Functional dyspepsia in children
Indigestion or simple dyspepsia is more common in children in the first years of life. It can develop acutely or against the background of precursors: anxiety, loss of appetite, regurgitation, increased bowel movements. After 3-4 days, the frequency of stool reaches 5-7 times a day, it becomes liquid, heterogeneous in color, resembling the appearance of a chopped egg with lumps of white, yellow and greenish color, mixed with mucus. With dyspepsia, children experience bloating, frequent passing of gas, regurgitation, and vomiting. The child is bothered by intestinal colic: before defecation, he twists his legs and cries. Appetite decreases to the point of refusing to eat, which leads to a halt in weight gain. Simple dyspepsia in children lasts 2-7 days. Against the background of dyspepsia, children may develop thrush, stomatitis, and diaper rash.
Toxic form of dyspepsia
In weakened children, simple dyspepsia can transform into a toxic form. In this case, fever, uncontrollable vomiting, and frequent (up to 15-20 times a day) stools appear, which quickly become watery with lumps of desquamated epithelium. Significant loss of fluid during vomiting and diarrhea is accompanied by dehydration, decreased tissue turgor, retraction of the large fontanelle, and a sharp decrease in body weight. The child's face takes on mask-like features with his gaze directed at one point; the skin and mucous membranes become dry; reflexes decrease and convulsions may occur. Toxic dyspepsia in children can lead to impaired consciousness, development of coma and death of the child.
Functional dyspepsia in older children occurs with periodic abdominal pain (usually soon after eating), rapid satiety, nausea, feelings of fullness, belching, heartburn, alternating constipation or diarrhea. Digestive disorders with dyspepsia in children are often aggravated due to stressful situations and are combined with dizziness and sweating.
Diagnosis of dyspepsia
An examination of children with dyspepsia by a pediatrician should include the collection of anamnesis and complaints, a clinical examination, and comprehensive laboratory and instrumental diagnostics.
First of all, in the presence of dyspepsia syndrome in children, it is necessary to differentiate the organic or functional nature of the digestive disorder. For this purpose, the child undergoes an ultrasound of the abdominal organs (liver, gallbladder, pancreas), esophagogastroduodenoscopy, and radiography of the stomach.
Laboratory tests include stool testing for Helicobakter Pylori, biochemical liver tests, and determination of pancreatic enzymes in the blood and urine. With the help of bacteriological culture of stool, OCI is excluded, and by examining stool for helminth eggs - helminthic infestations. Lactase deficiency, celiac disease, and intestinal dysbiosis should be excluded. A study of the coprogram for dyspepsia in children reveals the presence of inflammatory changes in the intestines and signs of impaired digestion of food components.
Since functional digestive disorders are almost always associated with disorders of the nervous system, children with dyspepsia should be consulted by a pediatric neurologist and psychologist.
Causes
- Heredity (frequent diseases of the gastrointestinal tract in relatives).
- Features of early child development (dysbacteriosis, intestinal infections in the first year of life).
- Stress factors and chronic fatigue (strong emotional experiences, heavy workload at school).
- Excessive consumption of flour products, caffeine, chocolate, etc.
- Past intestinal infections.
- Hormonal changes (for example, during puberty).
IBS is a chronic disease, the symptoms of which can last a lifetime in varying degrees of severity. At the same time, for some people, the symptoms of the disease disappear completely over time.
Treatment of dyspepsia in children
Mild forms of nutritional dyspepsia in children are treated on an outpatient basis. The main component of the treatment of simple dyspepsia in children is the abolition of the product that led to indigestion, adherence to a diet and diet in accordance with the age of the child.
It is recommended for infants to replace 1-2 feedings per day with a water-tea break and reduce the volume of other feedings. The child is given glucose-saline solutions, carrot-rice broth, and weak tea to drink.
To improve digestion for dyspepsia in children, enzymes (pancreatin) are prescribed; for removing toxins - sorbents; to relieve pain - antispasmodics.
Since 75% of cases of dyspepsia syndrome in children are based on impaired gastric motility, it is advisable to prescribe prokinetics (motility regulators).
To restore the intestinal flora after dyspepsia, children can be given drugs with live cultures of bifidobacteria and lactobacilli.
