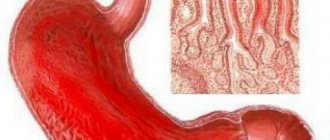
Intestinal paresis is a condition that accompanies many serious diseases and is characterized by a gradual decrease in the tone of the intestinal wall and paralysis of intestinal smooth muscles. Among surgical patients of all profiles, intestinal paresis is diagnosed in 0.2% of operated patients. Postoperative paresis requires timely treatment, in the absence of which complications may develop.
Specialists at the Yusupov Hospital are developing paresis treatment programs for patients, which include effective methods. The modern equipment of the rehabilitation center of the Yusupov Hospital and the professionalism of specialists allow patients with this pathology to return to a full life in the shortest possible time.
Causes
Intestinal paresis can be caused by a fracture of the lower thoracic and lumbar spine with damage and without compromising the integrity of the spinal cord.
It develops in the presence of a retroperitoneal hematoma, compressing the vessels and nerves of the mesentery. Doctors at the Neurology Clinic provide conservative therapy for intestinal paresis in patients with diseases of the nervous system using effective drugs registered in the Russian Federation. Intestinal paresis is a fairly common manifestation of diseases of the internal organs. Paralysis of the muscles of the intestinal wall in 25% of cases develops against the background of acute pathology of the abdominal organs, less often - with severe diseases of the pulmonary and cardiovascular systems, endogenous intoxication, and generalized infection.
More than 70% of patients suffering from intestinal paralysis are over 60 years of age, so intestinal paralysis is considered a disease of the elderly. Intestinal paresis can occur in newborns, children of different ages, and pregnant women. One of the reasons for the development of the disease is considered to be the formation of an inflammatory process localized inside or outside the abdominal cavity (with peritonitis, retroperitoneal phlegmon and other diseases).
The cause of paretic intestinal obstruction can be:
- impaired blood supply to the intestine with the development of ischemia (with irreversible myocardial ischemia, rupture of an abdominal aortic aneurysm, acute coronary insufficiency);
- disturbance of innervation (due to tumor or traumatic injury to the spinal cord, taking calcium channel blockers, inhibition of acetylcholine synthesis in nerve endings).
Reflex paresis develops with complicated pneumonia and renal colic. Impaired motor skills are also possible due to exogenous and endogenous intoxications.
Etiology, pathogenesis of motor-evacuation disorders of the gastrointestinal tract after operations on the abdominal organs
Paresis of the gastrointestinal tract is the second most common among the observed postoperative complications [7, 34, 38]. Its pathogenesis is very complex and, apparently, has not one, but several causes of development [38, 43, 49, 55, 64, 66]. Most often, paresis of the gastrointestinal tract occurs after major abdominal operations [15, 61, 64, 72]. Many authors explain this by the fact that during such surgical interventions, the peritoneum, rich in receptors, is injured, as a result of which circulatory disorders develop in the wall of the gastrointestinal tract, the tone of the sympathetic nervous system increases with the release of large amounts of catecholamines into the blood [5, 44, 50, 64, 75, 76]. In this regard, many authors assess the development of postoperative paresis of the gastrointestinal tract as a protective reaction to surgical trauma in the next 2-3 days after surgery [38, 54].
Gastrointestinal dysfunction is the most common and severe complication of peritonitis. According to most researchers, in the pathogenesis of peritonitis, one of the key factors in the progression of the disease is enteral insufficiency syndrome. It is considered as a pathological symptom complex that occurs during acute surgical illness and trauma to the abdominal organs and is accompanied by disruption of all functions of the digestive tract, when the intestine becomes the main source of intoxication and the development of multiple organ failure [6, 10, 17, 21, 52, 74].
With the development of inflammation in the abdominal cavity, one of the pathogenetic mechanisms that determine changes in the motor function of the gastrointestinal tract is a disruption of the relationship between the sympathetic and parasympathetic nervous systems. Hypertonicity of the sympathetic nervous system extends not only to the smooth muscles of the intestinal wall, but also to the vessels supplying them, which leads, on the one hand, to inhibition of intestinal motility, and on the other (as a result of increasing arterial spasm) to a sharp decrease in regional blood flow [6, 60, 78].
