Current recommendations
Volodymyr Nikishaev, Igor Tumak, Andriy Patiya Lviv National Medical University Ukrainian Scientific and Practical Center for Emergency Medical Care and Disaster Medicine
Liver disease is one of the leading causes of disability and death throughout the world. Zokrema in the United States, liver cirrhosis ranks fifth among causes of death in individuals up to 60 years ago. The average number of such diseases is 50 years old, which reinforces the socioeconomic importance of the problem.
Treatment of patients with cirrosis and scutulointestinal bleeding associated with portal hypertension (SBL) lies at the stage of portal hypertension, which the patient undergoes, starting from illness with cirrhosis om and portal hypertension, in those who have not yet developed varicose veins, and in those who are sick with acute illnesses varicose bleeding, in which the main method of treatment is to stop bleeding and prevent relapse. Since 1997, when the AASLD, ACG, AGA and ASGE recommendations for the diagnosis and treatment of varicose vein bleeding were published, three international consensus conferences were held (Baveno III in 2000, Baveno IV in 2005 and conference on one topic AASLD/EASL 2007), on which experts assessed the changes that occurred in the common pathophysiology and treatment of varicose vein bleeding.
PATHOPHYSIOLOGY OF PORTAL HYPERTENSION IN CIROSIS
The portal vein begins with the drainage of the superior mesenteric and splenic veins, the inferior mesenteric and left splenic veins variably drain from them or directly into the portal vein. These names reflect the fact that it is a “gate” for draining blood from organs of the liver, the depth of the portal vein is 6–8 cm, and the diameter is 1–1.2 cm. Inkova artery, however, the main source of blood flow is the portal vein itself and the renal flow (“flow-flow”) is determined by the very pressure of the portal system. Cirrhosis is the remaining stage of any chronic disease of the liver, which can lead to portal hypertension. This means that the portal venous pressure moves over 5–10 mm Hg. Art. In accordance with the basic principles of hydrodynamics, both increased blood flow and increased support (based on Ohm’s law - pressure gradient ΔP = Q × R, where Q is the flow from the front, and R is the support of the flow) can lead to Extension of the vice in the portal system.
The portal pressure in cirrhosis moves forward as a result of increased support for the hemorrhage, which is mainly due to the development of the architectonics of the liver after the growth of fibrous tissue and the establishment of regenerative nodes. According to Poiseuille's formula, R = 8nl/πr4, where n is the viscosity of the medium, in this case, l is the pressure of the hydrodynamic system (the quantities are actually stationary), and r is the radius of the vessel. For such reasons, small changes in the diameter of the vessels lead to a sharp increase in support. The liver normally has a circulatory system with low support. Greater support can be localized on the subhepatic, hepatic and suprahepatic levels (Table 1). Intrinsic portal hypertension (PH), in its own way, can be divided into presinusoidal, sinusoidal and postsinusoidal, but in the clinical situation it is important to clearly distinguish between them: in case of chronic In case of chronic hepatitis, presinusoidal and sinusoidal support is expected, and in case of alcoholic liver disease, sinusoidal support is expected (producing collagen in the vastness of Disse , which is secreted by the medial parts of the liver) and postsinusoidal (fibrosis of the terminal hepatic veins). With schistosomiasis of the liver, the presinusoidal support moves to the kidney, and later the sinusoidal area is affected.
On the appendage to the structural support of the bloodclin, in reality an active intranschinopynkov, the sound of the Sudin, yake vidpovіd for 20-30% of the visor, the ovias, in the main, in the main, the zmenses of the endogenic product monoxide nitrogen. Both in chronic and acute illnesses of the liver, the tissues of the liver develop rapid changes, such activated cells replace actin-like filaments and express the smooth muscle actin alpha gene. They react to vasoactive substances that are visible in the endothelium of the vessels, due to a decrease in the production of the vasodilator NO and an increase in the production of the vasoconstrictor endothelin.