Moderate and severe forms of dyspepsia in children must be treated in a hospital setting. Toxic dyspepsia in children requires the use of antibacterial drugs. A child suffering from dyspepsia needs careful care: maintaining an appropriate temperature, a calm environment, and maintaining hygiene. It is necessary to carefully monitor the dynamics of the child’s condition, examine the nature of vomit and bowel movements, and prevent aspiration of vomit into the respiratory tract.
Introduction
According to modern views, functional disorders occupy a leading position in the structure of diseases of the digestive system in young children, accounting, according to various sources, 90–95% of their total number.
Functional diseases (or disorders) are considered to be situations when, during the examination, it is not possible to detect anatomical, morphological, metabolic or other disorders that could explain the child’s symptoms.
According to the now classic definition by DA Drossman (1994), “functional disorders of the gastrointestinal tract (GIT) are a varied combination of gastrointestinal symptoms without structural and biochemical disorders.”
Some authors consider this definition to be somewhat outdated, since as medical science develops, new research methods appear that make it possible to identify inflammatory and other changes in organs and systems at a more subtle level. In particular, in some patients with functional dyspepsia, Helicobacter pylori infection is found, and in patients with irritable bowel syndrome (IBS), microsigns of colitis are found according to histological examination.
Recognizing the right to exist of this point of view, we, however, would like to propose focusing on the classical definition, at least until the time when histological, genetic and molecular studies become firmly established in everyday clinical practice.
The causes of functional disorders are organ regulation disorders caused by “extraorgan” (psycho-emotional, stress, endocrine, etc.) factors [1, 2].
Symptoms of functional gastrointestinal disorders develop due to a combination of several known physiological determinants:
- motor impairment;
- visceral hypersensitivity;
- changes in mucosal immunity and inflammatory potential, including changes in bacterial flora, as well as changes in regulation of the CNS-ENS (enteric nervous system) axis, influenced by psychological and sociocultural factors [2].
The Rome IV criteria, proposed by the Committee on the Study of Functional Disorders in Children and the International Working Group on the Development of Criteria for Functional Disorders in 2016, divide functional bowel disorders into two groups depending on age [3].
G. Functional disorders in newborns and young children (0–3 years):
- G4. Infantile colic.
- G5. Functional diarrhea.
- G6. Infantile dyschezia.
- G7. Functional constipation.
N. Functional disorders in children and adolescents (4–18 years):
- H2b. Irritable bowel syndrome.
- H3a. Functional constipation.
As can be seen from the presented data, the diagnosis of IBS is not valid for children under 4 years of age.
IBS is a functional bowel disorder in which abdominal pain or discomfort is associated with bowel movements, changes in the frequency and nature of bowel movements, or other signs of bowel movement problems [3].
Synonyms of IBS: spastic colitis, colon neurosis, spastic constipation, spastic colon, mucous colic, nervous diarrhea, functional colopathy, etc.
Epidemiology
The detection rate of IBS in adults ranges from 9 to 48% (average 20%), depending on geographic location, socio-economic conditions, diet, etc.
The frequency of detection of IBS in children is 0.2%, according to primary outpatient care, and 22–45%, according to specialized inpatient departments in Western European countries. According to other data, the prevalence of IBS ranges from 6 to 14% in children and 22–35.5% in adolescents [2, 4, 5].
The Rome IV criteria report an incidence of IBS in the general pediatric population ranging from 1.2 to 2.9% in the United States and 4.9 to 5.4% in Colombia and Sri Lanka [3].
Classification of IBS (F. Weber, R. McCallum, 1992):
- IBS, occurring with a predominance of diarrhea;
- IBS occurring with a predominance of constipation;
- IBS, which occurs predominantly with abdominal pain and flatulence.
In recent years, this classification has undergone some changes and in the modern edition is as follows:
- IBS occurring with a predominance of diarrhea (IBS-D);
- IBS occurring with a predominance of constipation (IBS-C);
- IBS mixed type (IBS-S);
- IBS nonspecific (IBS-N).
The above figure illustrates these points.
Codes according to the International Classification of Diseases, 10th revision (ICD-10):
- K 58. IBS.
- By 58.0. IBS with diarrhea.
- To 58.9. IBS without diarrhea.
- By 59.0. Constipation.
- To 59.1. Functional diarrhea.
- To 59.2. Neurogenic excitability of the intestines, not classified elsewhere.
- To 59.8. Other specified functional disorders of the intestine.
