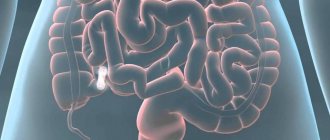
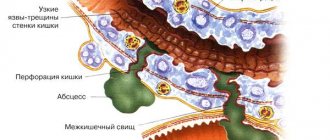
Paraproctitis is inflammation of the tissues near the rectum (pararectal tissue) due to the penetration of infection into them. An abscess (a limited space with purulent contents) often forms acute paraproctitis . An abscess of perirectal tissue can break out on its own, but the process often becomes chronic, forming rectal fistulas (chronic paraproctitis) .
Paraproctitis is one of the most common proctological diseases, the only more common ones are hemorrhoids, anal fissure and colitis. The most common causes of paraproctitis are infection of the perirectal tissue, most often through the rectal mucosa (microtraumas and cracks in the mucosa due to constipation, hemorrhoids, etc.). d.).
Reasons for development
There are many factors that can contribute to the development of pathology. Among them:
- Frequent bowel disorders or constipation
- Infectious bowel diseases, E. coli
- STD
- Haemorrhoids
- Traumatization of the rectum
- Untreated anal fissures
- Colitis
- Crohn's disease
- Decreased immunity
- Poor nutrition – predominance of fatty and spicy foods in the menu
- Alcohol abuse
- Specific physical activity, lifting heavy objects
- Failure to comply with hygiene standards
The combination of reasons determines the main category of patients of the proctologist - most often men aged 25-50 years turn to the doctor with paraproctitis.
Causes
Risk factors • Constipation or prolonged diarrhea • Prolapse and strangulation of hemorrhoids • Inflammatory diseases of the rectum (nonspecific ulcerative proctitis, Crohn's disease) • History of perirectal abscess. Pathomorphology • Inflammation of the Morganian crypt and anal glands • Inflammation of the cellular spaces with the formation of purulent cavities • Inflammatory infiltration and cicatricial changes around the ulcers and along the communication with the lumen of the rectum, in the long term - epithelization of the fistulous tract.
Symptoms of acute paraproctitis:
Symptoms of acute paraproctitis depend on the location of the source of inflammation and other factors. The most characteristic symptoms:
- Pain in the anal area; pain may intensify during bowel movements, physical activity, prolonged sitting, etc.; pain can be localized not only in the rectum, but also in the lower abdomen
- Deterioration of general condition - low-grade fever (37-38), weakness, loss of appetite
- Redness of the skin, swelling and tightening of tissue in the anus, sharp pain when pressed (when located in the subcutaneous tissue space)
With acute paraproctitis, symptoms may increase (pain intensifies, general health continues to deteriorate). In some cases, the condition improves dramatically, with pus and blood appearing in the stool. This means that the abscess has broken into the rectum. If there is a breakthrough or improper treatment of acute paraproctitis, there is a high risk of the disease becoming chronic. In addition, paraproctitis is dangerous due to the spread of purulent inflammation to the pelvic organs, abdominal cavity, and other serious complications.
Publications in the media
Acute paraproctitis (AP) is a fistulous form of abscess of peri-rectal tissues. The primary inflammatory focus is localized in the anal crypts (acute anal cryptitis). Subsequently, pus spreads through the ducts of the anal glands. The predominant age is 20–60 years; it occurs rarely in children. The predominant gender is male (7:3). Classification • By localization of abscesses, infiltrates, leaks: •• Submucosal - located under the mucous membrane of the rectum •• Subcutaneous - located under the skin of the perianal region and anal canal •• Ischiorectal (ischiorectal) - located below the levator ani muscle; accessible to palpation •• Pelviorectal (pelviorectal) - located above the levator ani muscle; •• Posterior rectal (retrorectal) - located in the presacral tissue• By the nature of the infection: •• Vulgar AP •• Anaerobic AP •• Specific AP (tuberculous, syphilitic, actinomycotic). Etiology and pathogenesis • Penetration of infection into the perirectal tissue from the ducts of the anal glands. The connection of the abscess with the rectum causes constant or periodic infection of the perirectal tissue • Microtrauma of the mucous membrane of the rectum and anal canal by undigested food particles, dense lumps of feces, foreign bodies; microtraumas are also possible as a result of therapeutic manipulations - enemas, pararectal procaine blockades, injections of sclerosing drugs • Hematogenous and lymphogenous routes of infection into pararectal tissue (rarely) • Mixed microflora: staphylococcus and its associations, gram-negative and gram-positive rods and their associations, monobacterial flora • Specific APs due to tuberculosis, actinomycosis, and syphilis are characterized by a torpid course and an infiltrative type of spread.
