Anatomy and physiology
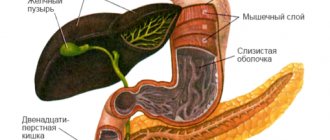
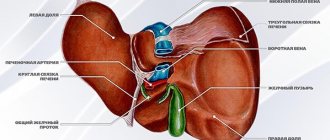
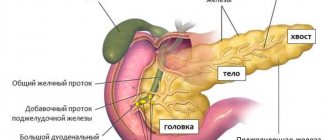
The organ with a grayish-pink surface has an oblong triangular-prismatic shape. The pancreas is one of the largest, second only to the liver in size. Its weight is 70-80 g, length is 16-22 cm, width 3-9 cm, thickness 2-3 cm. There are 3 parts in the gland. The widest is the head. Like a horseshoe, it is covered by the duodenum.
Further, tapering, the head passes into the body of the gland, and the body into an even narrower tail. All these parts smoothly replace each other from right to left: head, body, tail. There are no clear anatomical landmarks between them, except for the neck of the pancreas, a slight narrowing between the head and the body.
The pancreas is adjacent to the posterior surface of the abdominal wall at the level of JX-XII thoracic and I-II lumbar vertebrae. It is projected onto the anterior abdominal wall 5-10 cm above the navel. 1/3 of it is located to the right of the vertical midline of the spine, and the remaining 2/3 is to the left of this line. In this case, the gland is located extraperitoneally, outside the abdominal cavity.
Like other glandular organs, the pancreas is represented by stroma and parenchyma. The stroma is a connective tissue framework. Its function is mainly performed by the peritoneum, which covers the anterior and lower surface of the gland. The entire gland is covered with a thin, barely visible capsule. From it, septa or trabeculae extend into the thickness of the gland, which divide the parenchyma into lobules.
Parenchyma is the functional tissue of the gland. Parenchymal lobules consist of acini, tiny round accumulations of pancreatocyte cells. These cells secrete pancreatic juice or pancreatic juice. Each acini is equipped with a small excretory duct through which the juice flows.
Small channels merge into larger ones. And the largest - the main or Wirsung duct , stretches through the tail, body, and head of the gland, and comes off on the duodenum with an opening on the eminence, the major duodenal or Vater papilla.
Previously, it merges with the common bile duct , the common bile duct. The outlet on the papilla of Vater is equipped with a muscular valve or sphinketer of Oddi, which regulates the release of bile and pancreatic juice into the duodenum. Although in some people the common bile duct and the Wirsung duct on the nipple of Vater have different openings.
In addition to the main duct, the head of the gland also has an accessory or Santorini duct. Options are possible here too. In most, the main and accessory ducts merge in the thickness of the head. But in some Santorini, the duct opens into the duodenum independently through an opening on the small duodenal papilla. And it happens that the Santorini duct is absent, and this is the norm.
The pancreas secretes 1.5-2 liters of juice during the day. In addition to water, it contains sodium and potassium salts, as well as bicarbonates to alkalize the acidic contents of the duodenum coming from the stomach.
But the main components of pancreatic juice are enzymes - trypsin and chymotrypsin, amylase, lipase, nuclease , and many others. These enzymes break down proteins (proteolysis), fats (lipolysis), and sugars (glycolysis). Under the action of these enzymes, large molecular food compounds break down into intermediate products.
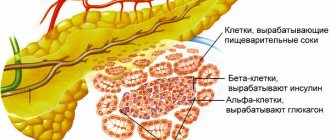
Juice-secreting acini with ducts are the exocrine or exocrine apparatus of the gland. It accounts for the bulk of the parenchyma. The gland also contains an intrasecretory or endocrine apparatus. These are the islets of Langerhans, small clusters of cells mainly in the tail of the gland.
Islets consist of different types of cells: alpha, beta, delta. These cells secrete hormones : insulin, glucagon, ghrelin, somatostatin. Hormones regulate carbohydrate metabolism, the secretion of other digestive glands, and stimulate appetite. Unlike the exocrine part of the gland, its islet endocrine apparatus does not have excretory ducts. Hormones are released directly into the blood.
Digestion.
