Statistics
This is one of the most aggressive malignant diseases. Esophageal cancer is the 8th leading cause of death worldwide. According to the International Agency for Research on Cancer, in 2018 the incidence was 7.49 cases per 100,000 people per year, and the mortality rate was 6.62. Calculations by Rosstat of the Russian Ministry of Health say that the incidence is 5.6 cases per 100,000 people. Among men – 9.43 per 100,000, among women – 2.29 per 100,000. The disease is most often diagnosed in the so-called “Asian belt”, that is, from the northern part of Iran, through Central Asia and to the central regions of Japan and China, also capturing Siberia. This is largely due to the peculiarities of the diet of people living in these areas.
Most often (up to 80% of cases), the neoplasm is located in the lower and middle thoracic sections of the esophagus. With a frequency of 10-15% of cases, cancer of the cervical esophagus is diagnosed.
Prevalence and etiology
Cancer is the most common disease of the esophagus.
It accounts for 70-90% of all esophageal diseases. In the structure of the incidence of human malignant neoplasms, esophageal cancer ranks 9-10th. Esophageal cancer is more common in men; women suffer from it 2-3 times less often. This is mainly a disease of older people; up to 80% of all patients with esophageal cancer are in the age group of people over 60 years old. Under the age of 30, esophageal cancer has been described only in isolated cases. There is significant unevenness in the geographical distribution of the disease. The incidence of esophageal cancer is especially high in Central Asian countries. Here it is 3-5 times higher than the average level and 10-12 times higher than the incidence observed in the southwest and west of the CIS. For example, in Moldova, Ukraine, Belarus, the incidence of esophageal cancer ranges from 1.7-2.6 per 100,000 population, in Kazakhstan and Turkmenistan it reaches 23.7-28.3 per 100,000 population. Fast passage:
- Anatomy of the esophagus
- Pathological anatomy and metastasis
- Classification
- Clinic (symptoms) of esophageal cancer
- Diagnosis of esophageal cancer
- Treatment of esophageal cancer
- Prognosis for esophageal cancer
Outside the CIS, esophageal cancer is most common in Iran, Switzerland, Panama, Brazil, and Japan. Esophageal cancer is rare in Cuba, Mexico, the southern states of the USA, and Nigeria. Thus, in the northern regions of Iran, the incidence is 114 per 100,000 population, and the highest incidence of esophageal cancer is registered in northern China and Korea - 140 per 100,000 population. Factors contributing to the development of esophageal cancer include systematic intake of hot, scalding, rough, poorly chewed food, consumption of strong alcoholic beverages and smoking. These factors cause chronic inflammatory processes, which, if prolonged, lead to the development of malignant neoplasms. Here we should also note the processes that entail the development of scars, chronic inflammatory changes - post-burn strictures, esophagitis due to hiatal hernia, “short” esophagus, etc. Along with this, esophageal cancer is also of an occupational nature - machine operators: tractor drivers are more likely to get sick , drivers, combine operators.
Diverticula, which support chronic inflammatory processes, also contribute to the development of esophageal cancer. Leukoplakia plays a major role in the development of esophageal cancer.
Leukoplakia of the mucous membrane develops into esophageal cancer in 48% of cases. Therefore, most researchers consider leukoplakia to be an obligate precancer. A connection has been noted between esophageal cancer and sideropenic syndrome, which occurs as a result of a decrease in plasma iron content (sideropenia). Sideropenic syndrome (sideropenic dysphagia, Plummer-Vinson syndrome) is characterized by dysphagia, achylia, chronic glossitis and cheilitis, early hair loss and tooth loss, severe hypochromic anemia. Polyps and benign tumors play a certain role in the development of esophageal cancer. However, these diseases themselves are relatively rare, and when cancer has developed, it is not always possible to establish traces of a pre-existing pathological process.
Risk factors
The main risk factors for the occurrence and development of such a disease:
- male gender, because men are more often susceptible to bad habits - smoking and drinking alcohol in large quantities;
- age - the older it is, the higher the risk; only 15% of patients were under 55 years old;
- excess body weight;
- smoking and alcohol abuse;
- drinking very hot drinks and food;
- Barrett's esophagus (when cellular degeneration occurs in the lower part of the esophagus caused by chronic acid damage);
- reflux;
- achalasia (when the obturator function of the opening between the stomach and esophagus is impaired);
- scars in the esophagus, leading to its narrowing;
- Plummer-Vinson syndrome (this syndrome is characterized by a triad, that is, three types of disorders simultaneously: impaired swallowing function, narrowed esophagus, iron deficiency anemia);
- contact with chemicals.
Approximately 1/3 of patients are diagnosed with HPV (human papillomavirus).
The risk of getting this type of cancer can be reduced by eating a varied diet, not drinking strong alcohol, and if you have Barrett's syndrome, monitoring changes in the mucous membrane.
There is no screening for this disease. However, if there is an increased risk of esophageal cancer, it is recommended to undergo an endoscopic examination, if necessary, with a biopsy of the suspicious area.
Causes
The main cause of esophageal metaplasia is gastroesophageal reflux - the reflux of gastric contents back into the esophagus. At the same time, aggressive gastric juice irritates the mucous membrane and provokes the replacement of stratified squamous epithelium with columnar epithelium that is more resistant to such effects, i.e. metaplasia occurs - the replacement of one type of tissue with another. Further irritating action leads to the fact that the metaplastic epithelium forms a clone of cells with a violation of the system of programmed death (apoptosis). This condition is called dysplasia and subsequently leads to cancer.
Additional risk factors are:
- Esophageal-diaphragmatic hernia. They lead to constant disposition of the stomach and, as a consequence, to constant reflux.