Hypertension is also caused by a disorder in sodium homeostasis and a hyperdynamic circulatory system. In patients with portal hypertension, there is an increase in cardiac index, a decrease in systemic vascular pressure and an increase in splanchnic arterial blood pressure (and, therefore, blood supply to the portal system). Please note that there are three main causes of peripheral vasodilation in portal hypertension. The first priority is to promote movement instead of circulating vasodilators due to their increased production and reduced catabolism (through impaired liver function and portosystemic shunting of blood past it). Another is an increased production of local vasodilators by the endothelium, and the third is a decreased response to vasoconstrictors. However, evidence for the importance of these three mechanisms is still insufficient and controversial.
As a result of systemic vasodilation, the hyperdynamic circulatory system increases plasma volume. It is important to note that splanchic and systemic vasodilation lead to a decrease in central arterial volume through an increase in the capacity of the venous pool. As a result, there is a compensatory activation of the renin-angiotensin-aldosterone system and the sympathetic system, and the production of vasopressin also increases. There is a retention of sodium and water and, apparently, an increase in plasma volume.
The inheritances of PG include blood stagnation in the spleen, hypersplenism and thrombocytopenia. Another dramatic legacy of portal hypertension is ascites. Portal hypertension causes the formation of porto-systemic collaterals. However, regardless of their developments, hypertension subsides for two reasons: 1) increased inflow to the portal vein as a result of dilation of the visceral arterioles, which occurs simultaneously with the formation of collaterals, and 2) insufficient portal decompression and through collaterals, the fragments of the stench create greater support, lower judgment healthy liver.
The supply between the portal and renal (caval) venous systems is normal, however, in PG the blood flow through them increases sharply. There are 4 main groups of portosystemic collaterals.
The first group is divided into two subgroups. Subgroup Ia includes the left sciliary vein and short sciliary veins, which in the dilation of the cardia of the Schulla create anastomoses with the intercostal veins, the phrenic vein, the veins of the splanchnic vein and other others. veins of the caval system (for example, lumbar veins). The most intense flow of blood in these paths should be achieved until the formation of varicose veins and varicose veins. Before subgroup Ib there is the superior rectal vein, which creates an anastomosis with the middle and inferior rectal veins of the caval system.
The other group is the paraumbilical veins, which run from the round ligament of the liver and anastomose with v v. epigastricae sup. et inf. Paraumbilical veins are a remnant of the fetal blood supply; in adults they are obliterated and in PG are recanalized.
The third group is formed by collaterals between the veins of the organs of the cervix and the caval veins of the adjacent tissues behind the cervix and the cerebral wall. This includes anastomoses between the veins of the liver and the diaphragm, the veins of the splenic-splenic ligament, between the veins of the anterior ducts of the colon and the lumbar veins. The same veins are located here that were formed at the adhesions after the previous abdominal operations.
The fourth group is an anastomosis between the splenic and left nirconic, inferior phrenic and left supranar veins (the third and fourth groups are not covered by all authors). The presence of portosystemic anastomoses indicates PG, however, with intense colateral blood flow, the pressure at the portal vein may fall. The short-lived PG may not be accompanied by obvious colateral circulation.
Both for the volume of blood flow and for the clinical values (bleeding), the most important are the portocaval anastomoses in the area of the schular-stravohod junction. Through them, blood is supplied to the azygos and superior veins. Through their clinical importance, it is necessary to thoroughly examine the anatomy of the venous vessels of this plot.
The diver can see that the venous vessels are moving around. Superficially, intraepithelial veins emerge to drain into the superficial venous plexus of the submucosal globule. The venous plexus was obtained from the deep veins of the submucosal ball. The perforating veins that penetrate the globulus musculosus through the vein pass behind the adventitial venous plexus.