Also on topic:
INSULIN
The part of the gland involved in digestion secretes pancreatic juice through the main duct directly into the duodenum. It contains 4 enzymes necessary for digestion: amylase, which converts starch into sugar; trypsin and chymotrypsin are proteolytic (protein-breaking) enzymes; lipase, which breaks down fats; and rennin, which curdles milk. Thus, pancreatic juice plays an important role in the digestion of essential nutrients.
Diseases and symptoms
Inflammation of the pancreas, pancreatitis , mainly develops against the background of other diseases of the digestive system - cholecystitis, gastroduodenitis. The disease occurs acutely and chronically. The inflammatory process is based on the mechanism of self-digestion of the gland. Normally, proteolytic enzymes (trypsin, chymotrypsin) are secreted in an inactive state and are activated in the lumen of the duodenum.
In diseases of the gastrointestinal tract (gastrointestinal tract), reflux , the reflux of the contents of the duodenum into the pancreatic ducts. Proteolytic enzymes activated in the duodenum digest their own gland.
A specific sign of pancreatitis is girdle pain in the upper abdomen. In chronic pancreatitis, it is aching, bursting. Patients complain of decreased appetite and nausea after eating. Insufficient supply of pancreatic juice leads to fermentation in the intestines.
This is manifested by flatulence, cramping diffuse abdominal pain, frequent foul-smelling loose stools (pancreatogenic diarrhea). Due to pain and digestive disorders, patients themselves limit their food intake. And this aggravates the existing weight loss.
The pain in acute pancreatitis is burning and intense. A characteristic connection of pain with heavy intake of fatty, fried foods, and alcohol. Therefore, pancreatitis often manifests itself after festive feasts. Hunger, combined with the effect of cold on the upper abdomen in the projection of the pancreas, leads to an improvement in well-being. When you bend your torso forward, the pain also decreases.
The pain is accompanied by intoxication, fever up to 38°C, dehydration due to repeated vomiting and diarrhea. Enzymes enter the blood in large quantities from the glandular parenchyma. This can lead to a state of shock due to depression of cardiac activity (enzymatic shock).
Pancreatic cancer is also severe with intense pain, weight loss and progressive deterioration with secondary damage to other organs.
As for the endocrine apparatus, the most vulnerable here are the beta cells that secrete insulin. A deficiency of this hormone leads to diabetes mellitus with hyperglycemia (high blood glucose levels) and other types of metabolic disorders.
Symptoms of the disease
At the beginning of stone formation, symptoms of pancreatitis are present. The presence of stones is indicated by the following signs:
- a burning pain that encircles the abdomen and radiates to the back or under the shoulder blade, which occurs after drinking alcohol or eating very fatty foods;
- nausea, vomiting bile;
- occasionally – the presence of a large amount of fat in the stool.
As the disease progresses, the secretory and enzymatic function of the gland deteriorates, and necrosis of its tissue appears. When palpating the epigastrium, the patient feels severe pain and drooling. If a stone enters the common duct, jaundice may develop. Most patients develop diabetes at this stage.
The work of the pancreas and its regulation
The pancreas is a vital organ
Like any other organ in the human body, the pancreas, or rather its work, is controlled by the central nervous system. From the research conducted by scientists, it can be noted that whenever an attractive smell of food appears, especially if a person is hungry, the work of the gland is immediately activated. It is worth mentioning separately that the vagus nerve is responsible for activating the work of this gland, and the sympathetic nerve is responsible for reducing activity.
Aberrant pancreas: diagnosis and therapeutic tactics. Clinical observation
Aberrant pancreas is the result of impaired embryogenesis. The clinical picture can be varied and disguised as various diseases. The complexity of diagnosis forces specialists to examine patients more carefully. Chronic recurrent pancreatitis is considered one of the risk factors for pancreatic cancer. Tobacco smoking and alcohol significantly increase the risk of developing an aberrant pancreas. The article provides a clinical example of diagnosing an aberrant pancreas in a 37-year-old man ten years after the onset of chronic pancreatitis. The disease began with an episode of acute pancreatitis, pancreatic necrosis, which was treated in the hospital. Four years later, pancreatic pseudocysts were diagnosed. However, only six years later cystic degeneration of the stomach walls and duodenal degeneration were diagnosed. Thanks to a correct diagnosis, it was possible to prescribe conservative treatment and avoid surgery.