Risk factors • Constipation or prolonged diarrhea • Prolapse and strangulation of hemorrhoids • Inflammatory diseases of the rectum (nonspecific ulcerative proctitis, Crohn's disease) • History of perirectal abscess. Pathomorphology • Inflammation of the Morganian crypt and anal glands • Inflammation of the cellular spaces with the formation of purulent cavities • Inflammatory infiltration and cicatricial changes around the ulcers and along the communication with the lumen of the rectum, in the long term - epithelization of the fistulous tract.
Clinical picture • Subcutaneous AP •• Rapidly increasing pain in the perineum, at the anus •• Increased body temperature (in the evening 38–39 ° C) •• Retention of stool; when the abscess is located in front of the anus - dysuria •• Palpation of the inflammatory infiltrate - sharp pain, fluctuation •• Pain during digital examination of the rectum. Pain and induration on endorectal palpation of the crypt, which was the source of AP. • Submucosal AP •• Moderate pain in the rectum, aggravated by defecation •• Low-grade body temperature •• Pus can break into the lumen of the rectum, in this case the disease ends in recovery •• Digital examination of the rectum - a painful rounded tight-elastic formation located under the mucosa shell above the dentate line. • Ischiorectal (ischiorectal) paraproctitis •• The disease begins gradually, there is a deterioration in general condition, weakness, sleep disturbances •• Later, a feeling of heaviness and constant sharp pain in the rectum and deep in the pelvis appear •• Body temperature rises to 39–40 ° C, chills •• When the inflammatory process is localized in the area of the prostate gland and urethra - dysuric disorders. • Pelvic-rectal (pelviorectal) AP •• Deterioration of general condition (fever, chills, weakness, loss of appetite) in the complete absence of pain. The duration of this period is 1–3 weeks •• With the appearance of an abscess, the disease takes an acute course: dull pain in the rectum and deep pelvis, accompanied by intoxication, hectic body temperature, stool retention, tenesmus •• Diagnosis - digital examination of the rectum. • Posterior rectal (retrorectal) AP •• From the very beginning of the disease, severe pain syndrome localized in the rectum and sacrum appears, intensifying with defecation and in a sitting position, dysuria •• Digital examination of the rectum - a sharply painful bulge in the area of its posterior wall. Laboratory research. CBC (leukocytosis, shift of the leukocyte formula to the left), bacterial examination of the purulent contents of the abscess, pathohistological examination of the scar tissue of the abscess capsule. Special studies • A test with a dye allows you to detect the connection of the abscess cavity with the intestinal lumen by puncture filling it with dye (usually 1% methylene blue solution) after preliminary emptying of pus • Probe test - passing a metal button probe through the abscess cavity into the intestinal lumen through the existing communication in order to detect the entrance gate of infection, as well as to establish the relationship of the fistulous tract to the sphincter fibers.
Differential diagnosis • Suppuration of pararectal dermoid cyst • Tumor of the sacrum • Abscess of the pouch of Douglas • Suppuration of the epithelial coccygeal tract • Purulent-inflammatory diseases of the skin of the perineum (abscess, boil, suppurating atheroma). TREATMENT. The main method is surgical. The operation must be performed immediately after diagnosis. The diet is easily digestible and slag-free. After surgery, a diet rich in plant fiber and plenty of fluids is prescribed. Regimen • Hospitalization in the department of purulent surgery or coloproctology to perform surgery under general anesthesia • The regimen of patients after surgery is generally active, but depends on the method of the operation performed.
Surgical treatment • The main surgical technique at present is opening the purulent cavity into the intestinal lumen. • Stages of the operation: opening of the abscess with an incision tangential to the fibers of the anal sphincter, revision of the purulent cavity, identification of communication with the “causal” crypt, excision of the affected crypt (cryptectomy) and tissue along the fistula according to the Gabriel operation, drainage of the wound. The wound in the anal canal and perianal area after radical opening of the OP into the intestinal lumen, as a rule, has the appearance of a “keyhole”. However, the configuration of the incisions in the perineum and in the anal canal may vary depending on the presence of purulent leaks. • In case of high transsphincteric and extrasphincteric communication of the purulent cavity with the lumen of the rectum, two-stage surgical treatment is possible, and at the first stage only the opening and drainage of the abscess cavity from the semilunar incision in the perineum is performed. Subsequently, after the formation of a fistula, surgical treatment is carried out as planned (see Chronic paraproctitis). In case of submucosal paraproctitis, the purulent cavity is opened from the lumen of the rectum with a linear vertical incision, also with excision of the affected crypt. With severe retrorectal and pelviorectal paraproctitis, infection may spread through the pelvic tissue spaces (pelvic cellulitis, retroperitoneal phlegmon). In such cases, additional drainage of the pelvic and retroperitoneal spaces is performed. Management in the postoperative period • Postoperative treatment after radical (or palliative) surgery must be carried out taking into account the phases of the wound process: in the first phase, before cleansing the wound surface, sorbents (application sorption), hydrophilic ointments (for example, Levosin) are used, and in the second, when granulations appear, fatty or jelly-like ointments are effective • Local ozonation, laser and UV irradiation of the surface of wounds, ultrasonic cavitation are effective • On the 3rd day after surgery, the patient is prescribed 20–30 g of castor oil during the day and at night.
