Pancreatic enzymes play a critical role in the digestion of food from the stomach in the small intestine. Biocarbonate, which is the main component of pancreatic juice, creates an alkaline environment, neutralizes the acidity of the food mass with gastric juice in the duodenum, creating the pH range necessary for pancreatic enzymes. Participation in digestion and regulation of metabolism are the main functions of the pancreas, which is both an exocrine (secreting) and endocrine (increting) organ.
Exocrine function of the pancreas
The secretory function of the pancreas is divided into two types:
- • Ecbolic function - consists in the synthesis of more than twenty enzymes and proenzymes by its cells and their release into the duodenum. Digestive enzymes make up more than 90% of the proteins in pancreatic juice and are involved in the breakdown of food in the intestines.
- • Hydrokinetic function - consists of producing water, bicarbonates and other electrolytes. This function affects the neutralization of stomach contents, creating an alkaline environment in the intestines, favorable for the activity of pancreatic and intestinal enzymes.
Pancreatic enzymes
There are several types of pancreatic enzymes:
- • Amylolytic enzymes (amylases) break down complex carbohydrates into dextrins, maltose, maltooligosaccharides and glucose.
- • Lipolytic enzymes (lipases) break down fats into fatty acids and monoglycerides that pass through the enterocyte membrane.
- • Proteolytic enzymes (proteases) break down proteins by breaking internal bonds in the middle of amino acid chains and synthesizing peptides.
- • Nucleolytic enzymes (nucleases) break down nucleic acids. Phosphodiesterases present in pancreatic juice are divided into two groups: ribonuclease breaks down ribonucleic acid, and deoxyribonuclease hydrolyzes deoxyribonucleic acid.
Pancreatic secretion
The quantity and quality composition of food consumed is directly proportional to the amount of pancreatic juice secreted and its enzymatic activity. Increased secretion of pancreatic juice causes a large volume of food, which stimulates an increase in acidity in the stomach. Ingestion of liquid food causes faster secretion from the pancreas than solid, fatty food slowly evacuated from the stomach. The production of pancreatic juice increases 2-3 minutes after food enters the stomach, its duration ranges from 6 to 14 hours. The greatest secretory response is caused by mixed food. Strong stimulants that affect the secretory enzymes of the pancreas are neutral fats, products of their digestion and proteins. Amino acids entering the intestinal tract (especially phenylalanine, choline, methionine) affect a sharp increase in the blood level of cholecystokinin, a hormone responsible for local stimulation of the activity of acinar cells that have the function of enzyme synthesis. The predominance of carbohydrates in the diet facilitates the activity of the pancreas, so this group of products is especially recommended in diets for patients suffering from exacerbation of chronic pancreatitis. Foods and drinks that stimulate the appetite (fruits, dairy products, products containing spices and seasonings, marinades, juices and alcohol) increase the production of gastric juice and hydrochloric acid, which, in turn, stimulates the onset of exocrine pancreas.
Magazine "Child's Health" 8 (43) 2012
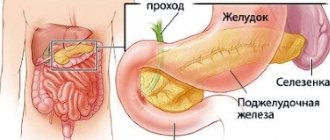
The pancreas, as is known, is the largest and most important gland of the digestive system and at the same time the most important endocrine gland, taking part in the regulation of carbohydrate metabolism. It secretes hormones into the blood (endocrine function) - insulin, glucagon, gastrin, somatostatin, pancreatic polypeptide, amylin. Moreover, insulin, produced only in this organ, is essential for life due to its role in ensuring normal metabolism. On the other hand, the pancreas produces a secretion (exocrine function) that is important in the functioning of the digestive tract. The enzymes contained in the secretion are involved in the hydrolysis of all nutrients. Diseases of the pancreas can lead to both digestive disorders (changes in gastrointestinal secretion, absorption, motility) and metabolic changes in the body.
The human pancreas produces every day from 50 to 1500 ml of juice (depending on age), containing enzymes that play a key role in the digestion of nutrients. The proportion of digestive enzymes from the salivary glands (amylase), stomach (pepsin), and intestinal epithelium (proteases) is relatively small. Pancreatic enzymes break down proteins, fats, and carbohydrates into small molecules, which are subsequently either broken down by intestinal membrane enzymes into individual molecules or can penetrate the intestinal mucosa (dipeptides, monoglycerides). Bicarbonates, which pancreatic juice is rich in, provide the reaction of the environment necessary for the activation of enzymes and the optimum of their action [1, 5].