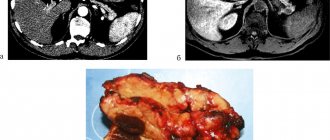
Rice. 1. Computed tomography with intravenous bolus contrast. Pancreatic phase. A – thickened stomach walls. B – increased size of the stomach, abundant contents inside – signs of gastrostasis
Rice. 2. Endoscopic ultrasonography. A – cystic degeneration of the gastric body wall
Rice. 3. Computed tomography with contrast in 2022. Pancreatic phase. A – decrease in the thickness of the stomach walls. B – reduction in the size of the stomach compared to the 2022 study, formation of folds in the gastric mucosa
Introduction
Aberrant pancreas (P) is one of the rare malformations, expressed by heterotopia of pancreatic tissue into other organs, such as the stomach, duodenum (DPC), small and large intestine, liver, gall bladder [1].
The pancreas begins to form in the fifth week of intrauterine development from the dorsal and ventral primary protrusions, originating from the duodenum and the liver anlage, respectively [2]. By the seventh week, both primordia merge. The lower part of the head of the pancreas and its uncinate process are formed from the ventral rudiment, and the body and upper part of the head of the gland are formed from the dorsal rudiment [3]. The aberrant gland has no anatomical or vascular connection with the main one. In the wall of the stomach, ectopia of the pancreas occurs in 25–60% of cases, in the wall of the duodenum – in 25–35% of cases [4].
In 1970, French scientists F. Potet and N. Duclert first described aberrant pancreas [5]. Among the Western population, the prevalence of aberrant pancreas reaches 10%; in Asians and Africans the frequency is lower – 1–2% [6].
Clinically, the disease can manifest itself with inflammatory and necrotic changes in the accessory pancreas and surrounding tissues. Aberrant pancreas can cause intestinal obstruction, pancreatitis and pancreatic necrosis, gastric ulcers, obstructive jaundice that develops as a result of compression of the bile ducts.
More than 95% of patients with aberrant pancreas are asymptomatic. Detection of the disease is an accidental finding during esophagogastroduodenoscopy (EGDS), computed tomography (CT), magnetic resonance cholangiopancreatography, endoscopic ultrasonography (EUS).
The pathogenesis is not completely clear, but it is believed that due to the presence of an additional pancreatic duct with a smaller diameter, which causes inadequate drainage of pancreatic juice, flow obstruction is caused. Increased intraductal pressure and distension of the pancreatic duct can lead to pancreatitis of the ectopic pancreas [6].
Differential diagnosis is complex and is a key link aimed at developing adequate treatment tactics for patients. Differential diagnosis is carried out with neoplasms of the hepatobiliary tract, congenital anomalies, and inflammatory diseases. The main symptoms include pain in the upper abdomen, nausea, vomiting, weight loss, anemia, and electrolyte imbalance [7]. It is known that both cancer and neuroendocrine tumors can develop in an ectopic gland [8, 9]. With the help of X-ray, ultrasound and endoscopic research methods, the correct diagnosis can be established with a high degree of probability.
One of the most important diagnostic methods is EUS; if necessary, elastography and contrast enhancement are used. The elasticity coefficient is assessed, the average value of which can differentiate inflammatory and tumor lesions of the pancreas. The use of EUS contrast enhancement makes it possible to evaluate the wall, septa and nodular component of cystic formations, which expands the possibilities of differential diagnosis of cystic formations of the pancreas [10].
In the absence of clinical manifestations, this pathology requires only dynamic observation [3].
If clinical manifestations are present, therapeutic and surgical treatment methods are considered. Therapeutic treatment is based on cessation of alcohol, smoking, and principles similar to those for the treatment of chronic pancreatitis. Drug therapy with analgesics, proton pump inhibitors, nonsteroidal anti-inflammatory drugs, and synthetic somatostatin analogues is used. If necessary, nutritional support is provided in the form of parenteral and (or) tube nutrition [11].
If therapeutic treatment is not possible, the main method is surgery [12]. However, the choice of surgical treatment is complex. Depending on the pain syndrome, the degree of narrowing of the duodenal lumen and damage to the pancreas, a certain method of surgical treatment is used: pancreatoduodenal resection, circular resection of the descending part of the duodenum and resection of the head of the pancreas, resection of the descending part of the duodenum with duodenoplasty and reimplantation of the major papilla of the duodenum, pancreas-preserving subtotal resection of the duodenum, resection of the head of the pancreas (Beger's, Frey's, Bernese resection operations) and palliative operations [13]. Most often, pancreaticoduodenectomy becomes the method of choice. In case of ectopia, pancreato- or cystoenteroanastomosis can also be performed in the stomach and other organs, and gastrectomy can be performed [11]. However, in the absence of clinical manifestations of pancreatic ectopy in the stomach, dynamic observation is recommended [3].