At the beginning of development, paresis of the gastrointestinal tract may be caused by dysfunction of endocrine regulatory mechanisms: 1) release of catecholamines; 2) activation of the kallikrein-kinin system with excessive release of histamine, bradykinin, proteolytic enzymes and other biologically active substances into the bloodstream; 3) a decrease in the biological activity of cells of the APUD system (serotonin [substance P] and motilin) involved in the work of the migrating myoelectric complex of the intestine and peripheral hemocirculation [3, 36, 53]; 4) dysregulatory intake of secretin, cholecystokinin and enteroglucagon [21]. Based on data from experimental and clinical studies, a concept has been formulated regarding the role of serotonin and serotonin receptors in the genesis of smooth muscle dysfunction, which is an integral part of the clinical syndrome of serotonin deficiency. It is now known that serotonin plays an important role in regulating the functions of the gastrointestinal tract. The largest supply of serotonin in the body is found in the gastrointestinal tract, which accounts for more than 95% of the serotonin content in the entire body [16, 28, 30].
The bulk of serotonin is contained in enterochromatophyte epithelial cells, within which serotonin is synthesized from L-tryptophan and stored in secretory granules. Enterochromatophyte cells are interspersed in the intestinal epithelium, mainly in the crypt region [68, 70]. Serotonin is also present in serotonergic neurons of the small intestinal nervous system. Some functions of “small intestinal” serotonin have already been studied.
First, serotonin acts as a mediator of interneuronal connections in the muscularis propria of the small intestine [77].
Second, serotonin, released from enterochromatophyte cells in response to chemical or mechanical stimulation, affects gastrointestinal motility and intestinal electrolyte transport [59, 69]. Peristalsis of various parts of the intestine is coordinated by neurons of the small intestinal nervous system, which, after activation of serotonin mechanisms, release other mediators [57]. In addition, extrinsic sensory neurons activated by serotonin initiate physical sensations from the gut, which may include feelings of nausea, flatulence, and pain [56].
Serotonin, found in enterochromatophyte cells, also regulates the growth of neighboring epithelial cells [73] and may slow the intestinal absorption of sugar [37] and L-alpha-aminoisocaproic acid [71]. Smooth muscle dysfunction is based on a violation of the interaction of serotonin with serotonin receptors of smooth muscles [28]. Apparently, during peritonitis its synthesis is disrupted [58] and, as a consequence, serotonin deficiency occurs, leading to smooth muscle failure. It has been established that in peritonitis, the level of endogenous serotonin is reduced by 2.5 times compared to normal levels [29].
According to this concept, smooth muscle dysfunction, resulting from disruption of the interaction of serotonin with its receptors, leads to disruption of endogenous vasomotility, disruption of microcirculation, local and regional hypoxia, tissue damage and necrosis. Based on experimental and clinical studies [27, 30], it has been established that under pathological conditions in the body the number of serotonin receptor ligands increases. Serotonin receptor ligands are divided into agonists and antagonists. Serotonin antagonists, when interacting with serotonin receptors, cause paralysis of smooth muscles. Agonists, on the contrary, cause spasm of smooth muscles. Subsequently, myocytes are unable to perceive nerve impulses due to pronounced metabolic changes and intracellular electrolyte disturbances. All this leads to stretching of the intestinal loops and increased intracavitary pressure and contributes to damage to both the entire digestive system and other functional systems of homeostasis [27, 28].
The resulting stagnation is accompanied by a local increase in venous pressure, leading to inhibition of gas resorption and a further increase in intraintestinal pressure. When the latter reaches the level of diastolic pressure, fluid absorption stops, which in turn causes even more pronounced distension of the small intestine and malnutrition of the intestinal wall [21, 38].