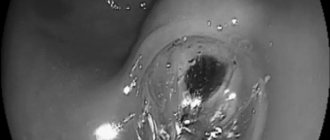
Vianna A. et al. (1987) saw 4 zones of the veins of the cardioesophageal vein: one zone in the schulca and 3 zones in the vein. The distal zone is located, in number, of small diameter, with a later vein located at the lamina propria and the submucosal ball, creating anastomoses. The distal veins of the sciliary zone are formed, forming vessels of large diameter, which are connected to the portal and splenic veins via the left sciliary vein and the short scilial veins. Proximally, above the cardia, the palisade zone of the vein begins, 2–3 cm, in which vein, which comes from the cardia, passes through the meatal plate of the mucous membrane and then goes to the middle of the cardia. This transition occurs on the level of the stravokhodno-schluk connection. Venous vessels in this zone are oriented laterally, they are parallel, however, in size. There is a perforating zone of about 2 cm in the vein, in which veins are formed, creating 4-5 large stovburs, which penetrate through the meatal plate of the mucous membrane and spread into the submucosal ball; There are also numerous communicative veins, as are the veins of different levels. In the Stovbur zone, proximal to the perforant, there are late submucosal veins, the diameter of which changes in the oral direction (it is assumed that in the Stowbur zone the blood in the veins flows in the caudal direction). With the advancement of the portal pressure and the development of hypertension in the venous pools, there is an increase in the deep veins of the submucosal globule, especially in the perforant zone. These veins themselves are identified endoscopically as varicose veins. The intraepithelial veins and veins of the superficial plexus are also dilated and transformed. Endoscopically, the stench looks like “red signs” that are located on the varicose veins themselves.
1.What are esophageal varicose veins?
Varicose veins of the esophagus
- This is the dilation of blood vessels in the esophagus.
Sometimes the veins expand in the stomach. This disease does not cause any unpleasant symptoms if there is no rupture or bleeding from the vessels. , portal hypertension
can occur , a life-threatening condition.
Portal hypertension
- this is an increase in pressure in the portal vein system (the vein through which blood flows from the digestive organs to the liver), which is often associated with a blockage of all blood flow to the liver. Increased pressure in the portal vein leads to the development of large, swollen veins (varicose veins) in the esophagus, stomach, rectum and umbilical area. Varicose veins are fragile and rupture easily. And if a vein ruptures, large blood loss can occur. The most common cause of portal hypertension is cirrhosis of the liver. Cirrhosis is scarring of the liver as liver damage caused by hepatitis, alcohol, and other less common causes heals. In cirrhosis, scar tissue can block the flow of blood through the liver and slow its functioning.
A must read! Help with treatment and hospitalization!
CLINICAL DIAGNOSIS OF PORTAL HYPERTENSION
With physical examination, it is easiest to detect ascites, redness, spider-like angiomas on the skin, erythema pubis, testicular atrophy, gynecomastia, Dupuytren's contracture and muscle atrophy. Portosystemic encephalopathy may also manifest itself - in some cases, asterixis (blurring or “purrying” tremor of the hands) and lethargy, in the lungs - restlessness, sleep disturbance. Most patients with PG also exhibit splenomegaly, although the size of the spleen correlates poorly with portal venous pressure and its increase may be associated with other illnesses. A hyperdynamic state can be indicated by a high (jumping) pulse, warm, well-perfused endings in conjunction with arterial hypotension.
Signs of portosystemic collaterals are dilated veins on the anterior surface of the abdomen (umbilical-epigastric shunts), the “jellyfish head” becomes a sinuous colateral vein near the navel. Internal hemorrhoids are not a specific finding, but may also manifest collateralization. The weakness of the visible collateral vessels on the back does not indicate portal hypertension, but obstruction in the level of the inferior venous vein. A rare cause may be compression of the lower empty vein by regenerative nodes in liver cirrhosis.
Ascites is easy to detect when there is a lot of swelling in the stomach - in such cases the life is tight, and swelling of the spine can be detected. However, minor ascites can be diagnosed instrumentally, for example, with the help of ultrasonography. Paracentesis helps to establish the etiology of ascites: the portal (non-peritoneal) etiology indicates a serum ascitic albumin gradient (SAAG) of over 1.1 g/dL (11 g/L). However, this method cannot distinguish between hepatic and suprahepatic PH, for example, in cases of persistent heart failure. Recent studies indicate a decrease in SAAG following the placement of transjugular portosystemic shunts (TIPS).
Laboratory testing of gas is not specific for the detection of PG. Indicates an increase in prothrombin hour and thrombocytopenia. In patients with cirrhosis of the liver, these indicators are considered predictors of the presence of varicose veins (see below). Hypoalbuminemia indicates the importance of liver function and, indirectly, PG. Similarly, hyponatremia and low sodium concentrations in the tissue may contribute to water and sodium retention in portal hypertensive syndrome.