Clinical observation
In December 2022, to the Moscow Clinical Scientific and Practical Center (MCSC) named after. A.S. Loginov, patient R., aged 37 years, was hospitalized with complaints of aching pain in the epigastrium and left hypochondrium, aggravated after eating, a feeling of fullness in the stomach, periodically - nausea, vomiting of eaten food, a constant sour taste in the mouth, general weakness, noise in the mouth. ears, weight loss of 10 kg in six months.
He abused light alcohol every day for ten years. I have not consumed alcohol for the last six months. I smoked a pack of cigarettes a day.
From the anamnesis it is known that in February 2010, against the background of an error in diet, intense pain in the upper abdomen, nausea, and vomiting of eaten food first appeared. According to emergency medical services, he was hospitalized in a hospital, where acute phlegmonous calculous cholecystitis and pancreatic necrosis were diagnosed. Laparotomy was performed: cholecystectomy, drainage of the abdominal cavity. Complex therapy was carried out aimed at suppressing pathological processes and preventing complications.
Subsequently, good health was noted. In October 2014, there was a closed abdominal injury with a splenic rupture. Splenectomy and drainage of the subphrenic abscess on the left were performed. At the same time, according to multislice computed tomography (MSCT) with contrast, a postnecrotic cyst of the body - tail of the pancreas measuring 70 × 35 × 47 mm was identified. Subsequently, the patient noted periodic intense pain in the upper abdomen, and therefore repeatedly underwent inpatient treatment with a diagnosis of exacerbation of chronic pancreatitis. MSCT with contrast from 2016 showed signs of pseudotumor pancreatitis. Dilation of the Wirsung duct to 6 mm, reduction in the size of the cyst to 60 × 10 mm. Since September 2022, I have been bothered by intense pain in the upper abdomen, nausea, and vomiting. Twice he was hospitalized in the hospital at his place of residence. Last hospitalization – October 2022
A clinical blood test revealed anemia - red blood cells - 3.54 × 106/μl, hemoglobin - 101 g/l, leukocytosis - 13.6 × 109/l, thrombocytosis - 636 × 103/μl, hyperamylasemia - 452 U/l, increased creatinine up to 127 µmol/l. According to ultrasound examination (US), CT without contrast, signs of acute pancreatitis. After conservative treatment, the patient was discharged with slight positive dynamics: the pain syndrome was relieved, nausea persisted. However, in December 2022, the above symptoms reappeared, and therefore the patient was sent for treatment to the Moscow Medical Research Center named after. A.S. Loginova.
Upon objective examination: the condition is satisfactory, the diet is low - body mass index (BMI) - 16.7 kg/m2. Physical examination revealed no significant abnormalities in the cardiovascular, respiratory or urinary systems. Pulse 68 per minute, satisfactory filling, correct rhythm, blood pressure 110/70 mm Hg. Art.
A clinical blood test showed signs of anemia (hemoglobin - 9.4 g/dl), thrombocytosis (716 × 103 μl).
Results of a biochemical blood test: hyperamylasemia (331 U/l), hypoalbuminemia (27.3 g/l), electrolyte disturbances (potassium - 3.47 mmol/l, chlorine - 95.2 mmol/l), iron - 2.7 µmol/l.
In addition, markers of inflammation were identified (C-reactive protein - 78.7 mg/l, erythrocyte sedimentation rate - 94 mm/h, leukocytes - 14.52 × 109/l, band neutrophils - 11%).
Urine diastasis – 2701.1 U/l.
Ultrasound results: gastrostasis (volume approximately 200–300 ml), the walls of the stomach are infiltrated, thickened to 10 mm. The contours of the pancreas are unclear, uneven, the boundaries are poorly differentiated from the surrounding tissues. Intra- and parapancreatic fluid accumulations ranging in size from 10 to 62 × 12 mm were identified. Peripancreatic tissue is infiltrated. The splenic vein is thrombosed, the blood flow is not localized. Thin layers of fluid in the abdominal cavity. Conclusion: Ultrasound signs of splenic vein thrombosis, recanalized portal vein thrombosis, pronounced changes in the pancreas with the formation of intra- and parapancreatic fluid accumulations, infiltration of parapancreatic tissue, slight effusion in the abdominal cavity, gastrostasis, with thickening of the stomach walls.