- Obesity. Firstly, obesity leads to increased intra-abdominal pressure, which provokes reflux. Secondly, in this condition there is an increase in the volume of peri-esophageal fiber, which releases pro-inflammatory cytokines that negatively affect the esophageal mucosa.
- Smoking.
- Metabolic syndrome.
Symptoms
Usually, esophageal cancer is detected in late stages, when therapy is already complicated, or by accident.
The most common symptoms include the following:
- Dysphagia . This symptom represents impaired swallowing function. Patients describe their condition as a feeling of “a lump in the throat.” Patients begin to reduce food portions and avoid solid foods. In later stages, only liquid food can be consumed.
- Increased salivation . More saliva begins to be produced in the mouth to help move the bolus through the narrowed lumen of the esophagus.
- Discomfort and pain in the sternum . These symptoms do not always relate to esophageal cancer; they can be caused by intercostal neuralgia, angina pectoris, and gastroesophageal reflux. Therefore they are not specific.
- Losing body weight . With difficulty swallowing and general weakness, the sick person begins to refuse food, so weight loss often accompanies esophageal cancer.
There are also more rare symptoms:
- cough;
- hiccups;
- hoarse voice;
- vomit;
- bone pain (in the presence of metastases);
- esophageal bleeding (after the blood passes through the gastrointestinal tract, the stool turns black);
- as a result of bleeding - anemia (the person becomes pale, weak, gets tired quickly, and experiences constant drowsiness).
Important! Having these symptoms does not mean cancer. However, you should definitely see a doctor and get examined.
Classification of esophageal cancer
By area of origin:
- intrathoracic esophagus;
- cervical region (from the lower border of the cricoid cartilage to the entrance to the thoracic cavity);
- upper thoracic zone (from the entrance to the thoracic cavity to the tracheal bifurcation area);
- middle thoracic region (the proximal part of the esophagus extends from the tracheal bifurcation zone to the junction of the esophagus with the stomach);
- lower thoracic region (distal part of the esophagus approximately 10 cm in length, including the abdominal part of the esophagus, extending from the tracheal bifurcation to the junction of the esophagus and stomach).
According to the nature of tumor growth:
- into the lumen of the esophagus (exophytic);
- ulcerative (endophytic);
- circular shape (infiltrative sclerosing).
According to the degree of differentiation of the neoplasm:
- degree undetermined – Gx;
- highly differentiated education – G1;
- moderately differentiated – G2;
- poorly differentiated – G3;
- non-differentiable – G4.
Diagnostics
Diagnosis is carried out by instrumental and laboratory methods.
- X-ray with barium contrast . The patient takes barium sulfate orally, which coats the walls of the esophagus. This allows you to see the relief of the walls in the image and detect a narrowing of the lumen. At an early stage, cancer may appear as small, round bumps called plaques. At a late stage of development, the neoplasm takes the form of a large, irregularly shaped tumor, which can cause severe narrowing of the esophagus. X-rays also make it possible to diagnose a tracheoesophageal fistula, that is, when, due to the destruction of the entire thickness of the esophageal wall by a neoplasm, the esophagus begins to communicate with the trachea.
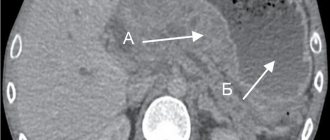
- CT (computed tomography) . This study makes it possible to visualize the extent to which the process has spread to nearby organs, determine the presence of distant metastases and damage to the lymph nodes.
- MRI (magnetic resonance imaging) . With its help, lesions of the spinal cord and brain are identified. The advantage of the study is the absence of radiation exposure to the patient. Recently, MRI of the esophagus has been performed; this technique is new, but has already proven itself well.
- PET (positron emission tomography) and PET-CT (PET combined with computed tomography) . They “highlight” all foci of cancer in the body and determine the level of activity in them. They are used to detect distant metastases and lymph node involvement. PET-CT makes it possible to correlate those areas where tissues containing rapidly dividing cells have accumulated radioactive substances with CT images.
- Examination by endoscopic method . It makes it possible to establish the reason why the swallowing function is impaired, to see the size and extent of the tumor, and to take a biopsy of the suspicious area.
- Endo-ultrasound , that is, the use of an endoscope with an ultrasound sensor. With such an examination, it is possible to accurately determine to what depth the tumor has grown into the wall of the esophagus, whether neighboring tissues are involved, including lymph nodes, and also to perform a biopsy.
- Bronchoscopy . Allows you to assess the condition of the trachea if there is a possibility that the neoplasm has spread to its wall.
- Thoracoscopy . During this examination, the condition of the chest organs and lymph nodes is assessed.
X-ray diagnosis of esophageal cancer
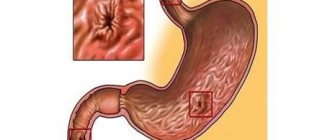
Endoscopic picture of adenocarcinoma
Endoscopic picture of squamous cell carcinoma
Laboratory research
- Clinical blood test. Allows you to identify anemia that occurs due to bleeding or a poor diet.
- Blood chemistry. It shows the condition of internal organs, namely kidneys, liver, etc.
- Analysis for tumor markers CA 19-9, CEA.
- Study of biomaterial taken during a biopsy. It detects HER2 protein receptors. If they are present, targeted therapy can be used against the tumor.
Treatment
The main method of treatment is surgery, but an integrated approach can improve results. Therefore, various techniques are combined.
Surgery
During the operation, the esophagus is removed entirely or part of it, it all depends on the extent and localization of the pathological process.
When the tumor is in the cervical region, most of the esophagus is removed. After this, the stomach is lifted and sutured to the remaining part of the esophagus. In addition, instead of the removed part through plastic surgery, part of the large or small intestine can be used. If it is possible to perform resection of the cervical esophagus, intestinal plastic surgery with microvascular anastomosis of vessels in the neck can be performed.