Drug therapy • Antiseptics: solutions of hydroxymethylquinoxylindioxide, hydrogen peroxide, nitrofural, aqueous solution of chlorhexidine • Suppositories with methyluracil • Ointments “Levosin”, “Actovegin” • Vaseline or castor oil orally • Antibacterial agents are indicated for severe general reactions (intoxication syndrome with high body temperature), as well as in patients with diabetes. Outpatient observation . Regular physical examination in the postoperative period until the postoperative wound is completely healed and the function of the anal sphincter is restored. Complications • After the usual opening of an abscess during AP, without eliminating its internal opening, 30–50% of patients subsequently develop rectal fistulas or relapse of AP • Insufficiency of the anal sphincters (associated with the suppurative process affecting the anal sphincters, or the surgical technique) • Recurrence of the abscess if the “causal” crypt was not excised. Current and prognosis . Long-term results depend on the form of AP, timing and methods of surgical intervention. Prevention • Prevention of constipation • Hygiene of the perianal area • Early diagnosis and timely treatment of purulent-inflammatory diseases of the pelvic organs • Compliance with the technique of performing enemas and other transanal medical procedures. Age characteristics • Children. Occurs more often in newborns and infants • Elderly. Often occurs against the background of chronic colostasis. Synonyms • Anorectal abscess • Cryptoglandular abscess Abbreviation. OP - acute paraproctitis
ICD-10 • K61 Abscess of the anus and rectum.
Chronic paraproctitis (rectal fistulas)
If acute paraproctitis is not treated correctly, the abscess breaks out on its own, or under the influence of other factors, the internal opening of the abscess forms a fistula. A perianal fistula is a thin canal that connects the anus to an opening in the skin near the anus. Chronic paraproctitis is characterized by stages of remission and exacerbation.
Pus can accumulate in the lumen of the fistula, causing swelling and pain. The fistula can drain (break through) on its own, in which case the symptoms disappear for a while and return when the lumen of the fistula becomes clogged again. Periodic exacerbations occur due to constant infection of the fistula with pathogenic flora of the rectum.
Short description
Acute paraproctitis (AP) is a fistulous form of abscess of peri-rectal tissues. The primary inflammatory focus is localized in the anal crypts (acute anal cryptitis). Subsequently, pus spreads through the ducts of the anal glands. The predominant age is 20–60 years; it occurs rarely in children. The predominant gender is male (7:3).
Code according to the international classification of diseases ICD-10:
- K61 Abscess of the anus and rectum
Classification • By localization of ulcers, infiltrates, leaks: •• Submucosal - located under the mucous membrane of the rectum •• Subcutaneous - located under the skin of the perianal region and anal canal •• Isciatic-rectal (ischiorectal) - located below the levator ani muscle; accessible to palpation •• Pelvic - rectal (pelviorectal) - located above the levator ani muscle; •• Posterior rectal (retrorectal) - located in the presacral tissue• By the nature of the infection: •• Vulgar AP •• Anaerobic AP •• Specific AP (tuberculous, syphilitic, actinomycotic). Etiology and pathogenesis • Penetration of infection into the perirectal tissue from the ducts of the anal glands. The connection of the abscess with the rectum causes constant or periodic infection of the perirectal tissue • Microtrauma of the mucous membrane of the rectum and anal canal by undigested food particles, dense lumps of feces, foreign bodies; microtraumas are also possible as a result of therapeutic manipulations - enemas, pararectal procaine blockades, injections of sclerosing drugs • Hematogenous and lymphogenous routes of infection into pararectal tissue (rarely) • Mixed microflora: staphylococcus and its associations, gram-negative and gram-positive rods and their associations, monobacterial flora • Specific APs due to tuberculosis, actinomycosis, and syphilis are characterized by a torpid course and an infiltrative type of spread.