The predominant part of the secretion (water electrolyte) is formed in centroacinar cells, the epithelium of intercalary ducts and ducts, the smaller part (enzymatic) is formed in acinar cells. Under all conditions, pancreatic secretion entering the duodenum is isotonic with blood plasma. The composition of electrolytes in it is not constant, the content of anions especially varies.
In 24 hours, the human pancreas synthesizes about 15–20 g of enzyme protein, i.e. its synthetic ability is superior to many organs. The release of enzymes from acinar cells occurs spontaneously (basal cholinergic tone), but mainly as a result of stimulation. Exocytosis from cells is independent of enzyme synthesis [1, 4]. In the whole organism, direct regulation of the production and release of protein by acinar cells is carried out by secretogens: acetylcholine, pancreozymin and secretin. Gastrin is a weak stimulant. Acinar cells synthesize and secrete a complex of powerful digestive enzymes: aamylase for the breakdown of starch, lipolytic enzymes for the digestion of fats, proteolytic enzymes for the breakdown of proteins, nucleases for the hydrolysis of nucleotides. Non-enzymatic proteins are found in small quantities in pancreatic juice [3].
All proteolytic enzymes enter the juice in an inactive form, which is the gland’s self-defense from digestion. Activation of proenzymes occurs in the duodenum. In its lumen, under the action of enterokinase produced by the mucous membrane, in the presence of calcium ions, trypsinogen is converted into trypsin and the cascade phenomenon of trypsin activation of other trypsinogen molecules and other proteolytic enzymes is triggered.
Among the proteolytic enzymes, there are endopeptidases that cleave peptide bonds inside protein molecules and polypeptides - trypsin, chymotrypsin, elastase - and exopeptidases that cleave amino acids located at the terminal end of polypeptides and proteins - carboxypeptidase [3, 5].
In humans, 19% of the proteins in pancreatic juice are trypsinogen I and II, which differ in molecular weight. The catalytic properties of both enzymes are similar, their optimum action is at pH 8. Trypsin breaks down almost all denatured proteins without affecting living tissues.
Chymotrypsin in humans exists in two molecular forms: A and B. The active form of the enzyme is excreted in the feces. Determination of chymotrypsin activity in feces has diagnostic value for assessing pancreatic function.
Using electrophoresis, the existence of two forms of proelastase has been proven. Elastase is involved in the digestion of elastin and denatured proteins. It is stable over a wide range - pH 4–10.5.
Carboxypeptidases A and B have larger molecular weights than other proteolytic enzymes. Carboxypeptidase causes the degradation of proteins and polypeptides.
Pancreatic juice contains a trypsin inhibitor. It accounts for 0.3–0.6% of all proteins. Trypsin inhibitor exists in several chromatographic forms. Of all the characterized pancreatic enzymes, it has the smallest molecular weight. Its inhibitory effect occurs at pH 5.5–11.
In pancreatic juice, 10% of the total protein is aamylase. This is a glycoprotein that cleaves the α1,4 glycosidic bond inside the starch molecule to form dextrin, maltotetraose, maltotriose and maltose. In humans, there are 6 isoenzymes of pancreatic amylase, distinguishable by electrophoresis. The optimum pH is in the range of 6–7. Determination of amylase activity in urine was the first enzymological study in medicine, carried out by J. Wohlgemuth in 1908. Determination of amylase activity in urine, blood serum and exudates is currently used as a test marker (albeit nonspecific) for inflammation of the gland [2].
Lipolytic enzymes secreted by the pancreas are lipase, colipase, phospholipase, and carboxylester hydrolase. Lipase accounts for 1–3% of all proteins in pancreatic juice. Lipase is a glycoprotein represented by two isoenzymes. Pancreatic lipase acts on the surface of thin fat droplets. Under its influence, triglycerides are hydrolyzed to 1,2 diglycerides and then 2 monoglycerides, releasing fatty acids. Bile acids promote the emulsification of fats into small droplets, but, in turn, they inhibit the activity of the enzyme. It is restored by colipase, a small protein (molecular weight less than 11,000), which acts only together with lipase. For the full effect of lipase, a complex of 1 lipase molecule, 1 colipase molecule and 1 micelle is formed on the surface of thin emulsified fat droplets [1, 2, 5].
Phospholipase A is found in pancreatic juice in an inactive form. The proenzyme is activated by trypsin in the duodenum. Its premature activation in the pancreas plays a special role in the pathogenesis of acute pancreatitis.