- To 59.9. Functional bowel disorders, unspecified.
IBS – biopsychosocial functional pathology
The foundation of IBS as a biopsychosocial disorder consists of two main pathological mechanisms - psychosocial influence and sensory-motor dysfunction of the intestine, i.e. disturbances of visceral sensitivity and intestinal motor activity [3, 6, 7].
The biopsychosocial model of disease is based on the complex interaction of genetic, environmental, physiological and psychological factors and their influence on symptoms and disease. This serves as a cornerstone for understanding the etiology of IBS [8].
Genetic factors in the development of IBS are based on identification of symptoms and diagnosis of clusters within families and twins. In particular, it is known that children whose mother suffers from IBS also develop IBS in most cases [9, 10]. Specific genes that may be responsible for susceptibility to the development of IBS are unknown, but genes encoding the production of serotonin, proteins involved in noradrenergic signaling, and cytokines are being studied [11].
Etiopathogenesis
The pathogenesis of IBS is not fully understood, however, according to the currently dominant concept, its main factors are disturbances in the interaction in the brain-gut system, represented by the following provisions:
- disturbance of nervous regulation (cortical and subcortical centers - limbic system, hypothalamus, segmental level);
- violation of humoral regulation (gastrointestinal hormones - VIP, motilin, cholecystokinin; biologically active substances - histamine, serotonin; endocrine pathology);
- disturbance of perception and transformation of the peripheral afferent flow of impulses in the cerebral cortex;
- visceral hypersensitivity (hyperalgesia, allodynia).
IBS – “comes from early childhood.” This thesis does not seem strange when you begin to study more deeply the characteristics of a child’s development from the moment of birth. In particular, stress during early life is thought to lead to the development of hypersensitivity or altered responses to pathological stimuli later in life [8, 12].
The risk of developing IBS also increases with traumatic experiences early in life, such as neglect or the loss of a parent. The minimum level of stress or pain that can lead to long-term health problems has not been determined. In addition, it is not clear what makes some children more vulnerable and at what age children are most susceptible to adverse effects [8, 13]. For example, children who, for various reasons, had a nasogastric tube inserted in the neonatal period, are significantly more likely to suffer from abdominal pain in adolescence compared to their brothers and sisters [14].
The relationship between IBS and comorbid psychiatric disorders has been well studied in children. It is known that there is an increase in the prevalence of IBS in adolescence. While there are no gender differences in the incidence of IBS among children of primary and secondary school age, females predominate among adolescents, which is similar to the epidemiology of anxiety and depressive symptoms and disorders [15]. There is literature data according to which patients with IBS represent a risk group for the subsequent development of anxiety and depressive disorders [16].
However, the exact relationship between mental characteristics, psychiatric complaints and IBS remains unclear. There is evidence that anxiety and depressive disorders are likely to predict gastrointestinal complaints. It is possible that in these individuals the psychological characteristics predispose them to increased attention to complaints from the gastrointestinal tract and trigger the mechanism of pain. There is a view that psychological disorders and IBS may share common risk factors or may simply be different manifestations of a distinct causal process [8, 16].
It is known that environmental factors influence intestinal microbiocenosis. Children who have suffered acute bacterial gastroenteritis are at high risk of developing IBS compared to the control group [17, 18]. Similar data were obtained in another work; In the same place, female gender was noted as an additional predictor of inflammation [17, 19]. Among adult patients, the likelihood of developing IBS is higher in those who have had a bacterial infection compared to patients who have had a viral infection [20].
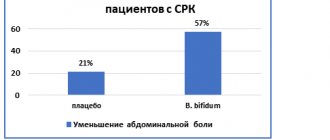
There are microbiological differences in the composition of the intestinal microflora between patients with IBS and healthy individuals. The epidemiological, physiological and clinical data available in the literature indicate the role of intestinal bacteria in the pathogenesis of the disease [8,21]. Some probiotic strains are known to reduce the risk of persistent IBS symptoms (particularly Lactobacillus reuteri and Lactobacillus GG).
Data on the use of probiotics in children with IBS are limited, but indicate a positive effect. However, the need to use different probiotics for specific conditions, symptoms and specific patients is indicated [21–23].