When the tumor is localized in the cervical esophagus with a large spread, it is necessary to perform an operation in the amount of: removal of pharyngolaryngoectomy with simultaneous plasty of the esophagus with a gastric graft, suturing it to the root of the tongue.
Surgical intervention to remove part of the esophagus with subsequent replacement with a graft can be performed openly or by thoracoscopy and laparoscopy.
With any type of intervention, regional lymph nodes are removed, which are then examined in the laboratory using histology. If cancer cells are found in them, then after surgery the patient is prescribed radiation treatment or chemotherapy in combination with RT.
There are also palliative surgeries. They are carried out so that the patient can eat if, due to the tumor, he cannot swallow. This type of intervention is called a gastrostomy, that is, the insertion of a special feeding tube through the anterior abdominal wall into the stomach.
Radiation therapy
Ionizing radiation is used to destroy tumor cells. This therapy can be carried out:
- Those patients who cannot undergo surgery for health reasons. In this case, radiation, usually along with chemotherapy, is the main treatment modality.
- When the tumor is localized in the cervical esophagus, chemoradiotherapy is the first stage of the combined treatment method.
- Before surgery along with chemotherapy. This is to shrink the tumor so it can be removed better (called neoadjuvant therapy).
- After surgery along with chemotherapy. In this way, residual tumor that could not be seen during surgery is treated (called “adjuvant therapy”).
- To relieve symptoms of advanced esophageal cancer. Allows you to reduce the intensity of pain, eliminate bleeding and difficulties with swallowing. In this case, it is palliative therapy.
Types of radiation treatment:
- External (remote). The source of ionizing radiation is located at a distance from the patient.
- Contact (called “brachytherapy”). The radiation source is placed endoscopically as close as possible to the tumor. Ionizing rays travel a short distance, so they reach the tumor, but have little effect on nearby tissue. Treatment can reduce the tumor and restore patency.
Dose distribution obtained with external beam conformal radiotherapy and intraluminal brachytherapy
Chemotherapy
This technique involves the introduction into the body of drugs that inhibit the vital activity of tumor cells or destroy them. Medicines are taken orally or injected into a vein, after which they enter the bloodstream and reach almost every area of the body.
Chemotherapy is given in cycles. This is due to the fact that the effect of the drug is aimed at those cells that are constantly dividing. The administration is repeated after a certain number of days, which is associated with the cell cycle. Chemotherapy cycles typically last 2-4 weeks, and patients are usually given multiple cycles.
Like radiation, chemotherapy is indicated in the adjuvant and neoadvant regimens. It is also used to relieve symptoms for those patients whose cancer is widespread and cannot be treated surgically.
Some drugs:
- "Cisplatin" and "5-fluorouracil" ("5-FU");
- "Paclitaxel" and "Carboplatin";
- "Cisplatin" together with "Capecitabine";
- ECF regimen: Epirubicin, Cisplatin and 5-FU;
- DCF regimen: Docetaxel, Cisplatin and 5-FU;
- "Oxaliplatin" together with either "Capecitabine" or "5-FU";
- "Irinotecan".
Targeted therapy
It is aimed at blocking the growth of a tumor by influencing certain targets, that is, those molecules that determine the division and growth of the tumor. If such protein molecules are found in biomaterial taken by biopsy, then targeted therapy may be effective.
Palliative methods
When carrying out palliative therapy, the following methods are used:
- Bougienage, that is, expansion of the esophagus.
- Installation of stents using the endoscopic method. Stents are hollow cylinders that are placed into the esophagus to allow food to pass through.
- Laser photocoagulation (the neoplasm is destroyed with a laser).
- Photodynamic effect. The patient is injected with a photosensitizer, which accumulates in tumor cells. Then, under the influence of a laser beam with a specific wavelength, these cells die.
- Chemotherapy and radiation. They can be used to reduce the size of the tumor and restore patency.
Stenting for esophageal cancer
Growing attention to the problem of Barrett's esophagus (BE) is caused by the high incidence of esophageal adenocarcinoma (EA) - a malignant tumor of the esophagus that develops from metaplastic intestinal epithelium of the mucous membrane in BE or (extremely rarely) from the esophageal glands (type I according to the classification of J. Siewert) . Over the past decades, there have been significant changes in the incidence of AP and squamous cell carcinoma of the esophagus. Now, in most cases of esophageal cancer in Western Europe and the USA, it is AP that is detected. In the United States, the incidence of AP has increased by almost 300% over the past 30 years (Fig. 1)
Rice. 1. Increase in the incidence of esophageal adenocarcinoma in the United States and a decrease in the incidence of squamous cell carcinoma (1975–2000) [3]. [12].
In Russia, this trend is not so clearly visible: 7-20% of diagnosed esophageal cancers have histological signs of adenocarcinoma. However, it is difficult to assess the reliability and accuracy of such data: the disease is detected in the late stages of advanced cancer, when it is impossible to determine where the tumor has formed - in the distal esophagus or in the proximal stomach. Among all oncopathologies of the digestive system, esophageal cancer and stomach cancer have the highest mortality rates. The prognosis after the diagnosis of adenocarcinoma is unfavorable - 5-year survival rate does not exceed 10-20%, and improvements in surgical treatment techniques do not make such a significant contribution to improving these indicators [4].
Understanding of the causes of AP and precancerous pathology, the possibility of early detection of precancerous processes and early forms of tumors have improved significantly in recent years. This makes it possible to form groups at increased risk of AP and organize expert centers for the identification and timely treatment of precancerous pathology. According to authoritative experts, adenocarcinoma of the digestive tract most often develops in pathologically altered mucous membranes. The chronic inflammatory process is considered as one of the important links in the chain of processes that ultimately lead to AP [5,6]. The main diseases against which cancer develops significantly more often have also been identified. For A.P. the underlying disease in most cases is BE.