ASSESSMENT OF PORTAL HYPERTENSION
The shortest, albeit indirect, method of assessing portal pressure involves wedged hepatic venous pressure (WHVP), which is determined by inserting a balloon catheter (from the jugular, brachial or stegnosus access) under the fluid. oroscopic guidance of the hepatic vein and “jamming” it in the small hilt (introduced all the way) or (which is still sticky) - by inflating the balloon and occluding the larger hilt of the liver vein. The correction of WHVP will ultimately involve the improvement of the internal hepatic vein pressure (for example, with ascites) by releasing the pressure in the hepatic vein - VTPV (free hepatic vein pressure - FHVP) or the internal hepatic vein pressure pressure in the empty vein, which is respected by the internal zero level. They survive when the balloon is deflated. The hepatic venous pressure gradient (HVPG) is determined by the pressure gradient in the hepatic vein. The indicator, as a matter of course, dies three times; If the values are technically correct, they are also well-designed and reliable. Although there is a gradient of pressure between the sinusoids (and not the portal vein veins) and the hepatic vein, HPVT will be advanced in case of intrahepatic causes of portal hypertension, such as cirrhosis, however normal for prehepatic causes, such as portal vein thrombosis. Normal HPVT readings are 3–5 mmHg. Art. It was found that WHVP closely correlates with portal pressure in both alcoholic and non-alcoholic cirrhosis. HPVT and changes in its symptoms, which are observed over time, may have a transferable value to the development of varicose veins in the vein, the risk of varicose bleeding, the development of non-varicose folds of portal hypertension And death. Single extinction is useful for the prognosis of both compensated and decompensated cirrhosis, while repeated extinction is useful for monitoring the effectiveness of drug therapy and the reversal of liver disease. The main considerations for the widespread viability of HPVT include the lack of evidence from practicing physicians and their continued recommendations on how to eliminate reliable and effective indicators, as well as other the simplicity of the procedure.
Duplex Doppler ultrasonography
Ultrasonography is a safe, inexpensive and effective method for screening for PG. The knobby structure of the liver, splenomegaly and the presence of colateral circulation give rise to suspicion of liver cirrhosis and PG. The diameter of the splanchnic vessels, directly and the fluidity of the bloodstream, changes in the diameter of the vessels during breathing, the index of portal venous stagnation, the pulsativity index and the resistance index of the liver, upper pars and glands also depend. Hepatic artery and hepatic vessel index. Normally, the diameter of the portal vein does not exceed 13 mm during quiet breathing, but during deep breathing it can increase by 50%. Expand a vein with a diameter of >15 mm while breathing calmly. When the pressure of the hemorrhage at the portal vein is increased and becomes monotonous (without breathing sounds) and resolves completely in the portal vein, straightened at the non-functioning portocaval shunts.
However, the creation of many ultrasonic parameters, their accuracy is insufficient, lies both in the tracking technology and in the form of additional rhythms, the development of the sympathetic nervous system, medicine, etc. So, for example, the diameter of the portal vein may increase in case of severe congestive heart failure (water from the inferior empty vein). Therefore, duplex Doppler ultrasonography itself is not recommended for establishing an accurate diagnosis of PG; it is unclear that its diagnostic value for the early stages of PG is lost (in most of these studies, patients were monitored with severe cirrosis and severe PG). Nevertheless, this method is valuable for primary closure, and also helps to identify the etiology of PG, for example, obstruction of blood vessels at different levels, highly sensitive to the identification of functional portocaval anastomoses. After placement of TIPS or portocaval anastomoses, Doppler ultrasonography is of great importance in monitoring shunt patency.
Computed tomography is a test with clear (not sharp) values, whereas ultrasonography did not give clear results. Apart from ultrasonography, CT results do not indicate the presence of gas in the intestine. Reconstruction of the vascular tree with the help of 3-D technology makes it possible to accurately create the actual portal venous system and collaterals. A common finding in portal hypertension during CT scanning is dilatation of the inferior vein. However, CT does not allow assessing the flow of blood through the venous and arterial vessels. MAR makes it possible, in addition to clear information, to obtain detailed information, for example, to assess venous flow in the portal and azygos veins. However, this does not exclude the need for invasive surveillance.
