Irritable bowel syndrome in children: main causes and approaches to treatment
Personal and psychological aspects, genetic predisposition, nutritional factors, changes in visceral hypersensitivity, disorders of motor activity and the neuroendocrine system (brain-gut axis), increased intestinal permeability, and disruption of the composition of the intestinal microbiota are important in the development of the disease. Qualitative and quantitative changes in microflora affect intestinal function, acting as a cause of disturbances in its motor activity, sensitivity and neuroimmune relationships, including impaired expression of mucosal receptors and changes in the function of the hypothalamic-pituitary-adrenal system. In pediatric practice, the possibilities of using medications are significantly limited by the age of the patients. In therapy, preference should be given to selective drugs that affect visceral sensitivity and probiotics with proven effectiveness and safety of strains.
Information about authors:
Zakharova Irina Nikolaevna – Doctor of Medical Sciences, Professor, Head of the Department of Pediatrics named after. G.N. Speransky Federal State Budgetary Educational Institution of Further Professional Education "Russian Medical Academy of Continuing Professional Education" of the Ministry of Health of Russia;; e-mail: [email protected]
Sugyan Narine Grigorievna – Candidate of Medical Sciences, Associate Professor of the Department of Pediatrics named after. G.N. Speransky Federal State Budgetary Educational Institution of Further Professional Education "Russian Medical Academy of Continuing Professional Education" of the Ministry of Health of Russia;
Berezhnaya Irina Vladimirovna - Candidate of Medical Sciences, Associate Professor of the Department of Pediatrics named after. G.N. Speransky Federal State Budgetary Educational Institution of Further Professional Education "Russian Medical Academy of Continuing Professional Education" of the Ministry of Health of Russia.
Key words : children, irritable bowel syndrome, prokinetics, trimebutine, neobutin, probiotics, maxilac.
Irritable bowel syndrome (IBS) is the most common functional bowel disease in adults, representing a significant social problem with a negative impact on quality of life. In most countries of the world, the incidence of IBS averages about 20%, ranging from 9 to 48%. There is little information on the prevalence of IBS in children; for example, in the USA, according to various authors, the frequency ranges from 1.2 to 2.9% [1]. It is difficult to estimate the prevalence of IBS in the population, since statistical data vary significantly even in different regions of the same country. Epidemiological indicators, summarized from questionnaires in different countries of the world, show that the numbers range from 10 to 25%. According to the meta-analysis, the overall prevalence of the disease was about 11.2% (95% CI, 9.8–12.8) with differences in regions of the globe: from the lowest in South Asia (7%) to the highest in South Asia. America (21%). It has been shown that in the first few months after the diagnosis of IBS, patients suffer up to 4 attacks with severe abdominal pain. Subsequently, in 30–40%, the light intervals between exacerbations increase. Approximately half of patients with IBS (45%) develop chronic diseases of the upper gastrointestinal tract (dyspepsia, chronic gastritis, GERD, etc.) after several years of IBS.
Functional disorders of the gastrointestinal tract are one of the most mysterious problems of modern medicine, since, despite the huge range of diagnostic procedures, it is not possible to fully understand the etiology and pathogenesis. We have been using clinical guidelines known as the Rome criteria for about 30 years. In 2016, a new edition of the clinical guidelines for functional gastrointestinal disorders - Rome IV was published, which includes sections on functional gastrointestinal disorders (FGID) in adults, children and adolescents. The emerging evidence base over the past 10 years has provided the basis for the Rome IV Revision recommendations. For some digestive disorders, the committee used clinical experience and consensus of committee members, still lacking reliable scientific evidence, for these sections. In the scientific literature one can find different interpretations of the translation “functional gastrointestinal disorders” - functional gastrointestinal disorders (FGID), functional disorders of the digestive organs (FDOP) and others. FROP more fully reflects the problem, since the consensus covers functional disorders of the upper and lower gastrointestinal tract and biliary tract disorders.
The Rome III criteria emphasized that organic disease must be excluded to establish functional impairment. In the Rome IV Revision Criteria, the phrase “no evidence of an inflammatory, anatomical, metabolic, or neoplastic process explaining the subject's symptoms” was removed from the diagnostic criteria [2]. Instead, it added “after appropriate medical examination, the symptoms cannot be attributed to another disease.” This change allows for random testing to confirm a positive diagnosis of FROP. It has also been noted that FROP can coexist with other organic and chronic inflammatory diseases [3]. In the same way, one patient may have different FROPs. The latest version of the international consensus recommendations, Rome IV, published in 2016, considers functional intestinal disorders as a spectrum of intestinal symptoms in 6 categories, which are defined as disorders of the gut-brain interface (GI-CNS). (central nervous system”) (disorders of gut-brain interaction) [4].
The new edition of the Rome IV criteria (2016) includes new disorders: functional nausea and functional vomiting. The wording “abdominal pain associated with functional gastrointestinal disorders” was changed to “functional abdominal pain disorders”. This group includes a new term, functional abdominal pain (aka “FAP-NOS”), to describe children with abdominal pain whose diagnostic criteria are insufficient for inclusion in a specific disorder, such as IBS, functional dyspepsia, or abdominal migraine.
The term “discomfort” in the new version of the Rome IV criteria has been removed from the definition, since it is difficult to distinguish between discomfort and abdominal pain in children. In addition, as a diagnostic criterion, there are no complaints such as bloating, stretching or other sensations of the patient [5].
FROP is based on combined physiological and morphological abnormalities associated with visceral hypersensitivity, disorders of gastrointestinal motility, protective mucosal barrier, immune function and composition of the intestinal microbiota, as well as disorders of the central nervous system.
In the development of FROP in children at an early age, factors such as morphofunctional immaturity, insufficient activity of pancreatic and gastrointestinal enzymes, impaired microbiocenosis, and insufficiency of mucosal immunity play an important role. At an older age, visceral hypersensitivity is caused by a combination of factors: social relationships, stress, overwork, disruption of the daily routine, and rest.
The development of IBS is based on the interaction of two main pathogenetic mechanisms: psychosocial effects and impaired motor function of the colon, which is manifested by an increase in visceral sensitivity and impaired motor function of the intestine (Table 1).
Table 1. Diagnostic criteria for IBS for children (signs must be present for at least 2 months) [ 1 ].
| The following symptoms must be present: | |
| 1. Abdominal pain at least 4 days per month associated with 1 or more of the following symptoms: | A. associated with defecation; b. associated with changes in frequency of bowel movements; c. associated with a change in the shape (appearance) of the stool. |
| 2. With IBS with constipation, the pain does not go away after a bowel movement (if the pain goes away after a bowel movement, then the child has functional constipation, not IBS). | |
| 3. After appropriate evaluation, symptoms cannot be attributed to another disease. | |
The pain can be of varying intensity and is usually localized in the lower abdomen, although it can also be noted in other parts of the abdomen. It often intensifies with errors in diet, psycho-emotional stress, worries, and also with physical activity. It is important to note that abdominal pain disappears at night when the child is sleeping.