To visually assess the organs of the upper digestive tract, confirm gastrostasis, and assess patency, an endoscopy was performed, which revealed signs of erosive esophagitis, infiltrative lesions of the stomach, deformation of the gastric outlet, gastrostasis, chronic duodenitis with pronounced lymphoid infiltration, in the area of the bulboduodenal junction the mucous membrane is sharply swollen with the formation of a circular narrowing that does not interfere with the passage of an endoscope with a diameter of 0.9 cm - signs of duodenal dystrophy.
Conclusion based on morphological examination: chronic inactive gastritis with foveal hyperplasia of the surface epithelium.
Due to nausea and periodic vomiting of eaten food, the patient underwent installation of a nasointestinal feeding tube.
As a result of the studies, signs of previous pancreatic necrosis, cysts in the projection of the pancreas, gastrostasis with possible infiltrative damage to the stomach, as well as indirect signs of impaired evacuation from the stomach were revealed.
In this situation, there was a need for a differential diagnosis between inflammatory and tumor lesions of the gastropancreaticoduodenal zone.
When performing MSCT with intravenous bolus contrast, attention was drawn to the presence of a minimal amount of fluid subphrenic on the left and along the left lobe of the liver. The stomach is filled with liquid and contents and is not fully expanded. From the middle third of the body of the stomach with the transition to the antrum and pyloric sections, an uneven circular thickening of the stomach wall up to 42 mm is noted. Against this background, liquid inclusions measuring up to 18 × 62 mm and up to 6 mm, respectively, are visualized in the walls of the stomach and duodenal bulb. The contours of the stomach are uneven, the accumulation of contrast agent is weak, and the differentiation of the layers is impaired. The formation infiltrates the isthmus of the pancreas; at this level its parenchyma is not traced. Paragastric tissue is infiltrated throughout, the infiltration is adjacent to the left lobe of the liver, extends to the hepatoduodenal ligament and the mesentery of the small intestine. Along the left gastric artery and the common hepatic artery there is a chain of secondary changed lymph nodes up to 20 mm, in the hepatoduodenal ligament and porta hepatis up to 9 mm, mesenteric up to 9 mm. The portal vein, superior mesenteric vein and splenic vein are compressed at the level of confluence. In the greater omentum, venous collaterals up to 5 mm are visualized. Intra- and extrahepatic bile ducts are dilated: lobar up to 11 mm, sectoral up to 9 mm, segmental up to 7 mm, common bile duct up to 13 mm, in the intrapancreatic section it is compressed by infiltrate. The pancreas has a lobular structure and is correctly located. Dimensions: head – 30 mm, body – 16 mm, tail – 15 mm, density unchanged. The contours of the gland are clear, uneven; at the level of the infiltrate of the isthmus of the gland, the contours are not visible. A cystic structure measuring 14 × 7 mm is visualized in the head of the pancreas. It seems that there is a connection with the main pancreatic duct (MPD). The gastrointestinal tract is expanded to 7 mm with a block at the level of the pancreatic isthmus. Parapancreatic and paraduodenal tissue are edematous.
Conclusion: thickening of the walls of the middle third of the body of the stomach with a transition to the antral and pyloric sections (differentiate gastric lymphoma and cancer/neoplastic tumor, inflammatory changes are less likely, further examination is recommended), with pronounced infiltration of the surrounding tissue and involvement of the pancreatic isthmus, development of pancreatic and biliary hypertension, secondary intra-abdominal lymphadenopathy. Signs of previous destructive pancreatitis (cystic structure in the head of the pancreas to differentiate between a pseudocyst and intraductal mucinous neoplasia of the pancreas of the lateral type), duodenal degeneration (Fig. 1).
To assess the layers of the walls of the esophagus, stomach, duodenum and obtain cytological material, EUS was performed.