In the pathogenesis of IBS, inflammation of the mucous membrane, dysregulation of intestinal immunity, disruption of microbiocenosis and permeability of the intestinal mucosa play a role [6]. According to some data, children with IBS have higher levels of fecal calprotectin compared to the control group [24]. Individuals with this pathology have bacterial overgrowth syndrome (SIBO). The cause of this condition, as is known, is microbial contamination of the small intestine with the microflora of the large intestine. SIBO results in excess gas production, changes in intestinal motility and sensitivity of the intestinal mucosa, and activation of the intestinal immune system [25].
One of the triggers for the development of IBS can be food allergies. Studies of adult patients have proven that when mast cells are activated, located in close proximity to the nerve endings in the intestinal wall, a change in visceral perception occurs, which provokes abdominal pain syndrome [26, 27]. In addition, the use of mast cell stabilizers (ketotifen) reduces visceral sensitivity and leads to increased abdominal pain in adult patients with IBS [28].
Interesting data were obtained by M.Yu. Tipikina (2014). In particular, it was shown:
- the most significant risk factors for the development of IBS in children and adolescents are past stressful situations, a burdened neurological history, long-term and repeated antibiotic therapy, past intestinal infections, regular diet and nutritional disorders;
- IBS is accompanied by signs of minimal chronic inflammation in the intestines: in 97.5% of cases, microscopic chronic distal colitis occurs. In children with IBS, the level of pro-inflammatory cytokines in the colon mucosa is increased (45% of patients have increased interleukin (IL) 8, 90% have increased interferon-γ); 27.3% of patients had increased levels of fecal calprotectin;
- IBS is characterized by changes in the composition of the microflora in all parts of the intestine: 100% of children have dysbiotic disorders in the colon, 85.1% are diagnosed with SIBO [38].
A serious problem is the variant of post-infectious IBS (PII). So, according to A.I. Parfenova et al. (2009), the proportion of PI-IBS after acute intestinal infection (AIE) is 24–32%. The incidence of PI-IBS after bacterial gastroenteritis is 98.2 per 10 thousand people per year [39]. According to N. Tomblom et al. (2007), the incidence of PI-IBS in children under 6 years of age is 7–33% [42].
According to A.I. Parfenova (2009), risk factors for PI-IBS include:
- female;
- treatment of infectious diarrhea with antibiotics;
- the presence of an organic disease of the digestive system;
- signs of socio-psychological maladaptation (anxiety, depression, tendency to somatization, chronic stress, dissomnia disorders).
The same authors provide a list of the most significant infectious agents in the genesis of PI-IBS: Salmonella, Escherichia coli, Shigella, Campylobacter jejuni, enteroviruses, rotaviruses, etc.
Among the genetic factors that determine predisposition to the development of PI-IBS, a decrease in the frequency of expression of the IL-10 allele, an increase in the frequency of the intermediate allele of tumor necrosis factor α, an imbalance of cytokines, a decrease in the levels of IL-10, IL-12 and transforming growth factor β are usually mentioned [ 39].
According to I.N. Ruchkina et al. (2006), the diagnostic criteria for PI-IBS include:
- compliance with Rome criteria-IV;
- detection of OCI markers in substrates from the patient;
- disturbance of microflora in bacteriological studies of the intestine;
- SIBO;
- changes in the mucosal microflora of the small intestinal mucosa;
- positive dynamics from treatment with antiseptics and probiotics [41].
Visceral hypersensitivity or hyperalgesia plays a certain role in the genesis of IBS. However, this statement in relation to children requires a systematic evidence base [29, 30]. Symptoms of IBS may have more to do with pathological amplification of physiological stimuli rather than true neurosensory hypersensitivity [31].
Motor disorders have long been considered the basis for the formation of functional intestinal disorders. At the same time, according to the literature, disorders pathognomonic specifically for IBS have not been identified [32]. Many of the motor disorders described in patients with IBS are found at lower frequencies in healthy people.
Impaired motility, as a rule, leads to secondary changes in the internal environment of the intestine, the development of changes in the composition of the microflora, and disruption of the processes of digestion and absorption. The latter inevitably increases the imbalance of intestinal microflora, aggravates motility disorders (which provokes pain), thereby closing a vicious circle.
Clinical picture
According to the Rome III criteria (2006), diagnostic criteria for IBS in children include:
1. Abdominal discomfort for at least 2 months (discomfort not described as pain) or pain associated with ≥2 of the following symptoms at least 25% of the time:
- relief after defecation;
- onset is associated with a change in stool frequency;
- the onset is associated with a change in the nature of the stool.