These processes are aggravated by progressive endogenous intoxication, which increases the degree of hypoxia of the intestinal wall, forming a “vicious circle”. Exo- and endotoxins, as well as “aggression factors” and metabolic products of continuously multiplying microflora colonizing the proximal sections, have a toxic effect on the intestinal wall directly and indirectly [52]. When the suppression of intestinal motility is accompanied by a decrease in intramural blood flow, the intensity of the digestion and absorption processes decreases sharply, reaching a critical level. The extent of disturbance of local blood flow primarily depends on the degree of dilatation of the intestinal loop and the force of compression of the vessels in its wall. When the pressure level in the intestinal lumen is above 100 mm Hg. there is a profound disruption of the filtration function of capillaries with a sharp limitation of oxygen consumption by tissues and an increase in ischemia of the intestinal wall, which develops when the blood flow in it decreases by 50% of the proper value [48].
Under conditions of intestinal ischemia, the content of oxygen and nutrients in tissues decreases (with an increase in the concentration of active toxic oxidants), tissue acidosis develops, and hyperproduction of paracrine substrates occurs (histamine, serotonin, bradykinin, nitric oxide, leukotrienes, thromboxanes, interleukins-1, 2, 4, 6, 8, 10, endothelins, complement and thrombin) [9, 18, 33]. At the same time, in our opinion, the reserves of these substances are depleted, which can ultimately lead to their persistent deficiency.
With the development of intestinal paresis and, as a consequence, a delay in the passage of intestinal contents, intensive growth and changes in the microflora of the small intestine occur. In conditions of inflammation of the peritoneum, an imbalance develops between different types of microorganisms and their distribution in different parts of the intestine. Increased reproduction of pathogenic allochthonous (foreign, not from a given part of the gastrointestinal tract) microflora weakens the local immune defense of the mucous membrane, leading to a decrease in its barrier function, inhibition of the functional activity of the lymphatic and reticuloendothelial systems, loss of the antagonistic properties of normal intestinal microflora in relation to pathogenic and putrefactive microbes, a decrease in vitamin-forming and enzyme functions [18].
This significantly affects the effectiveness of anti-infective protection in general. Capsular antigens of protein and polysaccharide nature secreted by pathogenic microorganisms provide the selective possibility of their adhesion to the surface of enterocytes. After fixation of microbial cells, their proliferation is observed. The enterotoxin (endotoxin) released in this case causes a disruption in the transport of electrolytes, which causes increased secretion into the intestinal lumen, water imbalance and severe dehydration of the body. Exotoxins produced by allochthonous pathogenic microorganisms lead to metabolic dysfunction of integumentary cells, disruption of the relationship between secretion and absorption of fluid, and give a cytotoxic effect, accompanied by the destruction of cell membranes of epithelial cells [6, 9, 52].
The multidirectional impact of these numerous pathogenic factors on the structural formations of the intestinal mucosa leads to a sharp change in its properties (especially barrier properties) and a “breakthrough” of pathogenic microflora into the lymphatic bed, portal blood flow and even into the free abdominal cavity. This process is called “bacterial translocation” [18, 21, 74].
Currently, it is this syndrome that is assigned the leading role in saturating the body with endotoxin (with the inclusion of the lipopolysaccharide complex), which is the main inducer of the development of systemic inflammatory response syndrome, abdominal sepsis and multiple organ failure. It is the intensity of bacterial translocation that is associated with the nature and severity of endogenous intoxication, the development and progression of multiple organ dysfunction syndrome [6, 9, 18, 21, 52, 74].
Thus, the pathogenetic causes of postoperative intestinal paresis are varied, but in our understanding, the theory of serotonin deficiency proposed by A.P. deserves attention. Simonenkov [27, 28].
Diagnosis of intestinal paresis in the early postoperative period
To date, objective methods of monitoring the activity of the gastrointestinal tract have not been sufficiently introduced into clinical practice. Many authors limit themselves only to indicators of the timing of the passage of gases and the appearance of the first stool [40, 62, 67]. At the same time, early diagnosis of postoperative paresis could be a significant addition to routine physical methods of examining the patient: general examination of the patient, auscultation of peristaltic sounds.