Treatment of paraproctitis
The most effective method of treating both acute paraproctitis and rectal fistula is surgical.
The operation is performed under anesthesia. Local anesthesia is not used in this case, since complete anesthesia of the surgical field and muscle relaxation are extremely important. During the operation, the abscess is opened, and the patient is drained of the pus. However, surgical treatment does not end there if we are talking about a chronic form of the disease - it is important to eliminate not only the abscess, but also the fistula itself. This cannot always be done at the time of active inflammation. Therefore, in some cases, two operations are performed - one to open the abscess, the second to excise the fistula. Sometimes, as part of preoperative preparation, the patient is prescribed a course of anti-inflammatory and antibacterial therapy; physiotherapeutic methods have also proven themselves to be effective.
At the Treatment and Diagnostic Center of the Central Clinical Hospital of the Russian Academy of Sciences, operations for acute paraproctitis and excision of a fistula are carried out in the operating room of a day hospital. The use of modern anesthetics eliminates discomfort during surgery. After the intervention, the person is under the supervision of a doctor for several hours, after which he can go home independently, having received the necessary recommendations for postoperative treatment.
How long does the healing process take?
In the first week after surgical treatment of a fistula, the patient may experience moderate pain, which can be controlled with painkillers. The period of forced disability is minimal. After surgical treatment of a fistula or paraproctitis, a period of follow-up treatment at home is necessary using sitz baths 3-4 times a day. It is recommended to add dietary fiber or laxatives to the diet. To prevent contamination of underwear, you can use gauze bandages or pads. Normal stool has no effect on wound healing.
Consultation with a proctologist
Any suspicion of paraproctitis is a reason for immediate contact with a proctologist and immediate surgery if the diagnosis is confirmed. If treatment is not carried out or the source of infection is not eliminated, paraproctitis becomes chronic and a fistula tract is formed over time. During the consultation, the proctologist will conduct a diagnosis through palpation and visual examination. Determine the severity of the condition and recommend the most effective way to combat the disease.
Diagnostics
Differential diagnosis • Suppuration of pararectal dermoid cyst • Tumor of the sacrum • Abscess of the pouch of Douglas • Suppuration of the epithelial coccygeal tract • Purulent - inflammatory diseases of the skin of the perineum (abscess, boil, suppurating atheroma). TREATMENT. The main method is surgical. The operation must be performed immediately after diagnosis. The diet is easily digestible and slag-free. After surgery, a diet rich in plant fiber and plenty of fluids is prescribed. Regimen • Hospitalization in the department of purulent surgery or coloproctology to perform surgery under general anesthesia • The regimen of patients after surgery is generally active, but depends on the method of the operation performed.
Predisposing factors
A number of factors contribute to the development of acute paraproctitis:
- chronic proctological diseases (hemorrhoids, cryptitis, anal fissure, etc.);
- functional gastrointestinal disorders (constipation, diarrhea);
- immune-mediated chronic bowel diseases (ulcerative colitis and Crohn's disease);
- vascular changes caused by diabetes mellitus;
- decreased immune defense due to the patient having a chronic infection, immunodeficiency, or due to severe hypothermia;
- poor personal hygiene of the perineal area, for example, with a spinal cord injury.
Types and stages of the disease
Rectal fistulas can be complete, incomplete, or internal.
- Complete ones
have two openings - located inside and opening into the rectum, as well as external, usually located next to the anus. - An incomplete fistula
has only an internal opening that opens into the rectum. There is an opinion that this is a transitional phase, because sooner or later the external opening will open and the fistula will become complete. - An internal fistula
has two internal openings that extend into the lumen of the rectum.
According to their location relative to the anal sphincter, fistulas are divided into:
- Intrasphincteric
, or marginal. The most common ones usually have a straight course without scars and an external opening near the anus. - Transsphincteric
- the course of such a fistula goes through the anal sphincter at different depths. The higher the course, the more it branches and the more often purulent streaks form, and scar tissue forms around the fistula itself. Scars can deform the anal sphincter and interfere with its function. - Extrasphincteric
- differ in the location of the internal opening - on the surface of the intestinal crypt. The stroke itself passes high and goes around without affecting the outer pulp. The incidence of such fistulas is 15-20% of the total incidence.
Extrasphincteric fistulas are usually tortuous and have a long course. With them, purulent leaks form and scars form around the fistula canals. With repeated exacerbations, new external openings appear. There is a classification that distinguishes four degrees of complexity of fistulas of this type:
- – there are no scars around the internal opening, the course is straight, infiltrates and purulent streaks do not form in the perirectal tissue.