2. There are no signs of inflammation, anatomical, metabolic or neoplastic changes that could explain the presenting symptoms.
The Rome Criteria-IV (2016) somewhat clarifies these provisions:
1. Abdominal pain (at least 2 months before diagnosis), at least 4 days during the month + the following signs:
- connection with the act of defecation;
- change in stool frequency;
- change in stool shape.
2. In children suffering from IBS with constipation, the pain does not go away after the constipation is eliminated (if it goes away, it is functional constipation).
3. After appropriate evaluation, symptoms cannot be explained by other causes [3].
I would like to draw special attention of clinicians to point 2 as an important differential diagnostic practical criterion.
Symptoms that support a diagnosis of IBS include:
- pathological stool frequency: ≥4 times a day and ≤2 times a week;
- pathological form of stool: lumpy/dense or liquid/watery;
- pathological passage of feces: excessive straining, tenesmus, urgency, feeling of incomplete evacuation;
- excessive mucus secretion;
- bloating and feeling of fullness.
Features of the clinical appearance of IBS are:
- abdominal pain: variability of intensity, lack of constant localization, recurrent nature, combination with flatulence and flatulence, decreased intensity after defecation and release of flatus;
- flatulence: not pronounced in the morning, increases during the day, mainly in the lower abdomen, is not constant, is associated with an error in diet;
- alternation of diarrhea and constipation with a predominance of one of the symptoms;
- Features of diarrhea: absence of polyfecal matter, loose stools 2–4 times only in the morning, after breakfast, against the background of a traumatic situation, imperative urges, a feeling of incomplete emptying.
Additional diagnostic criteria for IBS include:
- polymorphism of complaints: a variety of autonomic and neurological disorders, extraintestinal manifestations, signs of functional disorders of other organs;
- high appeal to doctors of various specialties;
- discrepancy between the duration of the disease, variety of complaints, satisfactory appearance and physical development of the patient;
- no progression of symptoms;
- absence of clinical manifestations at night;
- connection with a traumatic situation.
The English-language literature provides an original and easy-to-memorize ABCDE system that reflects the main diagnostic criteria for IBS (NJ Talley, 2012):
- A – abdominal (stomach or bowel) pain or discomfort (abdominal pain or discomfort in the projection of the stomach or intestines);
- B – bloating, feeling swollen, or even visible swelling, like one in pregnant (bloating, increase in its size as during pregnancy);
- C – constipation (constipation);
- D – diarrhea (diarrhea);
- E – extra-bowel symptoms in some cases, such as nausea, tiredness, backaches, or muscle aches (extra-intestinal symptoms in some cases, such as nausea, fatigue, back or muscle pain).
The above diagnostic criteria are of great clinical significance and allow one to at least suspect IBS in a child. The appearance in the patient of the so-called symptoms of anxiety (“red flags”, “red flags”) force the doctor to think about the organic (inflammatory, infectious, etc.), but not functional nature of the disease. Knowledge of these symptoms is mandatory for a pediatrician of any profile.
Symptoms of anxiety (“red flags”) include:
- stereotypical pain syndrome, irradiation of pain;
- constant pain in the right upper or lower quadrant of the abdomen;
- persistent vomiting;
- persistence of symptoms at night;
- presence of blood in the stool, vomiting mixed with blood, melena;
- dysphagia;
- impaired physical development, growth retardation;
- unmotivated weight loss;
- delayed sexual development;
- fever of unknown origin;
- joint pain, arthritis;
- perianal lesions;
- lymphadenopathy;
- persistent diarrhea, nocturnal diarrhea, polyfecal matter;
- constant enlargement of the abdomen;
- hepato-(splenno)megaly.
- any changes in clinical and/or biochemical blood tests;
- heredity burdened by intestinal cancer, inflammatory bowel diseases, celiac disease, peptic ulcer disease.
Differential diagnosis is usually made with the following diseases:
- intestinal infections, parasitosis;
- malabsorption syndrome (celiac disease, allergic enteropathy, short bowel syndrome, etc.);
- endocrine pathology (hypothyroidism, thyrotoxicosis, diabetes mellitus);
- neuroendocrine tumors of the gastrointestinal tract (vipoma, gastrinoma);
- gynecological pathology;
- inflammatory bowel diseases (ulcerative colitis, Crohn's disease, Whipple's disease);
- colorectal cancer, diverticulosis, intestinal polyposis;
- tuberculosis, intestinal amyloidosis;
- intestinal ischemia;
- Iatrogenic factors (long-term use of laxatives, iron supplements).