Carboxylester hydrolase is a nonspecifically acting esterase, the presence of which has been proven in human pancreatic juice. It breaks down water-soluble carboxylesters, such as cholesterol. It has the highest molecular weight of all characterized enzymes (100,000–300,000). Bile acids activate the enzyme.
The secretory cycle of acinar cells includes six stages: synthesis, segregation, intracellular transport, concentration of enzyme material, deposition and exocytosis. The main factor causing the release of the proenzyme from the cell is cholecystokinin. Together with enzyme precursors, a solution of electrolytes is secreted into the pancreatic lobules. A primary secretion is formed. Its electrolyte composition changes as it passes through the ducts (especially in the intralobular ducts) due primarily to the exchange of Cl– for HCO3– in them, resulting in the formation of secondary pancreatic juice that enters the duodenum [3].
The existence of the phenomenon of adaptation of pancreatic enzymes to the nature of the food consumed has been proven. We are talking about the phenomenon of adaptation not to a single food used, but to the characteristics of nutrition in general. Changes in enzyme formation in accordance with the nature of the food consumed occur within several days. During adaptation, an increase in synthesis is observed, rather than activation of existing enzyme molecules [4].
It is assumed that there is a feedback mechanism between the duodenum and the pancreas. It is believed that trypsin in the duodenum inhibits the release of pancreozymin, and its absence stimulates [6].
In children, the proteolytic activity of the digestive juice of the pancreas is at a fairly high level already from the first months of life, reaching a maximum by 4–6 years. Lipolytic activity increases during the first year of a child's life. The activity of pancreatic amylase by the end of the first year of life increases 4 times, reaching maximum values by 9 years. With natural feeding, the concentration of pancreatic enzymes in duodenal juice is low, with mixed feeding it increases by 1.5–2 times, and with artificial feeding it increases by 4–5 times [4].
In accordance with the two main functions of pancreatic juice - neutralization of gastric contents entering the duodenum and digestion of nutrients - the secretion of one component (fluid and bicarbonates) is determined mainly by the amount of acid it contains, and the other (enzyme) is determined by the presence of fat digestion products and squirrel. The pancreatic response to food represents an integrated response to nervous and humoral stimulation. There are cerebral, gastric and intestinal phases of pancreatic secretion.
Also I.P. Pavlov in 1896, in experiments with imaginary feeding of dogs, discovered that the sight and mental preparation for food stimulate pancreatic secretion. In humans, the existence of a brain phase of secretion has also been proven. These are conditioned and unconditioned reflexes that are transmitted through the vagus nerve. In humans, ingestion of food that is chewed but not swallowed causes strong enzyme secretion and pronounced bicarbonate secretion [5].
During the cerebral secretion phase, in addition to the direct effect on the gland, gastrin is released through vagal influence. This peptide is a pancreozymin agonist in influencing enzymatic pancreatic secretion. On the other hand, the secretion of acid induced already in this phase with subsequent entry into the duodenum and release of secretin (transition to the intestinal phase) stimulates the release of bicarbonates by ductal cells.
With the entry of acid and food gruel into the duodenum, the intestinal phase of pancreatic secretion begins. Both the quantity and duration of gastric contents entering the small intestine significantly determine further stimulation of the pancreas. Fats are removed from the stomach very slowly, carbohydrates quickly, proteins occupy an intermediate position. Stimulation of pancreatic secretion begins with food intake and is maintained for several hours (up to 8 hours or more) after gastric emptying. The intestinal phase accounts for approximately 80% of the gland's response to food intake. With the interaction of nervous and humoral regulation, further stimulation of pancreatic secretion occurs. During this phase, the role of humoral control is especially important, the study of which began with the discovery of secretin by WM Bayliss and EH Starling in 1902. The stimulator of the secretion of water and bicarbonates is secretin, released by endocrine cells of the duodenal and jejunal mucosa when exposed to hydrochloric acid. The secretion of the enzyme component is caused by pancreozymin, the release of which is stimulated by the products of fat and protein digestion [1, 2, 5].
Strong stimulation of enzyme secretion is caused by peptides (formed already in the stomach), amino acids and fatty acids located in the small intestine. The effect is achieved through both the release of pancreozymin, the main humoral stimulator of enzyme secretion, and the duodenopancreatic reflex, which is realized through the vagus nerve and a short reflex arc. The amount of pancreozymin released depends on the amount of incoming peptides, amino acids, fatty acids and on the size of the surface of the intestinal mucosa that comes into contact with them. Interestingly, calcium and magnesium in the duodenum increase enzyme secretion in a concentration-dependent manner, probably through the release of pancreozymin.