In cases of doubt about the genesis of liver disease, puncture biopsy may be necessary: necrosis of the third zone, for example, indicates portal hypertension, secondary to cardiac failure, and normal liver parenchyma - about subhepatic cause of PG.
Contraindications for liver biopsy include coagulopathy and ascites. Table 1. Causes of portal hypertension and pressure gradient in the hepatic vein
| GTPV | |||
| Rhubarb occlusion | Normal | Normal or movement | Advances |
| Subhepatic syndromes of PG | Portal vein thrombosis Thrombosis of the splenic vein Splanchnic arteriovenous fistula | ||
| Intrinsic hepatic syndromes of PG More important than the level of presinusoidal level | Schistosomiasis, primary biliary cirrosis, idiopathic PG (early stage)* Vuzlova regenerative hyperplasia* | Myeloproliferative diseases*† Polycystic disease*† Liver metastases*† Granulomatous disease † (sarcoidosis, tuberculosis)*† | |
| More important is the level on the sinusoidal or postsinusoidal level | Budd-Chiari syndrome‡ | Cirrosis of the liver Schistosomiasis, primary biliary cirrosis, idiopathic PG (late stage) Hospital and fulminant hepatitis Gostria alcoholic hepatitis Wilson's illness Veno-occlusive disease Budd-Chiari syndrome‡ | |
| Post-hepatic syndromes of PG | Obstruction of the inferior venous vein Right heart failure Clutching pericarditis Tristucular valve insufficiency | ||
* Rare causes of PG. † For the mind of the sinusoids. ‡ Sinusoidal symptoms are weak.
Features of treatment
Therapeutic measures can be divided into two groups. The first group is aimed at eliminating the cause that led to varicose veins of the esophagus and stomach. In addition to liver cirrhosis, it can be chronic hepatitis, echinococcosis, tumors that compress the portal vein and other diseases that were discovered during diagnosis.
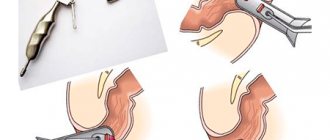
The second group of methods is aimed at eliminating varicose veins directly. For this purpose, conservative treatment is prescribed (drugs that contribute to the narrowing of blood vessels and strengthen their walls) and endoscopic operations that are aimed at eliminating pathologically dilated veins (sclerotherapy, ligation, etc.).
If these measures are ineffective, then they resort to more radical treatment, which consists of creating “bypass paths” (shunts) for the blood in order to unload the blood flow in the portal vein.
Our clinic’s specialists can offer several modern methods for treating varicose veins of the esophagus and stomach. The exact plan is drawn up individually, after consulting a doctor and the necessary diagnostics. Surgical interventions are performed in a well-equipped small operating room.
OVERBIG ZAVROYUVANNYA
Sculo-stravochoidal varicose veins (PVVs) are the most clinically important portosystemic collaterals, so their ruptures lead to varicose bleeding, the most common lethal form of cirrhosis - the first This episode is associated with a mortality rate of 30–50%. Varicose veins and varicose bleeding are aggravated by cirrhosis, which is directly caused by portal hypertension. Patients with cirrhosis and gastroesophageal varix show HPVT readings of less than 10–12 mmHg. Art.
Schlunkovo-stravochodnye varicose veins are detected in about 50% of patients with cirrhosis. Their presence correlates with the importance of liver disease (Table 2): while in class A for Child-Pugh, only 40% of patients have varicose veins, then in class C veins, 85% of patients have varicose veins. In patients with primary biliary cirrosis, varicose veins and varicose bleeding may develop at an early stage of illness, leading to the diagnosis of cirrhosis. It was also found that 16% of patients with hepatitis C and pontic fibrosis have varicose veins.
In patients without varicose veins, the remaining varicose veins develop with a frequency of 8% per river; the strongest predictor for the development of varicose veins in patients who are not visible during the initial endoscopic examination is an HPVT reading >10 mm Hg. Art. In patients with small varices, large varices develop with a frequency of 8% per population. Decompensated cirrhosis (Child B/C), alcoholic cirrhosis and the presence of “worm signs” during the first endoscopy are the head signs associated with the transition from small varixes to large ones (Merli M. et al., 2003). Japanese authors, in their classification, give a substantive meaning to the bluish bark of the VRV.