The results of the study showed that the wall of the cardiac part of the stomach is structural, five-layered, the differentiation of layers is not impaired. From the middle third of the body of the stomach along the lesser curvature, the posterior wall, in the antrum the circular wall of the stomach is thickened, up to a maximum of 2.5 cm. In the antrum there is no differentiation of the mucous and submucosal layers, the internal contour is smooth, the muscular layer can be traced. In the body, in the structure of the wall, multiple cystic neoplasms with echogenic contents, separated by hyperechoic septa, are identified (Fig. 2). To exclude atypia, a fine-needle puncture of the wall was performed and liquid contents were obtained. Conclusion: deformation of the gastric lumen due to circular thickening of the wall of the antrum of the stomach, cystic degeneration of the wall of the body of the stomach.
Study of the contents of a cystic formation of the stomach wall: in the preparation, all fields of view are covered by neutrophils, a small number of histiocytes and lymphoid cells, amorphous and cellular detritus. There is no other cellular composition. Conclusion: cytological picture of inflammatory changes. No elements of malignant neoplasm were found in the provided material.
As a result of the studies, a diagnosis was made:
- main: chronic pancreatitis of the first stage, moderate severity according to the M-ANNHEIM classification: C (13 points). Pancreatic necrosis in 2010;
- complications: developing postnecrotic cysts of the pancreas. Cystic degeneration of the stomach walls. Duodenal degeneration. Protein-energy deficiency of the 2nd degree. Circulating protein deficiency, grade 1. Moderate hypochromic anemia. Underweight;
- associated: cholelithiasis. Cholecystectomy in 2010. Gastroesophageal reflux disease in the stage of erosive esophagitis. Splenectomy for trauma in 2014
In the department, the patient received anti-inflammatory, enzyme replacement, antisecretory, infusion, antibacterial therapy, nutritional support (Nutricomp Peptide Liquid, albumin, Ringer's solution, Aminosteril H-Hepa, lipofundin) with a positive effect, the probe was removed on the seventh day from the start of treatment. The patient was able to feed independently without experiencing nausea or vomiting.
As a result of the treatment, the patient's weight stabilized and the pain decreased.
When monitoring laboratory parameters, an increase in hemoglobin level to 11.1 g/dL and a gradual decrease in platelet levels (687 × 103 μL) were noted. Biochemical blood analysis showed normalization of electrolyte parameters (potassium - 3.7 mmol/l, chlorine - 99.4 mmol/l, iron - 16.4 µmol/l), hyperamylasemia persisted (497 U/l).
Markers of inflammation decreased (C-reactive protein – 35.7 mg/l, erythrocyte sedimentation rate – 84 mm/h, leukocytes – 10 × 109/l, band neutrophils – 6%).
Urine diastasis decreased to 1888.6 U/l.
The patient was discharged on the ninth day from the start of conservative treatment.
The patient is recommended to continue treatment on an outpatient basis: taking multienzyme drugs in mini-microspheres or mini-tablets, probiotics (water-soluble forms), proton pump inhibitors, non-steroidal anti-inflammatory drugs, drugs containing potassium, iron supplements. High protein diet.
During a follow-up examination in 2022 (one year later), the patient complained of aching pain in the upper abdomen, heaviness that increased after eating. The patient gained weight, BMI returned to normal and amounted to 21.6 kg/m2.
Laboratory data: hyperamylasemia (201 U/l), urine diastasis – 768 U/ml. Other indicators are within normal limits.
According to ultrasound data, there was an absence of fluid accumulations in the projection of the pancreas and free fluid in the abdominal cavity.
During endoscopy, there were no signs of infiltrative lesions of the stomach; gastrostasis was not detected. However, indirect endoscopic signs of gastric and duodenal degeneration remained: starting from the lower third of the body of the stomach and distally, the circularly thickened folds did not completely straighten during insufflation with air. The lumen is narrowed, but is freely passable for an endoscope with a diameter of 9 mm. In the area of the bulboduodenal junction there are also circularly thickened folds that narrow the lumen.
MSCT data: absence of effusion in the abdominal cavity, pleural cavities and pericardial cavity, thickened stomach walls, reduction in the size of pancreatic cysts, reduction of lymphadenopathy, partial restoration of the structure of the pancreas. Cystic structures in the head of the pancreas are probably associated with post-necrotic changes. Signs of duodenal degeneration (Fig. 3).