The examination package for suspected IBS includes the following diagnostic methods:
- endoscopic examinations: sigmoidoscopy, fibrosigmoidoscopy, fibrocolonoscopy (FCS), esophagogastroduodenoscopy;
- ultrasound examination of the abdominal organs, kidneys, pelvic organs or computed tomography;
- laboratory blood test: hemogram, biochemical blood test;
- general urine analysis;
- stool examination: microscopy, parasitological examination, occult blood, elastase, microbiological examination for carbohydrates, etc.;
- hydrogen test to exclude hypolactasia and fructose malabsorption;
- exclusion of celiac disease (serological markers, genetic examination and examination of a biopsy of the duodenal mucosa).
Additional examination methods include:
- assessment of the state of the central nervous system and autonomic nervous system, psychological status of the patient;
- FCS with endobiopsy (according to indications, for constipation);
- colodynamic study;
- X-ray contrast examination of the intestines (irrigography);
- Dopplerography and angiography of abdominal vessels;
- serological blood test (pathogenic intestinal flora);
- allergy examination (food sensitization);
- immunogram.
It should be noted that in most cases, the pediatrician and pediatric gastroenterologist do not have clear grounds for conducting serious instrumental and invasive studies if IBS is suspected. Usually, a correctly collected anamnesis, physical examination and paraclinical data obtained are sufficient to make a diagnosis. The appearance of “anxiety symptoms” in a child forces the doctor to change tactics and conduct an examination aimed at excluding organic intestinal pathology.
Summarizing the provisions of the above part of the material, we can derive integral criteria for diagnosing IBS in children:
- compliance of clinical symptoms with Rome IV criteria;
- absence of “anxiety symptoms”;
- absence of organic pathology according to physical examination;
- adequate assessment of the child’s age and growth characteristics;
- presence of trigger factors according to medical history;
- features of psychological status, psychotrauma.
IBS is a diagnosis of exclusion, which can be made only after the organic nature of the disease has been reliably rejected (“inpatient diagnosis”).
IBS cannot be diagnosed in young children.
Treatment
Non-drug correction
Where should you start? It is necessary to reassure the child and his relatives, explain the characteristics of the disease and the possible reasons for its formation.
A set of measures for non-drug correction of signs of IBS in children is presented as follows:
- eliminating possible causes of intestinal symptoms;
- modification of the patient’s lifestyle (daily routine, eating behavior, physical activity, dietary preferences);
- normalization of the psycho-emotional state (elimination of traumatic situations, limitation of school and extracurricular activities, various options for psychotherapeutic correction, creation of comfortable conditions for defecation, etc.).
- dietary correction.
- physiotherapy, exercise therapy, massage with a sedative or stimulating effect (depending on the type of motor impairment);
- herbal medicine with sedative effect.
Diet therapy for a child with IBS should be structured in accordance with the following provisions:
- individualized diet in accordance with the child’s food stereotype;
- exclusion of individually intolerable foods, carbonated drinks, legumes, citrus fruits, chocolate, vegetables rich in essential oils;
- limiting the consumption of milk, foods with coarse fiber, as well as foods that cause flatulence.
Table No. 4 is usually taken as a basis, to which individual adjustments are made.
Medication correction
1. Correction of motor skills: drugs with a predominantly antispasmodic effect:
- topical intestinal modulators – selective blockers of sodium channels of intestinal smooth muscles: mebeverine, dicetel;
- myotropic antispasmodics: drotaverine, papaverine, incl. rectal suppositories;
- drugs with anticholinergic effects: hyoscine butyl bromide (Buscopan), Meteospasmil, belladonna preparations, incl. rectal suppositories;
- regulator of intestinal motility – trimebutine.
It should be noted that of the listed drugs, in addition to drotaverine, papaverine and belladonna preparations, hyoscine butylbromide (from 6 years old) and trimebutine (from 3 years old) are officially allowed in pediatrics.
2. Elimination of flatulence: simethicone preparations: Bobotik, Espumisan, Sab Simplex, Disflatil, combination preparations (Meteospasmil, Pankreoflat).