Variceal bleeding occurs with a frequency of 5–15% per river, and the most important predictor of bleeding is the size of the variceal vein, with the highest risk of first bleeding (15% per river) in patients with large variceal veins (The North Italian Endoscopic Club for the Study and Treatment of Es. ophageal Varices , 1988). Other predictors of bleeding are decompensated cirrhosis (Child B/C), bluish coloration of the veins, and the appearance of red signs during endoscopy. While bleeding from varicose veins usually disappears on its own in up to 40% of patients, regardless of treatment in the hospital for the remaining 10 years, stench is associated with a mortality rate of at least 20% between 6 years iv. Patients with HPVT >20 mm Hg. Art. (over a period of 24 years after varicose bleeding) it was found that there is a high risk of early recurrence of bleeding (re-bleeding in the first life after surgery) or distant spots of bleeding (83% versus 29%) and I see a single mortality rate (64% per against 20%) was equal to patients with lower pressure. Later, relapses of bleeding occur in about 60% of serious patients, mostly in 1-2 cases after the initial bleeding.
The portal pressure, the size of the vein and the thickness of its wall are related to each other by Laplace’s law - the stress of the vein wall (T) is directly proportional to the pressure in the middle of the vessel (P) and its radius (R) and is proportional to the thickness of the vessel Inki (W): T = P × R/W.
Apparently, dilatation of the node from the thinning of its wall is the main factor that causes stress on the wall and rupture of the varix. Under pressure, however, a vessel with a larger diameter will rupture, but a vessel with a smaller diameter will not. Around the diameter of the vessel, another source of stretching of the varicose wall is the pressure in the middle of the VRV, which is directly connected with the HPVT. Thus, a decrease in HPVT is likely to reflect a change in the stretching of the wall of the varicose vein and, apparently, a change in the risk of rupture. In fact, varicose veins do not bleed when HPVT decreases to <12 mmHg. Art. It has also been shown that the risk of re-bleeding significantly changes when GPVT is reduced by more than 20% of the cob level. Diseases in which HPVT decreases to <12 mmHg. However, at least 20% of the cob level (“HVPG responders”) may reduce the risk of recurrent variceal bleeding, as well as a lower risk of ascites, spontaneous bacterial peritonitis, and death. Table 2. Classification of the importance of cirrhosis according to Child-Pugh
| Items* | |||
| 1 | 2 | 3 | |
| Encephalopathy | No | Riven 1–2 (induced) | Riven 3–4 (chronic) |
| Ascites | No | Minor/moderate (responds to diuretics) | Tension (resistant to diuretics) |
| Bilirubin (mg/dl) | <2 | 2–3 | >3 |
| Albumin (g/dl) | >3,5 | 2,3–3,5 | <2,8 |
| Prothrombin hour (PT), daytime (s) or international normalized ratio (INR) | <4 <1,7 | 4–6 1,7–2,3 | >6 >2,3 |
*5–6 points: Child A. 7–9 points: Child B. 10–15 points: Child C.
Gastrointestinal bleeding - symptoms and treatment
First of all, the adrenal glands . They begin to “throw” special substances into the bloodstream - catecholamines. This reaction occurs on the first day after bleeding. It leads to spasm of peripheral vessels and compensation of hemodynamics - normalization of pressure and blood flow speed in the circulatory system. Thanks to this, sufficient blood supply to vital organs - the heart, brain and liver - is maintained.
tissue fluid “leaks” into the vascular bed . It makes the blood less viscous, promotes the removal of red blood cells from the “depot”, in particular from the spleen, and their entry into the bloodstream. Thus, the body, in the event of small short-term bleeding, creates conditions for the rapid restoration of the original volume and quality of circulating blood. But at the same time, metabolic disorders gradually develop at the tissue level, since tissue fluid is a liquid nutrient medium, thanks to which the exchange of substances occurs between cells and tissues on the one hand and blood on the other.
On days 4-5 after bleeding, the bone marrow begins to actively replenish the missing amount of lost blood elements, in particular red blood cells and platelets. If bleeding no longer occurs, the red blood cell count will return to normal after 2-3 weeks.
