cancer in a relative.
Metastasis
Colorectal cancers metastasize to surrounding lymph nodes; through lymphatic vessels, cancer cells can reach the supraclavicular region. The degree of involvement of the lymphatic collector correlates with the aggressiveness of the carcinoma and the duration of its existence. With well-differentiated adenocarcinoma, the course is more favorable, and metastases are not so abundant.
Through blood vessels, cancer screenings are carried to the liver and, a little less often, to the lungs. Bone metastases are not at all characteristic of CRC, but are not excluded in poorly differentiated adenocarcinoma.
Germination of the intestinal wall can lead to the spread of cells throughout the abdominal cavity and, often, carcinomatosis with ascites.
Surgery
Colon surgery
With the initial process in the colon, organ-preserving intervention is preferable using endoscopic equipment and removing only the affected area of the mucous membrane - endoscopic resection. Fundamentally, all malignant processes in the large intestine can be divided into two groups: operable or resectable and, accordingly, non-operable. A colorectal mass is removed in several ways, the scope of the operation depends on the size of the lesion: resection of part of the intestine, removal of half the intestine - hemicolectomy, almost the entire large intestine - colectomy and Hartmann's operation. The extent of surgery is not affected by how the patient came to the operating table - urgently for health reasons or as planned with high-quality preoperative preparation. The preferred operation is for a small number of metastases in the liver or lungs, but only if it is technically possible to remove them simultaneously with the affected intestine. If the tumor cannot be removed, in order to avoid the development of fatal intestinal obstruction, the intestine is removed to the abdomen - a stoma, and a bypass is created inside the abdominal cavity past the affected area.
Rectal surgery
The algorithm for surgical treatment of rectal carcinoma is similar to surgical approaches for colon cancer, with the only difference being that surgery is supplemented with local radiation therapy and chemotherapy.
The main operation is mesorectumectomy only for early carcinoma and is not supplemented by radiation.
A patient with an inoperable process is referred to radiology unless there is a need for palliative surgery, such as placing a stent in the narrowed area. After irradiation, the possibility of rectal surgery is again considered.
If it is impossible to perform an operation at the first stage, the question of it should be raised after each subsequent treatment stage - only surgical intervention has a significant impact on life expectancy.
Treatment for different stages
Early stages of colon cancer are highly treatable. The survival rate is more than 40%. However, even with an advanced form of cancer, there is a possibility of a successful outcome. It all depends on the treatment tactics chosen by the doctor and the body’s overall resistance to the disease.
- Stage 0 is a non-invasive type of cancer that can be removed without consequences to the body using polypectomy.
- Stage 1 - the tumor begins to grow deeper into the intestinal wall, the lymph nodes and nearby organs are not affected by tumor cells. The main treatment is anterior rectal resection. Additionally, radiation or chemotherapy may be prescribed.
- Stage 2 - cancer affects all layers of the intestinal mucosa and begins to gradually spread to nearby tissues. A cancer patient is shown two stages of radiation and/or chemotherapy in combination with surgery.
- Stage 3 - the disease metastasizes to regional lymph nodes, but there is no damage to neighboring organs. Combination therapy is indicated, combining preoperative chemotherapy, surgery and a postoperative course of chemotherapy.
- Stage 4 - there is extensive damage to other organs by malignant cells. Treatment involves resection of the intestine and subsequent partial removal of the organ affected by cancer. Next, the patient is prescribed an intensive course of chemotherapy. The oncologist, monitoring the patient’s condition and the dynamics of the disease, can prescribe additional treatment (chemotherapy, radiation therapy).
IMPORTANT:
If the tumor is not operable, the patient is prescribed only chemotherapy.
Chemotherapy for colorectal cancer
After radical surgery for advanced or metastatic colorectal cancer in the lymph nodes, prophylactic six-month chemotherapy is carried out; it should begin within the first 4 weeks. This is also done with simultaneous removal of the primary tumor and metastases in the liver or lungs.
If chemotherapy was given before surgery, after surgery, drug treatment is carried out, covering a total period of 6 months. There are several combinations of cytostatics, but they all necessarily include fluoropyrimidines.