Long-chain unsaturated fatty acids, in addition to the secretion of enzymes, also stimulate the secretion of bicarbonates. Bile acids, essential as fat micelle formers, also markedly enhance the secretion of water and bicarbonate, probably through the release of secretin. Carbohydrates do not have a stimulating effect on secretion.
As nutrients are absorbed in the small intestine, pancreatic secretion decreases; if there are large amounts of them in the duodenum and jejunum, secretion continues.
Along with the stimulating effect on pancreatic secretion, there is also an inhibitory effect. It is transmitted by sympathetic stimulation and vasoconstriction, as well as by the release of hormonal factors - inhibitors - from the lower part of the small intestine and colon.
Laboratory assessment of the exocrine function of the pancreas, as well as the diagnosis of pancreatic pathology in general, is traditionally considered a difficult task. In recent decades, however, the problem of lack of information has grown into another - the problem of choosing a diagnostic approach, choosing the most adequate research methods. Diagnosis of pancreatic diseases is based on medical history and characteristic clinical symptoms, assessment of the exocrine and endocrine activity of the organ and the results of instrumental studies that can determine the presence of structural changes. All methods for assessing exocrine pancreatic function are divided into direct (usually probe) and indirect (probeless). Direct methods are associated with the direct determination of enzyme activity in the duodenal contents, and indirect methods are associated with the assessment of the processes of digestion of standard substrates.
For diagnosis, in particular, of the key disease of the pancreas in children - exacerbation of chronic pancreatitis - it is important to determine the activity of pancreatic enzymes - amylase, lipase and trypsin in the blood, as well as amylase and lipase in the urine. A single determination of enzyme activity may not be enough, since amylase and lipase levels in the blood on an empty stomach usually increase 1.5–2 times for a short period of time (2–12 hours after an exacerbation and reach a maximum by the end of the first day, followed by a rapid decrease and normalization within 2–4 days). Amylase activity in urine increases approximately 6 hours later than serum activity. At the same time, an increase in serum amylase activity by two times or more in combination with an increase in the level of lipase and trypsin (or one of them) is a fairly reliable test for exacerbation of the disease. However, normal levels of enzyme concentrations in the blood and urine do not provide grounds to exclude the diagnosis of chronic pancreatitis. In such cases, a provocative test is used, in which enzyme activity is determined before and after stimulation. The most widely used is the determination of amylase in the blood after stimulation with pancreozymin or glucose, as well as the level of amylase in the urine during stimulation with proserin. After the introduction of irritants, the phenomenon of “evasion” of enzymes (hyperenzymemia) is observed, which indicates damage to the pancreatic tissue or an obstruction to the outflow of pancreatic juice. Sometimes provocative tests can be negative (enzyme levels do not change or even decrease), which is associated with a decrease in the number of acinar cells producing these enzymes in severe pancreatitis [2, 6].
It is known that the gold standard for determining the exocrine function of the pancreas is the secretin cholecystokinin test. In this case, pancreatic juice is obtained by gastroduodenal intubation using a two-channel probe after pumping out gastric juice and duodenal contents and introducing stimulants of pancreatic secretion. To stimulate secretion, secretin 1.5 U/kg is administered intravenously and, after 30 minutes, cholecystokinin 0.5 U/kg is administered. The secretincholecystokinin test is highly accurate, but its widespread use is impossible due to a number of disadvantages: the high cost of secretin and cholecystokinin, the need to probe the patient, the duration of the procedure, the need for intravenous administration of drugs and, as a consequence, the possibility of adverse reactions [5].
Indirect methods for assessing the exocrine function of the pancreas include scatological examination. A sign of exocrine pancreatic insufficiency is polyfecalia, when feces are greasy, grayish in color, mushy, viscous, with a strong putrid odor, and are poorly washed off the walls of the toilet. Microscopic examination may reveal undigested muscle fibers (creatorrea) - a sign of severe pancreatitis; the presence of drops of neutral fat (steatorrhea) is one of the early symptoms of pancreatic insufficiency [1].
In recent years, an alternative to the rather invasive secretincholecystokinin test has been the determination of elastase1 in feces using an enzyme-linked immunosorbent assay. This enzyme is secreted by the pancreas and is not metabolized in the intestine; its activity in feces objectively reflects the state of the exocrine function of the organ, and organ specificity eliminates the possibility of error associated with the activity of intestinal enzymes. This method has the highest specificity and sensitivity - more than 90%. Unlike other tests, elastase1 determination can be performed without stopping replacement therapy drugs. Normally, elastase1 activity in feces is more than 200 mcg/g of feces. A decrease in elastase concentration indicates exocrine pancreatic insufficiency, which can be a symptom of cystic fibrosis, acute and chronic pancreatitis, pancreatic trauma and some other conditions [1, 5, 7–11].