3. Staged correction of intestinal microbiocenosis disorders: intestinal “antiseptics” (ercefuril, enterofuril, nifuratel, etc.), enterosorbents, laxatives, pre- and probiotics).
Among enterosorbents, the undoubted leader is dioctahedral smectite (Smecta). The drugs Zosterin-Ultra, Latkofiltrum, Enterosgel, etc. can also be used.
Classification of laxatives:
- Drugs that cause chemical irritation of the intestinal receptor apparatus are derivatives of anthraquinones (preparations of senna, buckthorn, rhubarb) and diphenylmethane (Bisacodyl, Dulcolax, Guttalax), as well as fatty acids (castor oil). Of this group, only Guttalax is used in pediatrics (in children over 2 years of age).
- Agents with osmotic properties: magnesium sulfate, Carlsbad salt, lactulose, polyethylene glycol 4000 (PEG; Forlax), lactitol (Exportal).
- Agents that increase the volume of intestinal contents: bran, seaweed, Plantago ovatae seeds (Mukofalk), etc.
- Means that help soften stool and allow it to slide: petroleum jelly and almond oil.
Osmotic laxatives are first-line drugs both in the treatment of functional constipation and in the treatment of patients with IBS with constipation. Based on the results of long-term studies of the comparative effectiveness of drugs in this group, it can be concluded that PEG turned out to be more effective than lactulose in the following aspects: frequency of stools per week, stool shape according to the Bristol scale, relief of abdominal pain due to chronic constipation, and the need for strict adherence to a diet to obtain an effect. The findings apply to both children and adults. The exception is the relief of pain: differences in the frequency of this symptom when using PEG and lactulose were identified only in children [33].
4. Enzyme therapy: pancreatic enzyme preparations (Creon, Pangrol, Mezim Forte, etc.) are used according to indications (signs of exocrine pancreatic insufficiency).
5. Psychopharmacotherapy is used for special indications, but it should be remembered that, according to available data, the effectiveness of placebo for patients with IBS is comparable to that of amitriptyline (56 and 63%, respectively) [34].
6. Prebiotics: among prebiotics for IBS in children, lactulose is most often used, of course, when
having constipation. We have not found any convincing data on the high effectiveness of the drug Hilak Forte for IBS in children.
7. Probiotics: their fairly high effectiveness in some children with IBS was discussed above.
In clinical practice, both mono- and combined probiotics (symbiotics and synbiotics) may be effective -
Bifiform, Linex, Normobact L, Primadophilus, Relalife, as well as a drug containing Saccharomyces boulardii (Enterol).
8. The literature discusses the possibility of using an opioid receptor blocker, the drug loperamide, which slows down transit through the colon. It has been shown to be effective for adult patients with IBS and diarrheal syndrome [35]. Similar studies in children and adolescents are currently lacking.
According to a Cochrane review, despite the wide range of possible pharmacological interventions, there are no well-controlled pediatric studies of the treatment of IBS, so “the question of the true effectiveness of drugs in the treatment of functional bowel disorders in children remains open” [36].
The adoption of a biopsychosocial model of functional bowel disorders, in particular IBS, has created the basis for the use of psychosocial interventions, incl. family therapy, cognitive behavioral therapy, relaxation techniques, hypnotherapy, biofeedback. Many of these strategies not only have a direct impact on physical symptoms, but also promote the child's self-management of symptoms. Meta-analyses and systematic reviews have shown that psychological treatments are effective in treating physical symptoms in adults and children with IBS [37].
About 40% of children with IBS annually use so-called methods. alternative medicine. These include acupuncture, osteo-, homeopathy, herbal medicine, etc. Convincing evidence confirming the effectiveness of these methods for children with IBS is currently lacking [38].
Conclusion
Based on their own clinical experience and study of documentation from various medical institutions in our country, the authors came to the conclusion that IBS is underdiagnosed in children. The reason for this phenomenon, apparently, lies in the lack of sufficient knowledge of primary care pediatricians on the problem under discussion and, accordingly, in a decrease in alertness towards it. The above criteria for diagnosing IBS should help the pediatrician navigate the differential diagnostic intricacies and reach the correct diagnosis. Gastroenterologists, neurologists, and psychotherapists can provide further assistance in organizing treatment programs.