Screening
The risk of developing AP in patients with BE largely depends on the presence of intestinal metaplasia, dysplastic changes in the epithelium and the degree of their severity in the distal esophagus (in the segment of columnar cell metaplasia of the distal esophagus). Based on observational data from 8522 patients included in the Northern Ireland cancer registry (average follow-up of 7 years), the risk of developing adenocarcinoma increased from 0.04 to 0.23% per year in the presence of intestinal metaplasia with goblet cells, and in the presence of epithelial dysplasia low degree in the metaplasia segment - the risk increases more than 9 times. The cumulative risk of developing adenocarcinoma and high-grade epithelial dysplasia is also significantly higher in groups of patients who, at the time of inclusion in the registry, had morphological changes characterized by intestinal metaplasia and low-grade dysplasia (Table 1)
Table 1. Risk of esophageal adenocarcinoma in patients with Barrett's esophagus in the presence of intestinal metaplasia and dysplasia [7].
The risk of developing AP against the background of BE is even more significant in patients with epithelial dysplasia in the area of the segment of columnar cell metaplasia and especially high-grade dysplasia (Fig. 2).
Rice. 2. The risk of developing cancer in patients with a segment of columnar cell metaplasia without intestinal epithelium, with Barrett’s esophagus without dysplasia and with low- and high-grade dysplasia of the mucosal epithelium [8-11].
However, the appearance of AP is preceded by a gradual progression of dysplastic changes with the cells losing signs of differentiation. Progression from mild dysplasia (low-grade dysplasia) to severe (high-grade dysplasia) can occur on average within 29 months, while the subsequent development of adenocarcinoma takes 2 times less time - 14 months [7]. The widespread prevalence of GERD and the identification of the cascade of precancerous events are potentially attractive targets for screening for BE and AP. One of the latest American recommendations (American College of Gastroenterology) suggests that endoscopic examination of the upper digestive tract is necessary for all patients with symptoms of chronic GERD, due to their high risk of BE [12]. The European Society of Gastrointestinal Endoscopy noted that at present screening is advisable only in groups at high risk of developing adenocarcinoma: in the presence of a long history of GERD (more than 5 years), with a combination of such risk factors as the patient’s age over 50 years, male gender, the presence of blood relatives who were diagnosed with AP or BE, high body mass index and other factors [13]. Among all screening methods, endoscopic examination of the upper digestive tract is the “gold standard” in the diagnosis of pathological processes in the mucous membrane of the esophagus, as it allows not only to visually determine the presence of columnar epithelium in the esophagus, but also to perform a biopsy for morphological confirmation of the diagnosis, diagnosis of dysplasia and early cancer . This allows us to consider the endoscopic method the most important screening tool for BE and AP.
Definition
For a long time, the following definition of BE was generally accepted in clinical practice: this is a pathological condition in which part of the squamous epithelium of the mucous membrane of the distal parts of the esophagus is replaced by metaplastic columnar epithelium. A segment of columnar metaplasia should be identified by endoscopic examination, located above the esophagogastric junction or junction (Z-line) and confirmed morphologically by detecting specialized intestinal metaplasia (Fig. 3)
Rice. 3. Barrett's esophagus. a — endoscopic picture when examined in white light; b — endoscopic picture when examined in a narrow-spectrum mode (Narrow Band Imaging, NBI); c — histological picture of columnar cell metaplasia with diffuse intestinal metaplasia with many goblet cells — hematoxylin and eosin staining, ×100; d — Alcian blue staining, ×100. [14]. In 2022, the European Society of Gastrointestinal Endoscopy (ESGE) proposed a more modern definition of BE: it is a segment of columnar metaplastic epithelium of the distal esophagus, at least 1 cm long, which can be diagnosed by endoscopic examination proximal to the esophagogastric junction and confirmed histologically by the presence in biopsy material of specialized intestinal metaplasia [15].
In accordance with the recommendations of the Russian Gastroenterological Association (RGA), at the moment, to establish a diagnosis of Barrett's esophagus, mandatory histological confirmation of metaplasia of the epithelium of the mucous membrane of the esophagus of the intestinal type is required, since this is the only type of columnar epithelium characterized by an increased potential for malignancy [16].
From this definition it follows that endoscopic and morphological studies are the basis for the correct diagnosis of this pathological condition. Timely diagnosis of BE, dysplastic changes in the mucous membrane and early forms of AP depends on the endoscopist, his knowledge, methodological skills, correct interpretation of identified changes in the mucous membrane, experience and, finally, technical equipment. However, intestinal metaplasia is not always an area of AP growth, and therefore in Japan, to establish the diagnosis of BE, it is only necessary to detect a segment of columnar cell metaplasia in the distal esophagus during endoscopic examination.
Prevalence of gastroesophageal reflux disease and columnar cell metaplasia in the distal esophagus
BE is one of the most serious complications of GERD - a chronic relapsing disease caused by spontaneous, regularly recurring retrograde entry of gastric and/or duodenal contents into the esophagus, leading to damage to the distal esophagus and/or the appearance of characteristic symptoms (heartburn, retrosternal pain, dysphagia). As an independent nosological entity, GERD was officially recognized in the materials on the diagnosis and treatment of this disease, adopted in October 1997 at the Interdisciplinary Congress of Gastroenterologists and Endoscopists in Genval (Belgium) [17]. The results of studies conducted around the world and covering large populations show that symptoms of GERD are experienced by more than 1/3 of the population, and the presence of daily heartburn is 7-10%. Complications of reflux disease and heartburn (the main symptom of GERD) are much more common in whites (12.3% and 34.6%, respectively) and blacks (2.8% and 46.1%, respectively) compared with East Asians (0 and 2.6%, respectively [18]. It is quite difficult to estimate the prevalence of columnar cell metaplasia in the distal esophagus, since more than 80% of cases of the disease remain undiagnosed, as shown by data from an American study of autopsy material [19]. The results of a study conducted in the United States of this The same group of specialists (A. Cameron et al.) show a 28-fold increase in the number of clinically diagnosed cases of metaplastic changes in the distal esophagus for the period from 1965 to 1995. Data from endoscopic examination of the upper digestive tract in patients admitted to clinics with symptoms dyspepsia or for colonoscopy, indicate differences in the prevalence of columnar cell metaplasia of the esophagus depending on ethnic and geographical factors (Table 2).