Some proposed methods for diagnosing postoperative disorders of the motor-evacuation function of the gastrointestinal tract (balloonography, ionomanometry, direct myography, etc.) are of little use due to the severity of the condition of patients in the early postoperative period [28].
Some authors [25, 34] have proven the practical value of phonoenterographic examination of the abdominal organs.
A graphical representation of bowel sounds makes it possible to reliably diagnose decreased motility in the postoperative period.
Over the past decades, when studying the activity of the gastrointestinal tract, a graphic recording of the electrical activity of smooth muscles has been used - an electrogastroenterogram [7, 11, 19, 35].
It must be taken into account that the electrical potentials of the abdominal organs are very small and the existing electrophysiological equipment for studying such low-amplitude biopotentials must have amplification paths, which in turn can distort the signals.
In addition, researchers emphasize the laboriousness of mathematical and graphical processing of records, which also limits the use of such techniques in the clinic [19, 46].
Subsequently, in order to simplify the technique and obtain more objective data, it was proposed to place skin electrodes not in the projection of the stomach and intestines, but on the limbs, as in electrocardiography, proving the diagnostic value of this method and revealing a clear correlation between the signals obtained during the study from the limbs and the abdominal wall . The introduction of such peripheral electrography into clinical practice made it possible to assess the state of motor activity of the stomach and intestines in a number of diseases [12, 22].
The possibility of recording biopotentials from the surface of the body relieves researchers and clinicians from technically complex and not always safe invasive methods of studying the motility of the gastrointestinal tract [2, 3, 5]. However, according to J. Chen [46], the electrogastroenterogram does not provide useful information, since it is difficult to standardize.
There are attempts to analyze the electrogastroenterographic curve by entering the obtained information into a computer. The solution to this problem is reflected in most works of both domestic and foreign scientists [4, 14, 47].
Presenting data from electrogastroenterograms with subsequent computer processing, researchers speak with restraint about the clinical significance of electrogastroenterography, emphasizing the shortcomings of the method [39, 47].
Other authors [1, 20, 31] during the study revealed the informative value of peripheral computer electrogastroenterography in the objective diagnosis of motor-evacuation disorders of the gastrointestinal tract in patients with widespread peritonitis.
Thus, the most promising, reasonable and non-invasive method for assessing the motor-evacuation function of all parts of the gastrointestinal tract is peripheral electrogastrointestinography.
Modern principles of treatment of patients with postoperative intestinal paresis
Normal motility is the result of coordinated contractile activity of smooth muscles throughout the gastrointestinal tract. This activity is regulated by local factors that simulate the activity of smooth muscles, reflexes, the paths of which are closed within the autonomic nervous system, hormones and the influence of the central nervous system. According to many researchers, each of these systems plays a possibly independent pathogenetic role in the development of postoperative paresis, so treatment should be multicomponent.
Taking into account the above links of pathogenesis, an approximate program of therapeutic measures is being constructed aimed at resolving postoperative paresis and associated metabolic disorders. In each specific observation, an individual correction is carried out, each point of which fulfills the tasks of not one, but several pathogenetically based treatment directions.
Clinicians attribute unsatisfactory results of treatment of patients with subsequent postoperative paresis of the gastrointestinal tract to two main reasons. Firstly, in widespread clinical practice, the standard approach of doctors to the selection of therapeutic measures, without taking into account the pathogenesis of the disease, dominates [34]. Secondly, practical experience in the treatment of postoperative paresis indicates that the fight against it begins only when it has already developed. Meanwhile, measures aimed at preventing paresis should be carried out in the early postoperative period, before the appearance of clinical signs of paresis [7, 8, 11, 14].
As for treatment methods at the present stage, most authors are inclined to complex therapy taking into account the pathogenesis of the disease. In many surgical clinics, the treatment of postoperative paresis of the gastrointestinal tract remains routine and sometimes one-sided [5, 13, 15, 33].