Thus, positive dynamics were recorded in the form of a decrease in the severity of inflammation, which indicates the effectiveness of the treatment.
Over the next year, following a diet and conservative treatment, the patient’s well-being improved, nutrition improved, and quality of life increased.
The patient continues dynamic observation in the consultative and diagnostic department of the Moscow Scientific Research Center named after. A.S. Loginova and undergoes regular examinations in the department of pathology of the pancreas and biliary tract of the Moscow Scientific Research Center named after. A.S. Loginova.
Discussion
The described clinical situation shows the difficulties in making a diagnosis and determining treatment tactics.
The patient abused alcohol for a long time. He suffered from acute pancreatitis and pancreatic necrosis. Four years later, a postnecrotic cyst of the pancreas was detected, and subsequently regular exacerbations of chronic pancreatitis. Regular exacerbations led to disruption of duodenal patency and, as a consequence, disruption of food passage, nausea, vomiting, weight loss and electrolyte disorders. The clinical picture is similar to that in the presence of a malignant neoplasm, which could develop against the background of chronic pancreatitis [14, 15]. However, the results of the examination did not confirm the diagnosis of malignant neoplasm. The identified pathology was compensated by therapeutic treatment, which made it possible to avoid surgery. In this case, the patient requires regular examination and, if necessary, treatment adjustment.
The case considered is characterized by double localization of the ectopic pancreas: in the stomach and duodenum. Despite a fairly large number of descriptions of duodenal localization of ectopic pancreas [4, 11] and aberrant pancreas in the stomach [1], we were unable to find descriptions of dual localization in the literature.
Treatment options
Treatment based on examination data is prescribed by a gastroenterologist. Conservative therapy is usually indicated initially. With its help, they relieve inflammation, cope with swelling of the pancreas and ducts, and normalize phosphorus-calcium metabolism. A strict diet is prescribed, as well as enzyme replacement therapy. If the stones are small, they can pass into the intestines and then pass out of the body naturally.
In the early stages, conservative therapy is effective, but in the later stages, surgery to remove stones is required. It is performed endoscopically and has an easy postoperative period and quick recovery. If there are a lot of stones and they are large, then a classic abdominal operation is needed. It is more complex and difficult for the patient, but very effective.
If during the operation the surgeon sees that diffuse calcification is present in the tissues of the gland, then a decision is promptly made to remove the organ.
Indications for surgery:
- for several years, there are stones in the pancreas and/or its duct that increase in size;
- the patient showed signs of exhaustion;
- the inflammatory process progresses;
- attacks of excruciating sharp pain become more frequent.
The most popular operation today is ERCP, that is, endoscopic retrograde cholangiopancreatography. The surgeon uses an endoscope to remove small stones. If larger stones are found, the duct is slightly incised and the formations are pushed into the intestine.
The lowest morbidity is with ESWL - external shock wave lithotripsy. The stones are turned into powder and removed using an endoscope or allowed to pass naturally. The manipulation takes place under general anesthesia. The patient is placed stomach down on the emitter, which crushes the stones.
Prevention options
Today, science does not know the exact ways to prevent stone formation in the pancreatic duct. However, there are quite effective measures. The main one is diet. It is necessary to exclude fried foods, soups with strong meat broths, complex vegetable, meat and fruit salads, and fatty foods. All these products provoke increased production of bile and at the same time delay its outflow, that is, they form stagnation.
- Meat can only be eaten at lunch, in one sitting. In the evening - vegetables or fish. Twice a week it is useful to drink a bottle of mineral water - “Narzan”, “Borjomi” or others. Periodically, instead of usual tea, drink a choleretic and/or diuretic decoction, fennel infusion, marshmallow, rosehip decoction. All this improves the function of the excretory system.
- You need to eat 4-5 times a day in small portions. This reduces the viscosity of bile and thereby prevents its stagnation. Drink more water - up to 2 liters per day.
- Evacuation of bile is improved by vegetable oil, which also stimulates intestinal function. Butter can only be eaten as an additive to a side dish or porridge.
- It is necessary to completely exclude refractory fats - fatty fish and meat, lamb, lard. Chicken and turkey should be cooked without skin.
- In addition to diet, you need to give up bad habits and move more. Swimming in the pool is especially beneficial. If this is not possible, then at least do a little 15-minute gymnastics at home, stretching your back, neck, lower torso, and bending.