- – scars appear around the internal opening, there are still no ulcers or infiltrates.
- – the entrance to the fistula canal is narrowed, there are no scars, there are ulcers and inflammatory infiltrates in the perirectal tissue.
- – the inlet is enlarged, surrounded by multiple scars, and there are ulcers and infiltrates in the tissue.
Outcomes, complications and prognosis
Acute paraproctitis
- 1. In case of acute paraproctitis, in case of failure to seek medical help and, accordingly, failure to perform surgical intervention, the following development of events is possible:
- The first option is the generalization of the purulent process in the soft tissues of the small pelvis, the development of sepsis and death.
- The second option, which, fortunately, happens more often, is the independent opening of the abscess through the lumen of the rectum or the skin of the perianal area and the formation of a fistulous form of paraproctitis. In this case, the patient will require planned surgical intervention aimed at eliminating the abscess.
- 2. If the patient contacts a proctologist in a timely manner and opens the abscess with simultaneous elimination of the internal hole, according to medical statistics, complete recovery is noted in 80 - 90% of cases (that is, re-intervention is not required). Only 10–20% of patients develop a fistula (which will subsequently require surgery).
Chronic paraproctitis
This disease is characterized by temporary remissions. How does this happen? The fistula tract along one or another length is obliterated (closed by its own tissue, which acts as a “plug”). During such periods, the patient believes that he has been cured (although the external fistula remains, but no pus comes from it). However, pathogenic microorganisms continue to penetrate through the internal opening of the fistula and the abscess re-forms, which sometimes returns in a more severe form. The long-term existence of a chronic form of paraproctitis leads to the formation of rough scars that deform the anus and a decrease in the strength of the rectal muscle, which, in turn, can lead to impaired fecal continence. Also, in patients with a long history of the disease, a decrease in immunity is detected, since the body is forced to constantly “fight” the source of chronic infection, and the constant discomfort associated with the presence of a functioning fistula reduces the quality of life and leads to depression. Such severe consequences can be avoided. The prognosis for successful recovery after surgical treatment of the fistulous form of paraproctitis is favorable - more than 90% are cured.
The sooner a patient seeks medical help, the better. Long-term “untreated” paraproctitis, which the patient “endures” at home, leads to the formation of severe clinical cases, which seriously complicates treatment and worsens the prognosis.
Causes of paraproctitis
In most cases, paraproctitis is caused by mixed microflora. Most often, staphylococci and streptococci are found in combination with Escherichia coli and Proteus.
Predisposing factors in the occurrence of a purulent process in the perirectal tissue:
- weakening of local and humoral immunity due to exhaustion, alcoholism, due to acute or chronic infection (sore throat, flu, sepsis);
- vascular changes in diabetes mellitus and atherosclerosis;
- functional disorders (constipation, diarrhea);
- the presence of hemorrhoids, anal fissures and cryptitis.
Paraproctitis development mechanism
The anatomical boundary between the rectum and the anal canal runs along the anorectal line. At this level there are Morgani crypts - “pockets”, the bottom of which is located approximately on the border of the upper and middle third of the anal canal. At the bottom of the crypts, the excretory ducts of the anal glands open.
Anal glands are channels into which infection can penetrate from the lumen of the rectum. If a blockage of the gland duct occurs due to swelling of the rectal mucosa (with diarrhea), microtrauma or cicatricial changes in the excretory ducts (transferred cryptitis), then acute inflammation of the group of anal glands that open into the crypt (crypts) may develop and, as a result, the occurrence of a microabscess in the wall of the anal canal.
A microabscess that arises as a result of inflammation of the gland is initially localized in the crypt area, does not extend beyond the internal sphincter and, under favorable circumstances, can empty itself through the crypt. But if the abscess spreads deeper into the intersphincteric space, then paraproctitis occurs. Along the partitions of the intersphincteric space, pus can flow in different directions, causing the formation of ulcers in the perirectal cellular spaces.
According to the location of the lesion, they distinguish subcutaneous, submucosal, intermuscular, ischiorectal (ischiorectal), pelvic-rectal (pelviorectal) and, as one of the types of pelvic-rectal, retrorectal abscesses.
According to the localization of the crypt involved in the inflammation process, paraproctitis can be posterior, anterior or lateral. In first place in terms of frequency is posterior paraproctitis, in second place is anterior paraproctitis; crypts on the lateral walls are least often affected. More frequent damage to crypts along the posterior wall can be explained by the fact that the posterior crypts are deeper and less drainable; they are more often injured by hard feces due to the more rigid fixation of the intestinal wall along the posterior semicircle.