For inoperable rectal carcinoma, chemotherapy with fluoropyrimidine is carried out together with long-term radiation, and after completion of radiation, several more courses of chemotherapy are added.
For inoperable colon cancer, after the colostomy is removed, long-term chemotherapy with FOLFIRI, FOLFOX or other combinations with fluoropyrimidines begins.
A patient with newly diagnosed metastatic disease can count on long-term drug treatment; if the process stabilizes, the break in chemotherapy will last until symptoms of progression appear.
Without fail and repeatedly, the possibility and advisability of surgical intervention should be considered for each patient after completion of each stage of treatment. But there is one problem - urban oncology institutions suffer from queues for primary treatment, so they rarely take patients with a history of refusal of surgical intervention to the operating table.
Prognosis and survival of colorectal carcinoma
The prognosis is determined by many factors, but the main ones are the spread of the disease at the time of treatment and the morphological characteristics of the cells, that is, aggressiveness and resistance to drug effects.
The main cause of death in colorectal cancer is metastasis to the liver, either alone or in combination with damage to the lungs and lymph nodes.
After treatment for rectal carcinoma, recurrence at the surgical site or continued growth after chemoradiotherapy, as well as metastatic liver disease, often occur.
A good prognosis is promised by early colorectal cancer without lymph node metastases.
Types of colorectal oncology
Most diagnoses are adenocarcinomas
, starting in the cells that produce the mucus needed to lubricate the inside of the colon and rectum. The following types of neoplasms are much less common:
- Carcinoid tumors
: arise in cells that produce hormones - substances that are created by our glands, enter the bloodstream, travel with it to organs and tissues and tell them how to act - to work or rest, to secrete something or absorb something. - Gastrointestinal
, or gastrointestinal stromal
tumors
: arise in the interstitial cells of Cajal, which set the rhythm of intestinal contractions. Not all of them are malignant, that is, capable of growing into surrounding tissues and creating metastases - additional cancerous foci in other areas of the body. - Lymphomas
are an oncological disease of the immune system. They mainly begin in the lymph nodes, which retain and neutralize dangerous substances, but can also form in other organs - the spleen, bone marrow. Bone marrow is soft tissue located in some parts of the skeleton. It produces new blood cells and some lymphocytes - proteins that protect the body from bacteria and viruses., thymus gland The thymus, or thymus gland, is a small organ located in front of the heart. Some types of lymphocytes - proteins that protect the body from bacteria and viruses - mature and develop in it. tonsils Tonsils, or tonsils, are organs located in the back of the pharynx in which antibodies are produced - proteins that stop the proliferation of inhaled and ingested microorganisms. and the digestive tract. - Sarcomas
: Can develop in blood vessels, muscle layers, and other areas of the wall of the colon and rectum.
Prevention of colorectal cancer
It is impossible to prevent the development of many malignant diseases, but regular endoscopic examination of the intestines after 45 years of life can prevent the development of colorectal cancer - this is a scientific truth.
You can eliminate all risk factors for the disease, eat right and maintain normal weight, but with multiple polyps the carcinogenic risk approaches 100%, it is unlikely to avoid a malignant tumor by refusing regular colonoscopic monitoring and removal of villous polyps.
Several polyps or even one increase the risk of CRC up to 20%; Crohn's disease and other ulcerative lesions of the mucous membrane can result in cancer in every 20–25 patients.
Treatment and detection of oncological diseases is the specialty of our Clinic, our goal is to restore your health, if this is not possible, then we are ready to qualitatively change your life.
| More information about treatment at Euroonco: | |
| Proctologist-oncologist | RUB 5,100 |
| Oncologist-gastroenterologist | RUB 5,100 |
| Chemotherapy appointment | RUB 6,900 |
| Emergency oncology care | from 12,100 rub. |
| Palliative care in Moscow | from 44,300 rubles per day |
| Radiologist consultation | RUB 11,500 |
Book a consultation 24 hours a day
+7+7+78