Table 2. Frequency of detection of Barrett's esophagus during endoscopic examination of the upper digestive tract in patients with dyspepsia
The prevalence of GERD in Russia among the adult population is about 40%, and 45-80% of them have esophagitis: in 10-35% of cases it is severe esophagitis with multiple erosive lesions of the mucous membrane of the distal esophagus. BE of varying degrees of extent is diagnosed on average in 8-15% of patients with esophagitis. AP develops in 0.5% of patients with BE per year with low-grade dysplasia, and in 6% per year with high-grade epithelial dysplasia [26]. Risk factors for BE include middle and old age, male gender. In most patients, this pathology is diagnosed at the age of 50-60 years, in men 3-4 times more often than in women.
Pathogenesis
One of the main causes of the development of GERD and BE is gastroesophageal reflux - the reflux (entry) of gastric contents (primarily hydrochloric acid) into the esophagus. With the development of such reflux, the pH in the distal esophagus shifts significantly towards low values due to the ingress of acidic gastric contents. Prolonged contact of the esophageal mucosa with acid refluxate, which also contains pepsin, contributes to the development of its inflammation. Bile acids and enzymes, which can also be part of the refluxate, can have a strong damaging effect on the esophageal mucosa if the motility of the upper digestive tract is impaired. Esophagitis in some cases is accompanied by a structural restructuring of the epithelium of the esophageal mucosa with the formation of gastric or intestinal metaplasia, which is the background for the development of adenocarcinoma. An analysis of numerous studies shows that the risk of developing cancer in the segment of columnar cell metaplasia is primarily associated with the presence of intestinal metaplasia (incomplete intestinal metaplasia, type II and III) [27, 28]. In the esophagus, metaplastic changes begin with the appearance first of columnar epithelium of the gastric type, and then of the colonic type epithelium. Exposure to acid in the esophagus increases, on the one hand, the activity of protein kinases that initiate the mitogenic activity of cells and, accordingly, their proliferation, and, on the other hand, inhibits apoptosis in the affected areas of the mucous membrane. Approximately 50–80% of cases of dysplasia associated with BE and AP are characterized by mutations in genes involved in cell cycle regulation, DNA repair, and apoptosis [20]. Recent research in this area indicates the important role of the genes (proteins) P53 and P63 involved in the development of squamous epithelial cells. In the esophagus, P63 protein expression is detected only in squamous epithelial cells and is absent in columnar cell metaplasia. In the absence of P63, mucosal stem cells cannot begin differentiation along the path of squamous epithelial cells; as a result of this disorder, columnar epithelial cells are formed [29, 30]. The basis for the origin of intestinal-type epithelial cells can be both the stratified squamous and cuboidal epithelium of the ducts of the glands of the submucosal layer of the esophagus, and the cardiac-type epithelium in the distal esophagus, exposed to refluxate [31, 32]. Among the factors influencing the processes of carcinogenesis in the area of the esophagogastric junction, it is also necessary to take into account smoking, alcohol, increased body weight and bile reflux. Dynamic observations of patients with BE showed that the development of adenocarcinoma occurs through a multi-stage pathological process. This process is characterized by an increase in the degree of dysplasia, a pathology that precedes adenocarcinoma. An important promoter of this process is nitric oxide, which can accumulate in pathologically altered tissues of the distal esophagus and cause genetic changes. Genetic changes in cells occur parallel to the transition from metaplasia to dysplasia and then to adenocarcinoma [33].
Diagnosis and screening of Barrett's esophagus and esophageal adenocarcinoma
Endoscopic examination is key in diagnosing BE: while other methods (x-ray, scintigraphy) can only suggest this diagnosis, the endoscopic method can establish it with a high degree of probability. An endoscopic examination determines the extent of changes in the mucous membrane, the ratio of the zone of changes in the mucous membrane along the length to the esophagogastric junction, as well as the proximal border in relation to the incisors. In this case, the spread of the metaplasia zone is clearly visualized in the form of foci of hyperemia (“tongues of flame”) against the background of the “pearly white” epithelium of the esophagus. One of the important tasks of endoscopic examination is obtaining biopsy material. The objectives of the morphological study are to confirm metaplasia of the esophageal mucosa, identify areas of dysplasia and foci of adenocarcinoma.
From an endoscopic perspective, accurate diagnosis of BE presents several challenges. One of them is to determine the key landmarks - the zone of the esophagogastric junction or junction (EGJ), the Z-line and the boundaries of the segment of columnar cell metaplasia. Another is the accuracy of performing a biopsy in the focal distribution of areas of intestinal metaplasia and dysplasia in the segment of columnar cell metaplasia of the esophagus and the difficulties of endoscopic diagnosis of these foci [34].
Key guidelines for endoscopic diagnosis
1. The zone of the esophagogastric junction (junction) is the area of connection between the muscular layer of the esophagus and the muscular layer of the cardia of the stomach. It corresponds to the boundary between the tubular structure of the esophagus and the proximal part of the stomach with longitudinal folds. This anatomical boundary is defined in the region of the proximal edge of the longitudinal folds of the gastric mucosa (Fig. 4).
Rice.