According to E. Livingston [64], nasointestinal intubation is the only effective treatment for paresis.
Recently, early initiation of enteral (tube) nutrition has also been recommended, which contributes to a more rapid restoration of the functional activity of the gastrointestinal tract [13]. A number of authors note a positive effect on intestinal motility in the postoperative period of the use of chewing gum in the presence of postoperative paresis [51]. There is evidence of a positive effect on the motility of the gastrointestinal tract of the use of probiotics in the pre- and postoperative period in surgical patients [42].
Many authors propose various drug regimens to resolve postoperative paresis [13, 18, 24].
When evaluating drug therapy aimed at stabilizing gastrointestinal motility in conditions of paresis, it was noted that many drugs are ineffective and have side effects [34]. In clinical practice, anticholinesterase drugs (prozerin, ubretide, etc.) remain the main ones for the treatment of paresis. Their effectiveness is not always clear, and side effects are pronounced. Thus, prozerin and its analogues have a negative inotropic and chronotropic effect on the heart and, therefore, are contraindicated in bronchial asthma, angina pectoris, and bradycardia. In addition, the effect of proserin on the smooth muscles of the stomach and small intestine is short-lived, and it has no effect on the colon [14, 41]. D.B. Zakirov [14] notes that prozerin does not coordinate impaired intestinal motility, unlike ubretide, which significantly increases the electrical activity of all parts of the gastrointestinal tract and improves their rhythm. There is also evidence of a positive effect on the motility of the gastrointestinal tract of the use of besacodyl in patients who have undergone colon surgery.
Extensive reconstructive operations on the abdominal organs inevitably lead to irritation of interoreceptors, therefore the use of ganglion blockers is pathogenetically justified [17, 24]. The use of temporary ganglioplegia with pentamine [25] in combination with traditional methods of treating intestinal paresis in patients with widespread purulent peritonitis can improve microcirculation by restoring sympathetic influences and increase the efficiency of central hemodynamics. By normalizing the parasympathetic effects of the autonomic nervous system, it is possible to restore the motor-evacuation function of the gastrointestinal tract earlier and, as a result, reduce the severity of the systemic inflammatory response syndrome and abdominal pain syndrome. It should be noted that the pronounced vasoplegic effect of these drugs is an obstacle to their use in seriously ill patients who are prone to hypotension. Metoclopramide (cerucal) is widely used for the treatment of postoperative paresis, but after in-depth study it turned out that it reduces the total electrical activity mainly of the stomach and small intestine, inhibiting their contractile activity and promoting the restoration of contractions of the duodenum [14].
Analysis of the results of pharmacological treatment of postoperative paresis showed that a positive effect of adrenergic blockers, cholinomimetics and anticholinesterase drugs on gastrointestinal motility can be expected only with mild and moderate paresis.
Many works are devoted to the effect of serotonin on the motor-evacuation function of the gastrointestinal tract. PC. Klimov [15] found that serotonin at a dose of 0.1 mg/kg causes strong peristaltic activity of the stomach and small intestine. The results were confirmed by electrophysiological and radiological studies.
In the work of A.P. Simonenkova [26] proposed a method for treating postoperative paresis of the gastrointestinal tract with serotonin adipate, which is a natural biologically active substance that promotes the contraction of muscle cells, bypassing the autonomic nervous system. According to the author, after intramuscular administration of serotonin adipate at a dose of 0.2-0.3 mg/kg, the electrical activity of the jejunum increases and a more orderly and stable rhythm of contractile activity of the small intestine is observed.
Under the influence of serotonin, intestinal peristalsis is activated. N.S. Tropskaya et al. [32] after administration of serotonin adipate into the cavity of the small intestine in the early stages after operations on the abdominal organs, a spread of contractile activity from the stomach to the jejunum was observed, while the period of normalization of all parameters of gastrointestinal motility was reduced from 7 to 4 days.