If the pain goes away after a bowel movement, then most likely the child has functional constipation, rather than IBS with constipation. It has been found that 75% of children suffering from constipation have abdominal pain, which falls under the criteria for IBS. But patients with IBS are observed for a long time with a diagnosis of functional constipation without receiving effective medical care. The committee recommends that patients with constipation and abdominal pain be treated initially only for constipation. If abdominal pain goes away after treating constipation, then the patient has functional constipation. If the pain persists, your child may have IBS with constipation. Likewise for possible IBS with diarrhea. At the first stage, it is necessary to exclude intestinal infection with a recurrent, chronic course, celiac disease, carbohydrate malabsorption and, less commonly, inflammatory bowel disease. It is important to pay attention to alarming signals (“alarm symptoms”, “red flags”), in the presence of which it is necessary to exclude organic pathology of the gastrointestinal tract. It is indicated that the more alarming symptoms are present, the higher the likelihood of organic disease (Table 2). Fecal calprotectin is increasingly used as a non-invasive screening test to rule out inflammatory bowel disease (IBD) [6].
Table 2. Symptoms of anxiety in patients with FROP.
| family history of inflammatory bowel disease, celiac disease, or peptic ulcer disease; |
| Constant pain in the right upper or right lower quadrant; |
| Dysphagia, odynophagia; |
| Recurrent vomiting; |
| Bleeding from the gastrointestinal tract; |
| Nocturnal diarrhea; |
| Arthritis; |
| Perirectal lesions; |
| Unmotivated weight loss, weight loss, malnutrition; |
| Delayed puberty; |
| Unexplained fever; |
In children, as in adults, IBS can be divided into different types reflecting the predominant stool pattern (IBS with constipation, IBS with diarrhea, IBS with constipation and diarrhea, and unspecified IBS) and these types are now included in Rome IV [7].
Table 3. Functional disorders of the digestive organs: disruption of the interaction of the gastrointestinal tract and the central nervous system according to the Rome IV criteria (Rome IV and ICD-10 codes).
| C. Intestinal disorders | ||
| C.1 Irritable bowel syndrome (IBS) | IBS with constipation predominance (IBS-3) IBS with diarrhea predominance (IBS-D) IBS mixed type (SRK-Sm) Unclassified IBS (IBS-U) | |
| Formulation | Rome IV codes | ICD-10 |
| Functional constipation | C.2 | K59.0 constipation K58.2 Irritable bowel syndrome with predominant constipation |
| Functional diarrhea | C.3 | K59.1 Functional diarrhea K58.1 Irritable bowel syndrome with predominant diarrhea |
| Functional abdominal bloating/distension | C.4 | R14 Flatulence and related conditions K59.9 Functional bowel disorder, unspecified K58.8 Other or unspecified irritable bowel syndrome |
| Nonspecific functional intestinal disorder | C.5 | K59.2 Neurogenic excitability of the intestine, not classified elsewhere K58.3 Irritable bowel syndrome with mixed manifestations |
| Opioid-induced constipation | C.6 | K59.0 Constipation |
The complexity of the etiopathogenesis of the disease in children is explained by a combination of factors: personal psychological aspects, genetic predisposition, nutritional factors, the development of visceral hypersensitivity, impaired motor activity, changes in the neuroendocrine system (brain-gut axis), increased intestinal permeability, disruption of intestinal composition microbiota.
Scientific studies have shown that visceral hypersensitivity may be associated with childhood psychological stress (anxiety, depression, impulsivity, anger) [8].
Children with IBS may experience increased levels of self-reported stress, anxiety, depression, and emotional problems [9]. Severe illnesses requiring medically invasive intervention at an early age (eg, surgery) are associated with a higher risk of developing PDOP in children, including IBS [10].
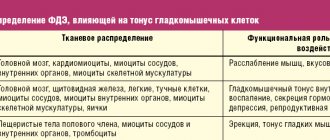
The pathophysiology of IBS includes the influence of external irritating factors (stress, high emotional stress, lack of sleep, etc.), which excites the central nervous system, the autonomic response, with an impact on the intestinal neuroendocrine system, including the hypothalamic-pituitary-adrenal (cortisol) system. This disrupts the motor activity of the intestines, increases spasm of smooth muscles, and disrupts the passage of intestinal contents. On the other hand, changes in intestinal motor activity lead to overexcitation of intraintestinal nerve fibers, Cajal cells, increasing spasm of smooth muscles, the microbiota changes, which affects the environment and the synthesis of SCFA. Thus, a vicious circle arises (Table 4).
Table 4. Pathophysiology of IBS development: the brain-gut connection [11].
| Dysfunction of the brain-gut axis | ||
| Corticotropin releasing factor (CRF) | Activation of the enteric nervous system | Change in microbiota |
| Activates CRF-1 receptors → increased motor activity under stress Pro-inflammatory activity → increased synthesis of IL-1 and IL-2 Enhanced response to endotoxins Increased cortisol and adrenaline levels Increased sensitivity of the enteric nerves | Excessive release Neurotransmitters → impaired peristalsis Increased mast cell degranulation → disruption of the serotonin signaling cascade Imbalance in the synthesis of pro-inflammatory and inflammatory mediators Increased intestinal barrier permeability | Violation of local immunity Expression of the synthesis of neurotransmitters (serotonin, GABA, histamine, acetylcholine, melatonin, etc.) Disruption of the mucosal barrier and microbial biofilm Modulation of intestinal sensitivity of afferent fibers |
IBS often develops after an acute intestinal infection; an increase in the content of pro-inflammatory cytokines in the gastrointestinal mucosa is noted, and post-infectious IBS develops [12]. A systematic review published in 2015 showed the incidence of post-infectious functional dyspepsia and IBS in the population, including children 10.0% (FD, n = 976; OGE, n = 9737) and 13.1% (IBS, n = 1128; OGE, n = 8624), respectively. The long-term persistence of disorders was also indicated; it was found that 1 year after the experience. gastroenteritis, post-infectious functional dyspepsia persisted in 13.5% of patients (n = 380; OGE n = 2807) and irritable bowel syndrome in 38.9% of children (n = 86; OGE n = 221) [13].
Another study indicated the role of parasitic infection (Balantidiumcoli, Strongyloidesstercoralis, Ascarislumbricoides, Necatoramericanus, Ancylostomaduodenale, Taeniasolium, Taeniasaginata and Hymenolepisnana) in the development of post-infectious FROP, in particular in the development of IBS (OR: 1.69), functional constipation (OR: 4.13 ) [14].
The role of intestinal microbiota in the pathophysiology of IBS is currently the subject of a large number of experimental and clinical studies. Changes in the gut microbiome have been demonstrated, although it is unclear whether these changes are a cause or a result of IBS and its symptoms [15].