4. Endoscopic picture of Barrett's esophagus. The arrows indicate the proximal edge of the folds of the gastric mucosa, corresponding to the area of the esophagogastric junction. Another additional landmark of the esophagogastric junction is the distal border of the longitudinal vessels of the mucous membrane visible during endoscopy (palisade or longitudinal intramucosal vessels)
. These vessels were first described by J. Carvalho in 1963 and are veins located in the mucosa above the muscularis propria. In the stomach they are located in the submucosal layer and extend into the superficial layers of the mucous membrane only in the area of the border of the stomach and esophagus. In the mucous layer of the esophagus, they run proximally for about 2 cm parallel to each other in the form of a “picket fence” and then plunge back into the submucosal layer, forming larger veins. The Japanese Society for the Study of Diseases of the Esophagus recommends defining the esophagogastric junction precisely along the distal edge of these longitudinal vessels (Fig. 5)
Rice. 5. Longitudinal vessels of the mucous membrane of the distal esophagus (examination in the narrow-spectrum NBI mode). The arrows indicate the distal edge of the palisade vessels, corresponding to the area of the esophagogastric junction. [35].
2. Jagged line or Z-line
- the border between the pale pink stratified squamous epithelium of the esophagus and the brighter and darker columnar epithelium of the stomach (see Fig. 3). The letter Z stands for zero - this is the zero mark of the zone where the squamous epithelium of the esophagus ends [36]. Normally, this uneven line coincides with the anatomical border of the esophagus and stomach. In the presence of columnar cell metaplasia in the distal esophagus, the Z-line does not coincide with the area of the esophagogastric junction.
3. In the case when the Z-line is located above the anatomical border of the esophagus and stomach (AGB), a segment of columnar cell metaplasia located between these two landmarks is determined. Modern diagnosis of the boundaries and extent of the segment of columnar cell metaplasia of the distal esophagus is based on The Barrett's Prague C&M Criteria, developed by The international working group for the classification of oesophagitis at the 12th European Gastroenterological Week in Prague in 2004. These criteria involve determining the proximal border of the circular segment of metaplasia, as well as determining the maximum extent and the uppermost limit of the longest “tongue” of metaplasia running from the circular segment to its upper edge (M value). The length of the circular segment of columnar cell metaplasia is measured from the edge of the gastric folds (GPL) to its proximal level (C value) (Fig. 6).
Rice. 6. Prague criteria for the diagnosis of Barrett’s esophagus [37]. a — the length of the circular segment of metaplasia is 2 cm, the length of the longest segment of metaplasia is 5 cm; endoscopic conclusion - “Barrett's esophagus C-2, M-5”; b, c - an endoscopic example of a correct endoscopic conclusion after the correct definition of the Prague criteria for Barrett's esophagus - “Barrett's esophagus C-1, M-6”. Small islands of metaplasia located proximal to the common segment, separate from it and unrelated to it, are not taken into account.
The presence in a patient of an axial esophagogastric hernia of the esophageal opening of the diaphragm (HH) significantly changes the position of the key diagnostic guidelines for P.B. Therefore, the diagnosis of this pathological condition is also an important element of endoscopic examination and involves determining the narrowing corresponding to the POD, located distal to the PVC zone [38].
Diagnosis of intestinal epithelium in the segment of columnar cell metaplasia
Histological examination in the BE zone reveals three types of glandular epithelium: epithelium of the fundus of the stomach (integumentary pit), epithelium of the cardia (or transitional type) and specialized (special) intestinal epithelium with goblet cells [39]. Verification of P.B. involves morphological confirmation of the presence of a specialized, intestinal-type epithelium in the distal segment of the esophagus. If histological examination reveals only cells of the fundic or cardiac types, one should not talk about BE, but only about columnar cell metaplasia of the esophagus, which is not associated with a high risk of AP. Until recently, endoscopic examination of a metaplastic segment of the distal esophagus with so-called “blind” biopsies at four points around the circumference of the esophagus and along each centimeter along the length of the segment was the “gold standard” for diagnosing P.B. However, the results of recent studies have shown that the accuracy of such diagnosis of intestinal metaplasia foci is 48.2% [40].
Is it possible to increase the efficiency of diagnosis and dynamic monitoring of patients with BE in order to timely detect not only specialized intestinal epithelium, but also dysplastic changes in the epithelium, as well as early cancer?
Currently, it is relevant to develop new and determine the effectiveness of existing methods for staining the mucous membrane of the distal esophagus, which can effectively diagnose metaplastic, dysplastic changes, as well as early forms of cancer. In addition, there are a number of new optical endoscopic techniques that make it possible to avoid performing “blind” biopsies. Among these techniques, the most effective are chromoendoscopy, magnifying and narrow-spectrum endoscopy.
Chromoendoscopy
The methylene blue staining technique, based on the absorption of the dye by metaplastic intestinal epithelium, is effective in diagnosing foci of intestinal metaplasia in the segment of columnar cell metaplasia of the distal esophagus. It is this technique that allows you to objectively assess the localization, size and distribution of foci of intestinal metaplasia (Fig. 7, a).
Rice. 7. Foci of intestinal metaplasia. a - after staining with a 0.5% solution of methylene blue, they look like blue spots against the background of pink gastric epithelium, which does not absorb the dye; b — false positive results of chromoscopy, methylene blue dye is adsorbed in the area of linear erosions and ulcers. And the staining technique with indigo carmine, which allows using a dye to highlight and emphasize structural surface changes in the mucosa, helps in the diagnosis of focal lesions of the mucous membrane, including early cancer. Performing a targeted biopsy of areas stained with methylene blue followed by a morphological examination of gastrobiopsy specimens allows one to assess the type of intestinal metaplasia and timely diagnose foci of epithelial dysplasia occurring against the background of metaplasia and early forms of cancer. However, chromoscopy techniques are not highly specific for metaplastic and dysplastic changes in the epithelium of the distal esophagus. Methylene blue dye can stain not only foci of intestinal metaplasia, but also be adsorbed in the area of erosions and ulcers, which leads to false positive diagnostic results (see Fig. 7, b).