Thus, currently in pediatrics there are various methods for assessing the exocrine function of the pancreas, both direct and indirect. The doctor’s task is to choose the most adequate research method, taking into account the main clinical symptoms of the disease.
Disorders of the pancreas
Disorders of the digestive process in the small intestine are directly related to changes in enzyme synthesis caused by pancreatic dysfunction. In the presence of pathological processes in it, the action of lipase, which hydrolyzes fats, is inhibited, which leads to their insufficient digestion and excretion with feces up to 80% of the mass entering the body. The breakdown of proteins is also impaired, as may be evidenced by creatorrhea, a characteristic feature of which is the presence of an increased volume of muscle fibers in the stool. Digestive insufficiency can lead to such negative consequences as dyspeptic syndrome, dehydration, acid-base imbalance and intestinal autointoxication. External secretion of the pancreas is caused by the presence in the body of a number of inflammatory diseases and some other pathological processes:
- pancreatitis in acute and chronic stages;
- cholelithiasis;
- the presence of parasites in the intestines;
- duodenitis, duodenal ulcer, which results in a decrease in the production of pancreatic juice;
- tumors;
- vaterites;
- excess body weight, hormonal imbalance, resulting from metabolic disorders, causing dystrophic lesions of the pancreas;
- allergic restructuring of the body;
- Vagal dystrophy, long-term atropinization;
- tumors that destroy the pancreas.
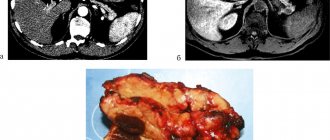
The development of obstructive processes in the pancreas that impede or completely block the outflow of pancreatic juice into the duodenum, and the resulting hypertension that results from this pathology can cause acute pain in this area, as well as internal ruptures and destruction of the parenchyma of the organ. As a result of the destruction of the pancreas, its enzymes and destruction products are absorbed into the blood and surrounding organs, which leads to the development of necrosis and the formation of a body intoxication syndrome.
Endocrine functions
The intrasecretory functions of the pancreas cannot occur without special substances - hormones, which this organ also produces. This function is called endocrine (internal secretory function) and its activation also largely depends on the food that a person eats throughout the day. However, it should be noted that the hormones that the gland synthesizes do not enter the digestive organs. They are released into the blood, where the body's humoral regulation of them is noted.
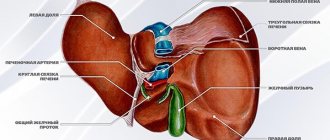
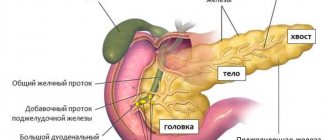
Cells that synthesize hormones are located inside the islets of Langerhans
The endocrine function of the pancreas is performed through special cells, the number of which does not exceed 2% of the entire body of the organ. These cells form clusters, which in medicine are called islets of Langerhans.
There are only 5 types of cells responsible for the production of hormones:
The structure of the pancreas
- alpha cells - secrete glucagon;
- beta cells - produce insulin;
- delta cells - produce somatostatin;
- D1 cells – provide the human body with vasoactive intestinal polypeptides;
- PP cells - synthesize pancreatic polypeptide.
Without these hormones, the functioning of the pancreas and metabolic processes in the body cannot occur normally. After all, they regulate metabolism and also support the functioning of the kidneys, intestines, liver and duodenum.
The most famous among people far from medicine is the hormone insulin. Its release into the blood ensures normalization of blood glucose levels. It binds to glucose molecules, breaks them into smaller structures and delivers them to the cells and tissues of the body, thereby saturating them with energy. If the functioning of beta cells is disrupted, insulin deficiency occurs, which leads to an increase in the concentration of sugar microcrystals in the blood and provokes the development of diabetes mellitus and sudden weight loss. After all, instead of wasting the energy that insulin provided the cells, they begin to use fat as fuel, which leads to degeneration of adipose tissue.
The endocrine function of the pancreas plays a very important role in the human body. Despite the fact that a small number of cells are involved in its implementation, without it not a single process in the body can occur. Since the intrasecretory function manifests itself in humoral control, which is an early evolutionary way of controlling the body. The pancreas synthesizes hormones, releases them into the blood and ensures hormonal balance. As a result, the work of all internal organs and systems is regulated.