It has been established that qualitative and quantitative changes in microflora affect intestinal function, acting as a cause of disturbances in its motor activity, sensitivity and neuroimmune relationships, including impaired expression of mucosal receptors and changes in the function of the hypothalamic-pituitary-adrenal system. It is known that intestinal microflora utilizes insoluble carbohydrates and releases metabolites, among which short-chain fatty acids (acetic, propionic and butyric acids) are important. It is these short-chain fatty acids (SCFAs) in different parts of the colon that have the opposite effect on its motor activity, through stimulation of certain receptors that produce biologically active substances. In the proximal parts of the colon, SCFAs stimulate L-cell receptors, which produce the regulatory polypeptide PYY, which slows down the motor activity of not only the colon, but also the small intestine. And in the distal sections, SCFAs stimulate the receptors of Ecl-cells that produce histamine, which, acting on the 5-HT 4 receptors of the afferent fibers of the vagus nerve, initiates a reflex acceleration of motor activity. Among SCFAs, butyrate (butyrate) is important for colonocytes, which is the most important energy source for colonocytes, membrane lipid synthesis, protective barrier and permeability of the colon mucosa, suppression of oxidative stress, inflammation, colorectal carcinogenesis, restoration of water and electrolyte balance. In addition, butyric acid (butyrate) has a regulatory effect on intestinal motility [16]. SAVanhoutvin et al. studied the effect of butyrate (butyrate) on visceral hypersensitivity in healthy volunteers. Study participants were given butyric acid rectally. The results of the study showed that butyric acid administration increased the pain threshold and reduced the pain and discomfort caused by rectal balloon inflation, as assessed by an analogue scale. It was noted that the administration of butyrate had a dose-dependent effect; the higher the dose of butyrate, the more visceral sensitivity decreased [17].
The use of one of the strains of Bifidobacterium bifidum in patients with symptoms of IBS led to a significant reduction in symptoms such as abdominal pain/discomfort, flatulence, and indigestion in 57% of patients compared to 21% in the placebo group [19].
The meta-analysis assessed the results of 24 clinical trials involving 2001 patients with IBS. The results of the analysis showed a significant reduction in pain and general symptoms in patients using multi-strain probiotics. Multistrain probiotics were evaluated in 12 RCTs involving 1197 patients with a significant effect on abdominal pain symptom (RR = 0.81; 95% CI 0.67–0.98, p
According to studies conducted in patients with IBS, using various probiotics and their combinations, it was found that:
- L. rhamnosus LGG., L. Rhamnosus, B. animalis B. lactis – help stabilize the intestinal microflora [20, 21].
- S.thermophillus, L. acidophilus, B. longum – lead to optimization of the intestinal mucosal barrier [21];
- L. rhamnosus LGG., L. Rhamnosus, B. animalis, B. lactis; L. acidophilus, B. Bifidum – help reduce abdominal symptoms [22];
- B. longum, L. Acidophilus, L. lactis, S. thermophilus, L. plantarum – increase the quality of life of patients [23, 24];
- L. Acidophilus, L. lactis, S.thermophillus (Drouault-Holowacz S et al. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol 2008), B. bifidum [25, 26 ] – help eliminate bloating [25].
The results of a systematic review (Simrén M. 2014) confirm the role of synbiotic complexes, a combination of multi-strain probiotics and prebiotics in the complex treatment of IBS manifestations (54).
One of the multi-strain probiotics is Maxilac , which includes bifidobacteria (Bifidobacterium longum, Bifidobacteriumbreve, Bifidobacteriumbifidum) and lactobacilli (Lactobacillusacidophilus, Lactobacillusrhamnosus, Lactobacilluscasei, Lactobacillusplantarum, Lactobacilluslactis), Streptococcusthermophilus 108 CFU, as well as the prebiotic component fructooligosaccharide. The innovative DRcaps™ capsule, in turn, neutralizes the negative effects of acidic stomach contents, bile salts and digestive enzymes.
The leading role in the structure of abdominal pain syndrome most often plays spastic visceral pain, which is based on involuntary contraction of intestinal smooth muscles, not accompanied by their immediate relaxation. Selective myotropic antispasmodics have age restrictions in children. In terms of clinical effectiveness, trimebutine is comparable to antispasmodics such as pinaveria bromide and mebeverine in the treatment of abdominal pain in IBS in adults [27].
The mechanism of action of trimebutine is to stimulate peripheral opioid (enkephalin) receptors (μ-, k-, δ-) throughout the gastrointestinal tract. Binding to k-receptors leads to a decrease in muscle activity, and binding to μ- and δ-receptors causes its stimulation. However, the drug does not affect other receptors. Trimebutine has a direct effect on smooth muscle cells through receptors of myocytes and ganglia of the enteric nervous system, imitating the action of enkephalins [28]. There is a generic version of trimebutine on the Russian pharmaceutical market: Neobutin® (available in tablet form in dosages of 100 and 200 mg), which is approved for children from 3 years of age. The drug has a normalizing effect on the motor function of the gastrointestinal tract: depending on the initial state of the gastrointestinal tract, it has a stimulating or relaxing effect on the gastrointestinal tract. In addition, trimebutine, influencing Na+ and Ca2+ channels, provides an anesthetic effect and a direct antispasmodic effect, thus indirectly normalizing gastrointestinal motility and visceral hypersensitivity.
A comparative assessment of the effectiveness of therapy with drugs that coordinate intestinal motility (trimebutine) both monotherapy and in combination with probiotics in children aged 5-17 years with symptoms of IBS was carried out. In the first group (n=15), treatment with trimebutine was carried out at an age-specific dosage for a course of 1 month; in the second group (n=15), combination therapy with trimebutine + probiotics was carried out. The authors determined the level of fecal calprotectin (a protein that reflects the severity of the inflammatory process) as an indicator of inflammation in the intestines. The level of calprotectin in patients with IBS was initially increased in 27.3% of children, the average level of the indicator was 2 times higher than normal values.
The results obtained showed the high effectiveness of trimebutine (in 82% of children) in monotherapy, and the best result (up to 98%) when combining trimebutine with a probiotic. In the placebo group, relief of abdominal pain was observed in 22% of children. Normalization of stool in 99% of children in the second group and 15% in the placebo group [29].
Another study assessed the prevalence of IBS in children and the effectiveness of trimebutine in correcting clinical symptoms. 345 children and adolescents aged 4-18 years were included. The prevalence of IBS according to Rome III criteria in children and adolescents was 22.6%, with IBS with constipation being the predominant subtype. 78 children and adolescents with normal laboratory parameters and an established diagnosis of IBS were randomized into two groups: 39 patients received trimebutine 300 mg per day for three weeks, 39 patients did not receive drug treatment. Clinical improvement was observed in 94.9% of patients treated with trimebutine versus 20.5% in the no-drug group (P
Thus, the use of synbiotic complexes containing a multi-strain composition of probiotic strains of lacto- and bifidobacteria, which have a positive effect on the qualitative and quantitative composition of the gastrointestinal microflora, and trimebutine maleate, which can have a direct normalizing effect on the motor function of the gastrointestinal tract, has grounds for inclusion in complex therapy of irritable bowel syndrome in children.