Clinical observations describe the positive experience of using serotonin adipate when administered intravenously in an amount of 20-60 mg/day in the early stages of the postoperative period to restore peristalsis in functional intestinal obstruction. In this case, the duration of drug administration ranged from 2 to 5 days, satisfactory clinical results were obtained associated with the rapid normalization of intestinal motility [28, 30].
In recent years, electrical stimulation of the gastrointestinal tract has attracted particular interest [23, 63, 65]. The basis for its use was fundamental physiological research, which proved that smooth muscle cells are electrically excitable and have an electrical rhythm [15], which can be controlled [7, 11, 19]. However, some authors believe that the literature data regarding the use of electrical stimulation for the treatment of postoperative paresis is not yet very encouraging [46].
The correspondence between the periodicity of changes in bioelectrical activity and the rhythm of peristaltic activity of the human stomach has been established. It has been shown that each section of the gastrointestinal tract has its own electrical rhythm, which is normally a constant value and can change under pathological conditions [35, 47].
Thus, the used physical and medicinal methods of conservative treatment of patients with postoperative intestinal paresis are not always effective. Apparently, the reason for this is unreasonably chosen drugs from the point of view of pathogenesis or drugs that give a positive effect for a short period of time and can sometimes only aggravate the patient’s condition. In our opinion, the use of serotonin for postoperative intestinal paresis is the most pathogenetically justified and can help improve treatment results.
Symptoms
Symptoms of intestinal paresis are similar to poisoning and manifestations of diseases of the gastrointestinal tract. Pain during intestinal paresis is colicky in nature, increased gas formation leads to an increase in the intensity of the pain syndrome, which does not have a clear localization. A characteristic symptom of this pathological condition is the passage of a small amount of stool with a liquid consistency.
The main manifestations of intestinal paresis after surgery are:
- uniform bloating;
- nausea;
- vomit;
- absence of bowel sounds;
- exicosis (dehydration);
- tachycardia (rapid heartbeat);
- secondary respiratory failure.
At the beginning of the disease, the vomit contains food eaten and gastric juice. Over time, the vomit becomes fecal in nature. About 40% of patients complain of constipation and lack of gas discharge. In the other half, gases and feces can pass even after the development of clinical intestinal paresis. Low-grade fever (increase in body temperature to 37.0-37.1 degrees) is observed in no more than half of the patients. Most often this indicates the presence of complications (perforation of the intestinal wall, peritonitis).
Significant bloating against the background of intestinal paresis leads to displacement of the diaphragm and compression of the organs of the chest cavity. Clinically this is manifested by the following symptoms:
- shortness of breath;
- shallow breathing;
- tachycardia;
- decrease in blood pressure.
Prolonged vomiting against the background of intestinal paresis can lead to dehydration, which is manifested by dry mucous membranes and skin, and a decrease in the rate of diuresis. Patients' abdominal circumference increases. On palpation, the abdomen is moderately painful; upon auscultation, a significant decrease in intensity or complete absence of bowel sounds is determined. Against the background of complete silence, respiratory sounds and heart sounds are heard in the abdominal cavity. Digital examination of the rectum reveals a dilated and empty rectal ampulla.
If these symptoms appear, you should contact a gastroenterologist or surgeon at the Yusupov Hospital for diagnostics.
Make an appointment
Diagnostics
Clinical manifestations of postoperative paresis are determined by a surgeon and gastroenterologist. When diagnosing pathology, various methods are used, including x-ray examination of the abdominal organs. The results of the study are reflected in the picture. Intestinal paresis is characterized by the absence of mechanical causes of obstruction, a single-level arrangement of fluid, and the accumulation of gases in the large intestine.
The most informative methods for diagnosing the disease are:
- multislice computed tomography;
- Ultrasound.
These procedures make it possible to determine the horizontal fluid level, as well as distended intestinal loops, due to the fact that the organs are scanned by the device in two- and three-dimensional images. The Yusupov Hospital is equipped with high-precision European equipment that allows detecting intestinal motility disorders.