Magnifying endoscopy
Magnifying endoscopy allows you to study in detail the microarchitecture of the mucous membrane of a segment of columnar cell metaplasia of the esophagus using an optical 115-fold magnification of its surface, to determine the types of patterns corresponding to metaplastic and dysplastic changes in the epithelium. This technique, in combination with 0.5% methylene blue chromoscopy, showed high specificity and sensitivity not only in diagnosing foci of intestinal metaplasia, but also dysplastic changes in the epithelium in patients with BE and made it possible to determine the types of microstructure of the surface of the mucous membrane (Fig. 8),
Rice. 8. Magnifying endoscopy (115x optical magnification) of a segment of columnar cell metaplasia of the distal esophagus. The type of pattern of “longitudinal ridges” corresponds to the intestinal epithelium (Barrett's esophagus). corresponding to these pathological changes [41].
Narrow band endoscopy
Narrow-spectrum endoscopy is one of the most modern endoscopic techniques registered by the Russian Ministry of Health and approved for use in the Russian Federation. Endoscopic examination with narrow band
) is based on the use of special narrow-spectrum optical filters that change the spectrum of the light flux. The depth of penetration of the light flux depends on the wavelength; light waves of a longer spectrum (for example, red) penetrate deeper into tissue, while the visible blue spectrum (shorter waves) is able to penetrate only the superficial layers of tissue, which allows for more detailed examination their microstructure. Thus, the use of special narrow-spectrum filters in this system, transmitting light fluxes with a wavelength of 415 and 445 nm and delaying light waves that penetrate deeper into the tissue, allows for improved visualization of the surface of the mucous membrane. In addition, narrow-spectrum light waves are well absorbed by tissue hemoglobin, which makes it possible to study the microvascular pattern of the capillaries of the mucous membrane and submucosal layer of the esophagus. Using this technique, it is possible not only to increase the efficiency of determining key landmarks for the diagnosis of BE (longitudinal vessels), but also to identify the smallest disturbances in the architectonics of the epithelium, characteristic of metaplastic, dysplastic changes and initial forms of cancer (Fig. 9).
Rice.
9. Barrett's esophagus. a — diagnostics in the normal light spectrum; b - narrow-spectrum endoscopy with optical image magnification - clearer visualization of a segment of metaplasia of the distal esophagus with a violation of microarchitecture and vascular pattern, areas of intestinal epithelium and dysplasia (indicated by an arrow). Using magnifying and narrow-spectrum endoscopy, we studied the pattern of various sections of the mucous membrane in the segment of columnar cell metaplasia of the distal esophagus, which made it possible to determine the types of epithelial pattern that do not have signs of dysplasia, as well as structural changes corresponding to dysplasia and early esophageal cancer (Fig. 10).
Rice. 10. Types of pattern of the epithelium of the distal esophagus without neoplastic changes. a - oval pattern of pits - epithelium of the cardia of the stomach in the distal segment of the esophagus; b - large and elongated oval pattern - “oval ridges” - characterizes intestinal metaplasia; c - pattern of longitudinal ridges - intestinal metaplasia; d - pathological vessels and destroyed type of epithelial surface pattern - high-grade dysplasia. The results obtained and the correlation of endoscopic findings with histological data showed high specificity and sensitivity of the methods in diagnosing the types of epithelium of the distal esophagus [42]. This allows us to talk about a new direction in endoscopic screening of BE - “optical biopsy” - and timely, highly effective detection of precancerous changes and early forms of cancer [43].
Endoscopic examination using optical image magnification (more than 100x) in combination with a narrow-spectrum contrast examination mode allows for accurate diagnosis of pathological changes in the epithelium, identifying areas of gastric and intestinal metaplasia and detecting pathological areas of dysplasia and adenocarcinoma. The specificity and sensitivity of the technique for metaplasia are 92 and 95%, respectively.
Treatment of patients with Barrett's esophagus
Most patients with BE have symptoms caused by reflux of gastric contents into the esophagus and characteristic of GERD: heartburn, regurgitation, chest pain, and sometimes extraesophageal manifestations (laryngitis, chronic cough and bronchial asthma). Treatment of patients with BE has two main directions: treatment of symptoms and manifestations of GERD, as well as reducing the risk of developing AP. Modern principles of effective therapy for any form of GERD and, in particular, erosive esophagitis, are reflected in the recommendations of the Russian Gastroenterological Association and consist of two main stages: induction of remission and maintenance of remission of erosive esophagitis. The first stage of treatment involves the administration of proton pump inhibitors (PPIs) in standard dosages for 4 weeks for single erosions and for 8 weeks for multiple erosive lesions of the mucous membrane. Maintenance therapy involves taking PPIs at half the dosage for 26-52 weeks. PPIs create optimal pH conditions for healing erosions and reducing hyperproliferation in metaplastic epithelium. This tactic makes it possible to achieve rapid improvement in clinical symptoms, positive dynamics of inflammatory changes determined by endoscopy, and reduce the time and costs of the treatment course. PPIs are the basis of both course and maintenance therapy for all forms of GERD and are 2 times more effective than histamine H2 receptor blockers in total clinical effectiveness, and 3 times more prokinetic than blockers [44]. After long-term use of drugs of this group, patients with BE experience a decrease in proliferation markers. And although it is believed that BE, as a rule, does not undergo reverse development, in some cases it is possible to achieve partial regression of a limited area of intestinal metaplasia. In one of the few randomized studies of 68 patients with BE treated for 24 months with omeprazole 40 mg (group 1) and ranitidine 150 mg (group 2) twice a day, a small but statistically significant regression of the metaplasia segment was established . A decrease in segment length and area by 8% was recorded only in group 1 of patients receiving omeprazole. No changes were observed with ranitidine [45].