To learn more
Bibliography
- Van Tilburg MAL, Walker L, Palsson O, et al. Prevalence of child/adolescent functional gastrointestinal disorders in a national US community sample. Gastroenterology 2014;144(Suppl 1):S143–S144.
- Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr 2010;51:579–583.
- Faure C, Giguere L. Functional gastrointestinal disorders and visceral hypersensitivity in children and adolescents suffering from Crohn's disease. Inflamm Bowel Dis 2008;14:1569–1574.
- Drossman DA, Hasler WL Rome IV — Functional GI disorders: disorders of Gut-Brain interaction // Gastroenterology. 2016. Vol. 150 (6). P.1262–1279.
- Jeffrey S. Hyams, Carlo Di Lorenzo, Miguel Saps, Robert J. Shulman, Annamaria Staiano, Miranda van Tilburg. Childhood Functional Gastrointestinal Disorders: Child/ Adolescent // Gastroenterology 2016;150:1456–1468.
- Henderson P, Casey A, Lawrence SJ, et al. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease. Am J Gastroenterol 2012;107:941–949.
- Rajindrajith S, Devanarayana NM. Subtypes and symptomatology of irritable bowel syndrome in children and adolescents: a school-based survey using Rome III criteria. J Neurogastroenterol Motil 2012;18:298–304.
- Iovino P, Tremolaterra F, Boccia G, et al. Irritable bowel syndrome in childhood: visceral hypersensitivity and psychosocial aspects. Neurogastroenterol Motil 2009;21. 940-e74.
- Waters AM, Schilpzand E, Bell C, et al. Functional gastrointestinal symptoms in children with anxiety disorders. J Abnorm Child Psychol 2013;41:151–163.
- Bonilla S, Saps M. Early life events predispose the onset of childhood functional gastrointestinal disorders. Rev Gastroenterol Mex 2013;78:82–91.
- Zakharova I.N., Berezhnaya I.V., Kholodova I.N., Zaidenvarg G.E. Abdominal pain in a child: pediatrician tactics. Training manual for doctors. – M.: FGBOU DPO RMANPO. –2020. – 128 p.
- Saps M, Pensabene L, Turco R, et al. Rotavirus gastroenteritis: precursor of functional gastrointestinal disorders? J Pediatr Gastroenterol Nutr 2009;49:580–583.
- S. Futagami 1, T Itoh, C Sakamoto. Systematic review with meta-analysis: post-infectious functional dyspepsia // Aliment Pharmacol Ther. 2015 Jan;41(2):177-88. doi: 10.1111/apt.13006.
- Blitz J, Riddle MS and Porter CK (2018) The Risk of Chronic Gastrointestinal Disorders Following in Acute Infection with Intestinal Parasites. Front. Microbiol. 9:17. doi: 10.3389/fmicb.2018.00017.
- Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011;141:1792–1801.
- Hamer HM. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104-19.
- Vahoutvin SA, Troost FJ, Kilkens TO. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21(9):952-76.
- S. Guglielmetti, D. Mora, M. Gschwender & K. Popp. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life –– a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011.
- Alexander C. Ford, MBChB, MD et al. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis. Am J Gastroenterol 2014; 109:1547–1561; doi: 10.1038/ajg.2014.202.
- Michael J Fox et al. Can probiotic yogurt prevent diarrhoea in children on antibiotics? A doubleblind, randomized, placebo-controlled study. BMJ Open 2015.
- Ki Cha B et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol 2012; 46.
- Williams EA, Stimpson J, et al. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo controlled study. Aliment Pharmacol Ther 2009.
- Drouault-Holowacz S et al. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol 2008);
- Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome — a randomized, double-blind, controlled study . Aliment Pharmacol, Ther 2010.
- A Randomized Clinical Trial of Synbiotics in Irritable Bowel Syndrome: Dose-Dependent Effects on Gastrointestinal Symptoms and Fatigue Sang-Hoon Lee, Doo-Yeoun Cho at al, The Korean Academy of Family Medicine. Received: May 16, 2022,
- L. plantarum (Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol 2012; 18.
- Rahman MZ, Ahmed DS, Mahmuduzzaman M. et al. Comparative efficacy and safety of trimebutine versus mebeverine in the treatment of irritable bowel syndrome // Mymensingh Med J. 2014; 23 (1): 105–113.
- Trukhan D.I., Bagisheva N.V., Goloshubina V.V., Grishechkina I.A. Trimebutin in the treatment of gastrointestinal functional disorders // International Journal of Applied and Fundamental Research. 2016; 11(6):1072–1076.
- Tipikina M.Yu., Kornienko E.A. Pathogenetically based strategy for the treatment of irritable bowel syndrome in children. Questions of children's dietetics. 2014; 12(1): 22-27.
- Gülcan S Karabulut. The Incidence of Irritable Bowel Syndrome in Children Using the Rome III Criteria and the Effect of Trimebutine Treatment. J Neurogastroenterol Motil. 2013;19(1): 90-3. doi:10.5056.
Newspaper "News of Medicine and Pharmacy" Gastroenterology (294) 2009 (thematic issue)
In the clinical practice of a gastroenterologist, functional intestinal dysfunctions are often encountered. Functional bowel disorders (FBD) include a group of heterogeneous clinical conditions that present with symptoms of the middle and lower gastrointestinal tract and are not accompanied by any structural, systemic or metabolic changes. Despite the lack of an organic basis, functional diseases reduce the quality of life of patients and cause great economic damage to society, both in terms of direct indicators of the costs of medical care and treatment, and indirect indicators, including compensation for temporary disability [3, 4]. For most practitioners, the diagnosis of functional intestinal disorders causes concern due to new terminology and a lack of clear understanding of their essence [7]. In 2006, experts from the International Group for the Study of Functional Diseases of the Digestive Organs reviewed and refined the definition, classification, diagnostic criteria and recommendations for the treatment of functional intestinal disorders, which was reflected in the materials of the III Rome Consensus [1, 4]. The classification of functional pathology of the gastrointestinal tract (GIT) includes the following categories of intestinal disorders:
Functional intestinal disorders (III Rome Consensus)
C1. Irritable bowel syndrome. C2. Functional bloating. C3. Functional constipation. C4. Functional diarrhea. C5. Nonspecific functional bowel disorder.
In ICD-10, functional bowel diseases correspond to codes K58–59.