Treatment
Treatment of patients with intestinal paresis is carried out in the intensive care unit of the Yusupov Hospital. Begin therapy with conservative measures:
- unloading the intestines by removing gases using a rectal gas tube and a thick gastric tube;
- cancellation of enteral load;
- treatment of the underlying disease (the cause of intestinal paresis);
- correction of water-electrolyte and metabolic disorders.
As measures that improve the patient's condition and accelerate the resolution of paresis, chewing gum is used (there are a number of scientific works in the field of gastroenterology that indicate stimulation of peristalsis during chewing), the patient's knee-elbow position, and moderate physical activity.
Conservative therapy includes drug stimulation of peristalsis with neostigmine. There are three techniques for non-surgical intestinal decompression:
- insertion of a thick probe under x-ray control;
- percutaneous puncture of the cecum and cecostomy;
- colonoscopy followed by drainage insertion.
These techniques are used in the presence of the following indications:
- an increase in the diameter of the large intestine by more than 100 mm;
- duration of intestinal paresis for more than three days in combination with the lack of effect from conservative therapy for 48 hours;
- lack of positive dynamics from treatment with neostigmine or the presence of contraindications to its use.
The method of choice for intestinal paresis is colonoscopy. The procedure is not performed for peritonitis or intestinal perforation. Isolated colonoscopy is effective in 25% of patients, while the combination of colonoscopy with the introduction of drainage tubes is effective in almost 90% of cases. Percutaneous cecostomy is performed in patients with a high risk of complications during surgery, when conservative therapy and colonoscopy with intestinal decompression are ineffective.
Open surgery is used in the absence of the effect of all of the above measures, in the presence of intestinal perforation and peritonitis. Surgeons perform open cecostomy, resection of the affected part of the intestine. After surgical treatment, narcotic analgesics are not prescribed, as they can inhibit the motility of the intestinal tube.
1.What is gastroparesis and its causes?
After eating, the stomach normally empties within one and a half to two hours. But in people with gastroparesis, this process takes much longer. The way food is digested changes, and this can cause unpleasant and sometimes very serious symptoms. One of the most serious complications of gastroparesis is bezoar.
. A condition where due to gastroparesis, food remains in the stomach for a very long time and forms into a hard lump that gets stuck in the stomach. However, the situation becomes so serious very rarely.
Causes of gastroparesis of the stomach
Gastroparesis occurs when the nerves in the stomach area are damaged or otherwise fail to function. Diabetes
– one of the most common causes of gastroparesis.
Other factors in the development of gastroparesis may include certain nervous system disorders
(Parkinson's disease and stroke) and
medications
(such as cyclic antidepressants, calcium antagonists, opiate pain relievers). Gastroparesis can result from complications after gastric surgery.
A must read! Help with treatment and hospitalization!
Rehabilitation
When working with patients diagnosed with intestinal paresis, doctors at the Yusupov Hospital develop not only a therapeutic complex, but also a rehabilitation program. Experienced rehabilitation therapists stimulate intestinal activity with abdominal massage. In addition, the patient is prescribed a special diet during the recovery period to reduce the load on the intestines.
The Yusupov Hospital provides high-quality medical care and maintains a high level of service. Modern equipment and highly qualified specialists ensure prompt diagnosis of the disorder and determination of the most rational methods of therapy. An important advantage of a multidisciplinary clinic is the absence of queues and quick appointments by phone.
Make an appointment
conclusions
1. The use of domperidone in the form of the drug Brullium Lingvatabs for 5 days for the preventive treatment of postoperative intestinal paresis in gynecological practice reliably contributes to the rapid relief of symptoms and improvement of the general well-being of patients.
2. The proactive nature of the proposed pathogenetically based program for the prevention and treatment of intestinal paresis contributes to the early restoration of the functions of the gastrointestinal tract and prevents the development of complications in surgical gynecology.
3. The study allows us to recommend the drug Brullium Lingvatabs for routine use in the prevention of PPC in the early postoperative period.