Preventing the progression of metaplastic changes and neoplasia in patients with BE remains a complex and poorly understood problem. Two studies showed that in 230 and 80 patients with GERD who received long-term therapy with omeprazole in various daily doses (from 20 to 80 mg), during follow-up (average 6.9 years) in 12 and 14.5% cases, accordingly, metaplastic changes were found in the distal esophagus, characteristic of BE [46, 47]. On the other hand, results of follow-up of 350 patients with BE for an average of 4.7 years showed that the risk of dysplastic changes was 5.6 times higher in those who did not receive regular PPI therapy (for at least 2 years) in throughout the entire observation period [48]. The results of work in this area generally indicate that PPI therapy cannot in all cases prevent metaplastic and dysplastic changes in the distal esophagus in patients with GERD. Further research is needed in this area, improving the mechanisms for recording the smallest structural changes in the mucous membrane, which is possible using new optical endoscopic techniques.
In case of ineffectiveness of drug therapy, as well as when dysplastic changes in the mucous membrane are detected, surgical and endoscopic treatment methods can be used. A retrospective analysis of the results of surgical operations—fundoplications—did not show significant advantages of this technique compared to drug therapy with PPIs. Fundoplication does not prevent the onset of BE in patients with GERD, nor its progression to AP [49]. Endoscopic techniques for ablation and resection of metaplastic and dysplastic changes in patients with BE are an alternative to surgical resection, which has a high risk of complications (Fig. 11)
Rice. 11. Stages of endoscopic argon plasma ablation of a segment of Barrett’s esophagus with low-grade dysplasia detected and confirmed by an expert morphologist without a visible pathological area. a, b - diagnosis of Barrett's esophagus when examined in a standard white light mode and using a narrow-spectrum mode (NBI)
Rice. 11. Stages of endoscopic argon plasma ablation of a segment of Barrett’s esophagus with low-grade dysplasia detected and confirmed by an expert morphologist without a visible pathological area. c—f — stages of hybrid argon plasma ablation using the new ERBE instrument (Germany), the first operation in Russia is performed by Dr. Thorsten Beina (Dusseldorf, Germany). [50, 51].
Endoscopic treatment should be accompanied by adequate antisecretory PPI therapy to ensure effective healing of mucosal defects in the area of distant foci of metaplasia or dysplasia and to create conditions for the appearance of stratified squamous epithelium of the esophagus in these areas. Despite the high efficiency and relative safety of argon plasma and electrocoagulation, as well as laser destruction, these ablation techniques do not always lead to histological eradication of intestinal metaplasia and cannot prevent the subsequent development of dysplastic changes in 100% of cases [52].
The algorithm for managing patients with BE in the presence of epithelial dysplasia is defined in the recommendations of the European Society of Gastrointestinal Endoscopy. Basic provisions of the algorithm:
- prophylactic endoscopic therapy, such as ablation, should not be performed if the patient has BE without dysplasia (due to the extremely low risk of developing adenocarcinoma
Treatment for esophageal cancer varies depending on the stage
Stage 0
A tumor at this stage is not true cancer. It contains abnormal cells. This condition is called “dysplasia”, it is a type of precancerous disease. The abnormal cells look like cancer, but are found only in the inner lining of the esophagus (epithelium); they do not grow into the deeper layers of the esophagus.
Endoscopic treatment methods are usually used:
- PDT, or photodynamic therapy;
- RFA, that is, radiofrequency ablation;
- EMR, endoscopic removal of a tumor of the mucous membrane (after this, long-term observation is provided using endoscopy in order to detect recurrence in time if it occurs).
Stage I
The neoplasm affects the muscular or lamina propria of the mucosa, but does not affect other organs and lymph nodes.
- T1 cancer. Disease at an early stage, when it is only in a small area of the mucosa and has not reached the submucosa (T1a neoplasm), can be removed by endoscopic resection within the mucosa or submucosal layer. Sometimes doctors recommend surgically removing part of the esophagus, followed by radiation and chemotherapy.
- T2 cancer. The tumor affects the muscular plate of the mucosa. Such patients undergo chemotherapy and radiation before surgery. Exclusive surgical removal is recommended only when the tumor is less than 2 cm in size.
When the cancer is located in the neck, radiation and chemotherapy may be recommended as the primary treatment instead of surgery.
Stages II and III
In the second stage, the tumor spreads to the main muscular layer of the esophagus or its outer lining. The neoplasm also affects 1 or 2 nearby lymph nodes.
In the third stage, the neoplasm grows on the outer lining of the esophagus, can spread to neighboring organs, and affects regional lymph nodes. Combination treatment is recommended, which includes surgery followed by chemotherapy or chemotherapy in combination with radiation. If, due to health reasons, the patient is at risk of not surviving the operation, then chemotherapy in combination with radiation becomes the main method of treatment.
IV stage
Cancer affects distant lymph nodes, there are metastases in distant organs (lungs, liver). At this stage, the main goal of treatment is to control the spread and size of the tumor for as long as possible. Patients are given symptomatic treatment to relieve pain, restore the ability to eat, etc. Radiation therapy and chemotherapy are used.
Complications
The main complication of Barrett's esophagus is malignant transformation into adenocarcinoma secondary to dysplasia.
On average, severe dysplasia is diagnosed in 20-25% of patients within 20-23 years after diagnosis. The likelihood of its development correlates with the length of the affected area and the degree of dysplasia:
- With low-grade dysplasia, the risks of malignancy are 0.8-1.9% per year.
- For severe dysplasia - 6-12.2% per year.
In addition, the number of foci of dysplasia is important. With multiple lesions, the likelihood of malignancy is three times higher than with single lesions.