Sections of functional intestinal disorders (ICD-10):
K58. Irritable bowel syndrome. K58.0. Irritable bowel syndrome with diarrhea. K58.9. Irritable bowel syndrome without diarrhea. K59. Other functional intestinal disorders. K59.0. Constipation. K59.1. Functional diarrhea. K59.2. Neurogenic excitability of the intestine, not classified elsewhere.
The leading role in the diagnosis of functional intestinal diseases is given to the analysis of clinical manifestations. A very difficult aspect of diagnosis in clinical practice is the distinction between functional conditions that are similar in symptoms: functional diarrhea and irritable bowel syndrome (IBS) with diarrhea, functional constipation and IBS with constipation, nonspecific functional bowel disorder with nonspecific IBS. The materials of the III Rome Consensus present diagnostic criteria that make it possible to distinguish between these clinical conditions. Knowledge of the characteristic symptoms and diagnostic criteria for each of the functional disorders is necessary to establish its type, carry out a differential diagnosis, and substantiate the treatment program for patients. Diagnosis of functional diseases is carried out in the absence of structural pathology, which can also cause the patient’s symptoms, therefore, laboratory tests, endoscopic and X-ray examinations of the intestine are important in verifying the diagnosis, allowing to exclude an organic cause of changes [1, 2, 4, 7].
The most common functional disease of the gastrointestinal tract is IBS. In Western European countries, IBS is detected in 10–20% of the population [4, 6]. The prevalence rates of IBS in Ukraine are most likely close to those in European countries. IBS occurs predominantly at a young age, and is more common in women. Among the clinical subtypes of IBS, mixed IBS is the most common [6]. Epidemiological data on the prevalence of functional constipation are variable, which is associated with different views of doctors and patients about functional constipation. In general, constipation affects almost 27% of the adult population; functional constipation is estimated to account for about 3%. It is also difficult to characterize the prevalence of functional bloating and functional diarrhea. It is known that about 2% of visits to a general practitioner are caused by diarrhea, including IBS with diarrhea and functional diarrhea [4, 6].
Irritable bowel syndrome
occupies a key place among functional diseases of the intestine and the entire gastrointestinal tract.
IBS is a functional disorder in which pain and discomfort in the abdomen are observed, the peculiarity of which is the relationship with changes in the frequency and nature of stool or other signs of impaired bowel movement. Most patients with IBS report a decrease in pain and discomfort after bowel movements. In the III Rome Consensus, based on factor analysis, groups of clinical symptoms were identified, which served as the basis for the formation of four clinical subgroups of IBS. In clinical practice, it is most practical to classify IBS subgroups based on stool shape. Stool consistency is assessed using the Bristol Stool Classification Scale (Table 1).
Division of IBS according to the predominant form of stool
1. Constipation-predominant IBS—hard or lumpy stools ≥ 25% and loose or watery stools < 25% of bowel movements. 2. Diarrhea-predominant IBS—loose or watery stools ≥ 25% and hard or lumpy stools < 25% of bowel movements. 3. Mixed IBS—hard or lumpy stools ≥ 25% and loose or watery stools ≥ 25% of bowel movements. 4. Nonspecific IBS - stool disorders that do not meet criteria 1–3.
The classification can be applied provided that the patient is not taking antidiarrheals or laxatives.
It is known that IBS is a multifactorial disorder, the basis of which is a disruption of the interaction in the brain-gut-brain system. Stress, changes in lifestyle and nutrition, and intestinal infections can lead to disruption of this interaction. Patients with IBS have a certain personality type and are prone to astheno-neurotic conditions, depression, and phobias. These disorders, in turn, lead to dysregulation at the interneuronal level in the wall of the colon, motor incoordination, increased visceral sensitivity of intestinal receptors to neurotransmitters, and a decrease in the threshold of pain sensitivity.
Functional constipation
is a functional bowel disease of unknown etiology, which is manifested by persistent difficult, infrequent bowel movements or a feeling of incomplete bowel movement. Functional constipation is based on disturbances in intestinal transit, defecation, or a combination of both.
Functional diarrhea
is a chronic or recurrent syndrome characterized by loose or loose stools without pain or discomfort in the abdomen. More often, functional diarrhea is a clinical variant of IBS, but if other diagnostic criteria are absent, then chronic functional diarrhea is considered as an independent disease. The etiology and pathogenesis of functional diarrhea are not fully understood, but it has been established that in such patients there is an increase in propulsive intestinal motility, which leads to a decrease in the transit time of intestinal contents. An additional role may be played by malabsorption of short-chain fatty acids as a result of rapid transit of contents through the small intestine with subsequent impaired absorption of water and electrolytes in the colon.
Functional bloating
, according to the III Rome criteria, is a recurrent feeling of fullness in the abdomen, which may not be accompanied by a visible enlargement of the abdomen and is not combined with other functional disorders of the gastrointestinal tract. Recurrent feeling of fullness in the abdomen or visible bloating has been observed at least 3 days per month for the past 3 months with the onset of symptoms 6 months ago.
Eligibility for all PRKs must have been met for at least the last 3 months, with onset of symptoms at least 6 months before diagnosis.
As follows from table. 2, all clinical subgroups of IBS are characterized by the presence of pain in the abdomen or discomfort that is not described as pain, while these symptoms are not typical for other FFRs. The pain syndrome is characterized by a variety of manifestations: from diffuse dull pain to acute, spasmodic pain; from constant to paroxysmal abdominal pain. The duration of pain episodes ranges from several minutes to several hours. In addition to the main diagnostic criteria, a patient with IBS may experience headache, back pain, increased urination, dysuria, nocturia, and dysmenorrhea. Almost 40–70% of patients with IBS exhibit mental changes in the form of anxiety and depressive disorders. The III Rome Consensus emphasizes that when examining a patient presenting with complaints characteristic of a functional disorder of the gastric tract, the nature of communication with the patient should include a psychotherapeutic component in everything, even in collecting an anamnesis.
Recommendations of the III Rome Consensus on establishing contact with the patient:
1. Collect anamnesis carefully, thoroughly, deeply, taking an interest in the patient. 2. Conduct the examination carefully, consider its cost and effectiveness. 3. Establish how familiar the patient is with the nature of his disease and what he considers to be the cause of the disease. 4. Provide a thorough explanation of the nature of the patient's distress in a way that he can understand. 5. Determine what improvement the patient expects to receive as a result of treatment and explain its possibilities. 6. If possible, assess the relationship of stressors to symptoms. 7. Set firm limits on the use of narcotic pain medications. 8. Involve the patient in the treatment process by suggesting some treatment options for consideration. 9. Give recommendations consistent with the interests of the patient. For example, “Antidepressants can be used to treat depression, but in low doses these medications can also be used to reduce pain.” 10. Establish a strong, long-term relationship with your family (local) doctor.
In the process of diagnosing PRK, one should analyze the clinical picture and make sure that the patient’s complaints are most likely associated with impaired intestinal function. It is necessary to exclude symptoms of anxiety (unexplained weight loss, repeated vomiting, progressive dysphagia, gastrointestinal bleeding). Unfortunately, the appearance of anxiety symptoms usually indicates an advanced process.