The patient’s well-being and the clinical picture of gastrointestinal bleeding are influenced by the volume and rate of blood loss. It depends on them how fully and quickly the body’s compensation and adaptation mechanisms will restore the volume of circulating blood.
In the case of spontaneous stopping of bleeding and loss of no more than 10% of the initial blood volume, the body’s condition, as a rule, is easily stabilized due to the processes described above.
In the first hours after significant blood loss, the hemoglobin concentration and the number of red blood cells also remain within normal limits. Their decline begins only at the end of the first day, which, at certain minimum threshold values, requires a transfusion of donor blood. In addition, the concentration of metabolic products in the blood increases - urea and creatinine, which is why intoxication is added to everything else. Taken together, these conditions lead to increasing multiple organ failure. In the absence of qualified medical care, a person in this condition usually dies.
Separately, it should be noted minor, frequently recurring bleeding , which is characterized by extremely insignificant blood loss (20-50 ml). This is possible with chronic hemorrhoids, the same ulcers (if they damage a small vessel) and other pathologies, including cancer. The danger lies in the fact that, against the background of small repeated blood losses, our body does not have time to compensate for the progressive lack of iron and/or vitamin B12 necessary for the production of hemoglobin. Thus, with frequent small blood losses, a person gradually develops a mild degree of anemia, which over time can develop into a more severe form. The risk of developing such a scenario is high among people who do not pay due attention to their health, are afraid of a medical examination, or, knowing about their illnesses, for various reasons refuse to treat them [2][4][7].
VARICOSE VEINS
Varicose veins, the veins are smaller, the lower varicose veins, and are now found in 5–33% of patients with portal hypertension, with a bleeding frequency of about 25%, with a length of 2 lines and with a large amount of bleeding. veins of the bottom of the scutum (Sarin SK et al., 1992). Risk factors for vulvar varicose bleeding include the size of the nodules at the bottom of the vulva (large > medium > small, values >10 mm, 5–10 mm and <5 mm uniform), classification of cyrrhosis as Child (C > B > A) and the presence of worms them signs on the surface of the varix during the hour of endoscopy (Kim T. et al., 1999). The schulci varicose veins are usually divided on the basis of their relationship to the splanchnic veins, as well as their localization in the scutum. Gastroesophageal varices (GEVs) are extensions of varicose veins along the line and are divided into 2 types. The most common types of varix are type 1 (GEV 1 – GOV1), which widen the lesser curve. The stinks are respected by the continued VRV voyage and are guilty of rejoicing in the same way. Type 2 slug ERVs (GEV 2) are expanding at the bottom and tend to be long and sinuous. Isolated hose VRV (ISHV) are identified for the presence of varicose veins in the traveler and are also divided into 2 types. VRV type 1 (ІШВ 1 – IGV1) are localized near the day and are often sinuous and complex, and VRV type 2 (ІШВ 2) are located near the body, antrum and near the hilum. The presence of varicose veins at the bottom of the scutum will require excluding the diagnosis of splenic vein thrombosis.
PORTAL GASTROPATHY
Portal hypertensive gastropathy is inherited from portal hypertension with capillary dilatation and rupture of internal mucosal arterial shunts. This indicates the importance of maintaining the liver, which may occur in patients with hepatic portal block. With morphological examination, dilatation of vessels of both the mucous and submucosal balls is indicated. Hemorrhage in the mucous membrane is associated with thrombocytopenia, which develops with liver cirrhosis. With portal gastropathy, the sensitivity of the mucous membrane to various ear-causing agents increases: aspirin, alcohol, etc. Regression of portal gastropathy after transhepatic portosystemic shunting and reduction - after sclerotherapy or ligation of varicose veins is indicated.
The New Italian Endoscopic Club (1997) recommends that the diagnosis of portal gastropathy be based on four signs: mosaic appearance of the mucous membrane, appearance of red spots (mild stage), cherry-red spots, black-brown spots (important). step). The body of the slut is struck in front of us.
It is important to note that portal gastropathy can occur in patients with arterial hypertension without PG, and also in patients without other illnesses, its manifestations can progress, and also disappear. Bleeding, referred to as portal gastropathy, is rarely massive and more often leads to chronic bleeding.