All functional intestinal disorders are characterized by a long course. Therefore, it is possible to establish a diagnosis of any of the PRKs only if clinical symptoms appeared at least 6 months ago. If during this period no other cause of intestinal dysfunction is identified, then this allows the doctor to assume its functional nature.
The scope of laboratory and instrumental studies depends on the patient’s age, severity and duration of symptoms, anxiety symptoms and family history of digestive diseases. Endoscopic examination of the intestine and radiological examinations are prescribed if necessary to exclude inflammation, tumor and melanosis of the colon associated with long-term use of laxatives. This especially applies to patients over 50 years of age with a short history of the disease.
Depending on the epidemiological situation, it is advisable to examine stool for worm eggs and Giardia cysts.
In some cases, it is necessary to determine antibodies to gliadin to exclude celiac disease.
Thus, the diagnostic recommendations proposed by the III Rome Consensus open up prospects for a more precise differentiation of functional intestinal disorders and the establishment of a clinical subgroup of IBS. A more precise definition of the pathophysiological variants of IBS requires the development of new drugs. This is probably the main positive meaning of the Third Rome Consensus. Knowledge of diagnostic criteria often allows you to establish a diagnosis of a functional disorder and avoid unnecessary diagnostic procedures. At the same time, one should remember the need to carry out additional diagnostic measures when clinical symptoms change, to exclude inflammatory and tumor diseases.
Functional disorders of the upper digestive tract in children
A feature of functional disorders of the gastrointestinal tract (GIT) is the absence of an obvious morphological substrate of the pathological process. According to the widely accepted definition of functional disorders according to DA Drossman (1994) and a similar formulation presented in the Rome II Criteria, the international consensus on functional diseases of the digestive system (1999), they include “a varied combination of gastrointestinal symptoms without structural or biochemical disorders" [1, 2]. In this case, the changes underlying functional disorders occur outside the affected organ and are associated with the regulation (nervous or humoral) of the impaired function. This explanation seems to be the most acceptable from both theoretical and practical positions.
Complaints associated with impaired motility of the gastrointestinal tract may be caused by changes at the level of the affected organ (“organic” pathology), may be a violation of the regulation of these organs by the nervous system, but can also be generated regardless of the state of the organ, due to the peculiarities of the psycho-emotional organization patient. In this case, it becomes possible for a symptom to be generated by an underlying organ without the participation of an overlying one or the generation of a descending stimulus at the segmental level in response to a pathological ascending impulse, for example, with receptor hyperreactivity. Any symptom (complaint) becomes one from scattered nervous impulses only at the level of mental activity, and if a true somatic complaint is determined by damage to one or another internal organ, and various parts of the nervous system serve as a connecting link and primary data processing, transmitting the latter to the level of the psyche or in the opposite direction, then the nervous system itself and its higher parts can become a generator of somatic-like complaints. At the same time, the mental level is absolutely self-sufficient, and here complaints can “emerge” that do not have their “prototype” at the somatic level, but are indistinguishable from true somatic symptoms. Differentiation of the primary level of the symptom (complaint) is of fundamental importance for the correct diagnosis and selection of the optimal treatment plan [3].
Despite the fact that the Rome II criteria indicate a stable and favorable course of functional disorders, practice shows that their evolution into organic pathology is possible. For example, diseases accompanied by gastroesophageal reflux (GER) can evolve into gastroesophageal reflux disease (GERD), functional dyspepsia into gastritis, and irritable bowel syndrome into colitis. Thus, the attitude towards functional diseases should be quite serious, and treatment measures should be adequate.
The latest classification of functional disorders of the digestive organs in children in our country was adopted in 2004 at the XI Congress of Pediatric Gastroenterologists of Russia (Moscow) within the framework of the “Working protocol for the diagnosis and treatment of functional disorders of the digestive organs in children.” The basis for this document was the classification proposed by the pediatric expert group working within the framework of the Rome II Criteria project. According to this classification, functional disorders with a predominant involvement of the upper digestive tract were included in two categories: functional disorders manifested by vomiting, which include regurgitation, rumination, cyclic (functional) vomiting and aerophagia, and functional disorders manifested by abdominal pain (functional dyspepsia).
The diagnosis of functional diseases is made on the basis of clinical data (including a carefully collected anamnesis) and the results of additional laboratory and instrumental examinations. The main difficulty in diagnosing functional disorders lies in the need to exclude all possible organic pathologies. Only after this can we speak with confidence about the functional nature of the disease.
Symptoms of functional disorders are varied, but complaints must occur over a long period of time (according to the Rome II criteria, the last 12 months or more (not necessarily continuously!).
Speaking about clinical and laboratory symptoms, it should be noted the so-called “symptoms of anxiety”, in the presence of which the development of functional disorders seems unlikely and requires a serious examination to identify their cause. “Symptoms of anxiety” include: fever, unmotivated weight loss, dysphagia, vomiting blood, blood in the stool, anemia, leukocytosis, increased ESR. Since functional disorders are almost always associated with certain disorders of the nervous system, the examination should always include consultations with a neurologist, psychologist, or neuropsychiatrist.
Based on ideas about the pathogenesis of functional disorders, the main directions of their treatment should be considered:
- elimination of the cause that led to their development, correction of the psychoneurological status, elimination of provoking factors, treatment of concomitant diseases that aggravate the course of functional disorders;
- correction of impaired motility of the digestive organs;
- correction of changes caused by impaired motor skills.
Functional disorders manifested by regurgitation and vomiting are based on GER, retrograde movement, leakage or reflux of gastric and/or intestinal contents into the esophagus. GER in a number of classifications, including ICD-10 (XI, K21) is often considered as an independent nosological unit, although, in essence, GER is a normal physiological process observed in healthy individuals, and pathological GER is a pathogenetic mechanism underlying at the basis of a number of diseases. In addition, pathological GER may be associated not only with a violation of regulatory mechanisms, i.e., act as a purely functional phenomenon. In some cases, it also develops against the background of organic processes, for example, anomalies of the esophagus, stomach, and duodenum. In this case, GER is considered as a manifestation of these diseases or their complication.
For the diagnosis of GER, the most informative is daily intragastric pH-metry, which allows you to determine the total number of reflux episodes during the day, as well as their duration. Normally, the pH in the esophagus is 5.5–7.0, and a reliable criterion for GER is a decrease in pH below 4. The criterion for pathological GER is the frequency of reflux episodes more than 50 per day and the total duration of reflux during the day exceeding 4.5% of the total observation period.
The basis of any GER is a decrease in the tone of the lower esophageal sphincter (or the inability to close it in a number of organic diseases) and an increase in intragastric pressure. A decrease in the pH of gastric contents plays a supporting role and is of greater importance for damage to the esophageal mucosa. The development of an inflammatory process in the esophagus (reflux esophagitis) or the occurrence of extra-esophageal pathological processes associated with functional GER (damage to the respiratory tract, oral cavity and teeth as a result of high reflux of gastric contents, as well as pathological reflexes - as a result of irritation of the esophageal mucosa) marks the formation of GERD and the transition from functional to organic pathology. Further evolution of GER through GERD may include metaplasia of the esophageal mucosa against the background of long-term persistent inflammation with the formation of Barrett's esophagus, which in turn is fraught with the development of esophageal cancer. The presented hypothetical chain of events completely rejects the attitude towards functional diseases as transient conditions without serious consequences.
One of the most common diseases of the group under consideration is regurgitation (regurgitation) (ICD-10, XVIII, R11) - the reverse reflux of food chyme soon after swallowing the food eaten. In children in the first months of life, regurgitation can be regarded as a physiological condition if it is rare, not abundant and occurs no later than an hour after feeding. On the contrary, at this age it is considered pathological if it is observed more than twice a day, occurs an hour or later after meals and is abundant. The development of regurgitation in children in the first months of life is predisposed by the structural features of the upper digestive tract and the immaturity of the neurohumoral regulation of the sphincter apparatus and gastrointestinal motility. Regurgitation is often caused by inadequate feeding (aerophagia, overfeeding, violation of the feeding regimen, inadequate selection of formulas, etc.), as well as perinatal damage to the central nervous system.
In some cases, regurgitation is caused by the presence of pylorospasm (ICD-10, XVIII, R19) - difficult emptying of the stomach due to spasm of the pyloric muscles. From the first days of life, with pylorospasm, regurgitation is observed; as the volume of food increases, “delayed” vomiting appears, with curdled acidic contents without an admixture of bile, not exceeding the volume of food eaten. The child, despite vomiting, gains weight, although not enough; if treatment is not started in a timely manner, malnutrition may develop. Radiologically, the pathology is not determined, although upon examination after 2 hours there may be a delay in the evacuation of the contrast mass. An endoscopic examination reveals a closed pylorus in the form of a slit, through which one can always pass with an endoscope, which excludes organic causes of pyloroduodenal obstruction.
Rumination (ICD-10, XVIII, R19) is repeated periodic attacks of contraction of the abdominal muscles, diaphragm and tongue, leading to the reflux of gastric contents into the oral cavity, where it is again chewed and swallowed. The onset is typical at the age of 3–8 months and there is no effect from changing the nature of nutrition, feeding through a pacifier or gastrostomy tube. There are no signs of discomfort. It can serve as a symptom of deprivation or a sign of severe organic damage to the central nervous system.
Cyclic (functional) vomiting (ICD-10, XVIII, R11) - acute attacks of nausea and vomiting lasting from several hours to several days, interspersed with asymptomatic periods lasting several weeks or months. A chronic course may be observed, with symptoms observed for at least 3 months (or with interruptions for a total of 3 months during the year). It occurs mainly in children over 3 years of age and requires a thorough neurological examination.
Aerophagia is the swallowing of air, leading to repeated belching and flatulence, observed for 12 months or more (not necessarily continuously) over the past year. Moderate aerophagia is often observed in children in the first months of life due to the immaturity of the nervous regulation of the swallowing process. Aerophagia is more pronounced in premature infants and children who are immature at the time of birth. Aerophagia is provoked by talking while eating, the habit of rushing when eating, chewing gum, and carbonated drinks. Persistent aerophagia in children over 1 year of age requires the exclusion of neurological pathology [4].
Treatment of the listed diseases is carried out in accordance with the general principles of the treatment of functional disorders and involves, first of all, eliminating the root cause, which often requires the involvement of a neurologist, psychologist or neuropsychiatrist.
Correction of impaired motor skills in GER includes routine, dietary and medicinal interventions.
Patients with GER are recommended to sleep with the head end of the bed raised at least 15 cm, avoid wearing tight clothes and tight belts, exercise associated with overstraining the abdominal muscles, deep bends, prolonged stay in a bent position, lifting weights weighing more than 8– 10 kg.
You should also limit or reduce the content of animal fats, increase the protein content in the diet, avoid consuming irritating foods, carbonated drinks, and reduce the amount of food taken per meal (you can increase the frequency). In addition, you should not eat before bed. Obese patients are advised to lose weight.
In children in the first months of life who are bottle-fed, special anti-reflux mixtures should be used, the peculiarity of which is a change in the ratio of casein and whey proteins towards casein, as well as the inclusion of thickeners in their composition (most often locust bean gum, E410).
If possible, you should avoid taking drugs that reduce the tone of the lower esophageal sphincter, including sedatives, hypnotics, tranquilizers, theophylline, anticholinergics, beta-agonists. It is also necessary to stop smoking.
Drug therapy includes the use of prokinetics (domperidone), as well as antisecretory drugs that increase the pH of the refluxate and, to a certain extent, the tone of the lower esophageal sphincter (H2-histamine receptor blockers and proton pump blockers).
The action of domperidone (Motonium, Motilium), as well as metoclopramide (Cerucal), is associated with antagonism to dopamine receptors of the gastrointestinal tract and, as a result, with increased cholinergic stimulation, leading to increased sphincter tone and accelerated motility. Unlike domperidone, metoclopramide penetrates well through the blood-brain barrier and can cause serious side effects (extrapyramidal disorders, drowsiness, fatigue, anxiety, as well as galactorrhea associated with an increase in prolactin levels in the blood), which makes it necessary to avoid its use in pediatric practice. Domperidone is prescribed at a dose of 2.5 mg per 10 kg of body weight 3 times a day for 1–2 months. Side effects (headache, general fatigue) are rare (0.5–1.8% of patients).
The use of motonium in children with GER of functional origin reduces, according to intraesophageal pH-metry, the frequency of reflux episodes by 68%. Clinical improvement (reduction in the severity of heartburn) is observed in at least 70% of children, although the success of treatment largely depends on the nature of the pathological process.
The use of antisecretory drugs is indicated only for severe forms of GER that cannot be eliminated by other means, in older children and in adult patients. The effectiveness of drugs in this group for GER is associated primarily with a decrease in gastric secretion, an increase in the pH of gastric contents thrown into the esophagus, and a decrease in the irritating effect of refluxate on the mucous membrane of the esophagus.
Currently, there are two approaches to choosing therapeutic tactics for GER. In the first case (the so-called “step-up” therapy), treatment begins with organizing the patient’s regimen and nutrition, and if there is no effect, prokinetics and then antisecretory drugs are prescribed. If the doctor uses an alternative (“step-down”) treatment regimen, therapy begins with a full range of drugs, which is subsequently reduced as indicators improve.
The content of the term “dyspepsia” has undergone significant evolution in recent decades. Traditionally, dyspepsia in domestic medicine meant alimentary dyspepsia, which most accurately corresponds to the translation of this term (“indigestion”) and which is understood as a discrepancy between the capabilities of digestive enzymes and the volume and/or composition of food taken. The term “nutritional dyspepsia” was most often used in pediatric practice in relation to children of the first year of life, but it is suitable to describe the condition of patients of any age.
At the end of the 20th century. A new definition of dyspepsia, which came from Western publications, has appeared in the domestic literature.
Dyspepsia syndrome, in accordance with the definition of the Committee on Functional Diseases of the World Congress of Gastroenterology (1991), is a complex of disorders including pain or discomfort in the epigastrium, a feeling of fullness in the epigastric region after eating, early satiety, nausea, vomiting, belching, heartburn . Subsequently, in 1999, dyspepsia with heartburn was classified as gastroesophageal reflux disease, and abdominal pain or discomfort combined with defecation disorders was classified as irritable bowel syndrome.
The term “dyspepsia syndrome” is used at the initial stages of the examination or in cases where an in-depth diagnostic process is not possible. Upon further examination, the diagnosis can be interpreted as organic dyspepsia, i.e., a diagnosis of gastritis, peptic ulcer, etc., or as functional dyspepsia, referring to functional disorders. Essentially, at present, functional dyspepsia is understood as those cases of dyspepsia when a thorough gastroenterological examination cannot determine its cause. Functional dyspepsia is based on motor disorders of the stomach and duodenum, resulting from disturbances of nervous and/or humoral regulation, including those caused by visceral hypersensitivity.
Functional dyspepsia (ICD-10, XI, K30) is a symptom complex isolated in children over 1 year of age and includes pain, discomfort or a feeling of fullness in the epigastric region (not necessarily associated with eating or exercise), early satiety, bloating, nausea, regurgitation, intolerance to fatty foods and other symptoms observed for at least 12 weeks over the past 12 months, if the examination fails to identify any organic disease. It is also important that symptoms are not related to bowel movements or changes in the frequency and nature of stool.
The following types of functional dyspepsia are distinguished: ulcer-like (localized pain in the epigastrium, “hungry” pain that goes away after eating, antacids or antisecretory drugs), dyskinetic (discomfort in the upper abdomen, aggravated by eating) and nonspecific (complaints are difficult to attribute to a specific option).
An obligatory component of the treatment of functional dyspepsia is the normalization of the vegetative status and psycho-emotional state, consultation with a neuropsychiatrist or psychologist.
The diet for functional dyspepsia is largely determined by individual tolerance to foods. Also excluded are all foods that can cause epigastric pain, heartburn, belching: fatty foods, smoked sausages, strong meat, fish and mushroom broths, cabbage soup, borscht, rye bread, fresh pastries, pancakes, carbonated drinks, coffee, radishes, hot seasonings . Patients are allowed to eat white bread, better than yesterday's bread, white bread crackers, dry unsweetened cookies, vegetarian soups and soups with weak broths, cream soups, boiled meat, steamed cutlets, meatballs (beef, chicken, rabbit, turkey), boiled fish, porridge (semolina, rice, buckwheat, oatmeal) with the addition of milk, pasta, soft-boiled eggs, steam omelets, whole and condensed milk, cottage cheese, kefir, yoghurt, mild cheese, jelly, jelly, boiled compotes of sweet varieties of berries and fruits vegetables (beets, potatoes, zucchini, pumpkin, cauliflower), raw grated carrots, sweet pears without peel, bananas, baked apples. It is recommended to eat 4–5 times a day [4].
Antacid and antisecretory drugs are indicated for ulcer-like variants of functional dyspepsia. Antacids may be prescribed if their use reduces the severity of clinical manifestations. Antisecretory drugs, if necessary, are prescribed taking into account the nature of gastric secretion.
Prokinetics are prescribed primarily to patients with a predominance of symptoms such as a feeling of fullness, rapid satiety after eating, and bloating (dyskinetic variant of dyspepsia). The drug of choice for the dyskinetic variant of functional dyspepsia is domperidone, prescribed at a dose of 2.5 mg per 10 kg of body weight 3 times a day for 1–2 months. The use of the domestic drug motonium in children with functional dyspepsia reduces in most cases the severity of discomfort in the epigastric region, the feeling of heaviness and nausea, and also reduces or eliminates pain symptoms in a large number of patients.
Antispasmodic drugs are prescribed for spastic conditions: myotropic antispasmodics - mebeverine hydrochloride (duspatalin) is used in children over 12 years old, 200 mg 2 times a day 20 minutes before meals; a new release form of 200 mg in microgranules enclosed in a capsule ensures not only the greatest effectiveness of the drug, but also prolonged action over time and throughout the entire gastrointestinal tract; papaverine (for children, depending on age, 0.005–0.06 g 2 times a day), drotaverine (no-shpa, spazmol) — for children under 6 years of age, 0.01–0.02 g 1–2 times per day daily, children 6–12 years old, 0.02 g 1–2 times a day.
It is important to take into account the patient’s vegetative status and prescribe appropriate drugs, in particular anticholinergic drugs for vagotonia.
Currently, diagnosis and treatment for dyspepsia syndrome are divided into two stages. At the first stage, the doctor, based on clinical data (including excluding symptoms of “anxiety”) and the results of a screening examination (complete blood count, scatological examination, stool examination for occult blood, ultrasonography), can with a high degree of probability assume the functional nature of the disease and prescribe treatment for a period of 2–4 weeks. The lack of effect from the therapy is considered as an alarm signal and serves as an indication for a thorough gastroenterological examination in a consultation center or specialized hospital. This approach is justified not only from a medical, but also from an economic point of view [5].
Comprehensive, timely and adequate treatment of functional disorders of the digestive organs in children is an important factor in preventing the development of more serious pathological conditions in young patients.
Literature
- Drossman DA The Functional Gastrointestinal Disorders. Diagnosis, Pathophysiology, and treatment. A Multinational Consensus. Little, brown and Company. Boston/ New York/ Toronto/ London. 1994; 370.
- Drossman DA The Functional Gastrointestinal Disorders and the Rome II process//Gut. 1999; 45; 2: II1-II5.
- Antropov Yu. F., Belmer S. V. Somatization of mental disorders in childhood. M., 2005.
- Belmer S.V., Gasilina T.V., Khavkin A.I., Eiberman A.S. Functional disorders of the digestive organs in children. M., 2005. 36 p.
- Pechkurov D.V., Shcherbakov P.L., Kanganova T.I. Dyspepsia syndrome in children: modern approaches to diagnosis and treatment: Information and methodological materials for pediatricians, gastroenterologists and family doctors. Samara, 2005. 20 p.
S. V. Belmer , Doctor of Medical Sciences, Professor T. V. Gasilina , Candidate of Medical Sciences A. I. Khavkin , Doctor of Medical Sciences, Professor A. P. Ponomareva Russian State Medical University, Moscow