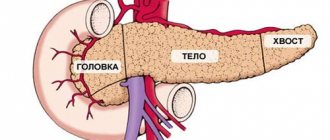
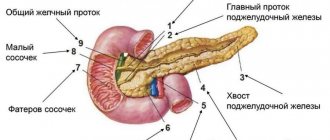
Benign neoplasms of the pancreas are a group of tumor diseases based on the pathological process of uncontrolled cell division of the tissues of the pancreas and its ducts, characterized by a benign course.
The main diseases of this group are insulinomas (tumors originating from glandular tissue that produces the hormone insulin), fibromas (from connective tissue), hemangiomas (benign neoplasms growing from blood vessels), and cystic formations.
Benign pancreatic tumors are quite rare. In general, in the general statistics of all types of pancreatic tumors, benign tumors account for approximately 0.01 to 0.1%. Men and women suffer from this disease with equal frequency.
Causes
The causes of the development of diseases in this group are very diverse. Among the main ones are the following:
- Smoking.
- Alcohol abuse.
- Metabolic diseases (obesity, type 2 diabetes).
- Poor nutrition (consumption of fast food, fatty and fried foods, lack of plant fiber in the diet).
- History of chronic inflammatory diseases of the pancreas (pancreatitis).
- Living in an unfavorable environmental environment, in conditions of high levels of asbestos, cadmium, benzene, petroleum products, soot, phenolic resins in the atmosphere; harmful working conditions (heavy dust, high temperature).
Symptoms and signs
Benign pancreatic tumors do not manifest themselves until they reach large sizes. The diagnosis is often made accidentally, during a routine ultrasound of the abdominal organs.
An exception is insulinoma: even with its small size, it causes disturbances in insulin secretion, and in patients the blood sugar level significantly decreases, which is accompanied by:
- increased appetite and rapid weight gain;
- weakness;
- an unreasonable feeling of fear;
- increased sweating;
- rapid heartbeat;
- episodes of dizziness, double vision, and sometimes loss of consciousness.
As the tumor grows and as a result of impact on adjacent structures, the following symptoms may be observed:
- Pain localized in the right or left hypochondrium, epigastrium (the area under the sternum), near the navel. They are often encircling in nature and can radiate to the back. Occurs regardless of food intake.
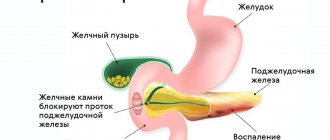
- Jaundice. The tumor disrupts the patency of the bile ducts, the outflow of bile is disrupted, which is accompanied by yellowing of the skin and sclera of the eyes, itching of the skin, discoloration of feces, and urine takes on the color of “strongly brewed tea.”
- Intestinal obstruction. As a result of the impact on the duodenum, its obstruction develops. The condition may be accompanied by nausea, vomiting, and a feeling of heaviness after eating.
Diagnostics
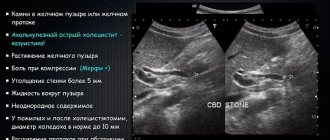
- Using imaging methods: ultrasound (ultrasound diagnostics), MRI (magnetic resonance imaging), PET-CT, the size of the tumor formation and its location are determined.
- Percutaneous biopsy of a pancreatic tumor under ultrasound guidance is an important method for determining the nature of the tumor process. A thin needle is inserted through the skin into the area of the pathological formation, and a tissue fragment of the pathological formation is taken. Subsequently, the test sample is sent for histological examination to detect/exclude cancer cells and determine the type of tumor.
- Endoscopic retrograde cholangiopancreatography (ERCP). A method for diagnosing the condition of the mucous membrane of the stomach, duodenum, and pancreas by introducing a thin endoscope equipped with an ultrasound sensor into the cavity of the esophagus. The method also allows you to assess the condition of the bile ducts (biliary system). A radiopaque substance is injected into the lumen of the common bile and main pancreatic duct (which open into the cavity of the duodenum). From the image transmitted to the monitor, one can determine the general patency and condition of the bile ducts, areas of narrowing, obstruction, and the presence of tumor growths.
- Diagnostic laparoscopy. An endoscope equipped with an optical device is inserted directly into the abdominal cavity. This imaging method also allows you to record tumor formations in the abdominal organs, determine their size, location, extent of the process (metastatic damage to neighboring organs) and take a biopsy.
What does the detection of a hypodensity formation mean?
If after a diagnostic procedure in the conclusion you were written that a hypodensity formation was detected, then this indicates the presence of a pathological process.
But hypodense formation is not the final diagnosis. Reduced tissue density may indicate the presence of:
- cysts filled with fluid;
- hemangiomas;
- other benign formations;
- primary cancer tumors;
- hypodensity of the kidney,
- hypodensity of the liver,
- hypodense formation of the thyroid gland,
- metastasis.
In order to find out what kind of pathology is taking place, it is necessary to conduct an additional examination. This may include laboratory tests, biopsy and other more specific methods that will accurately diagnose the disease.
Specialized clinic
Doctor: Klaus Thiemann, heart diagnostics
Heart treatment in Germany
9.9/10
Germany, Munich
German Heart Center (Osypka Herzzentrum Muenchen)
Heart treatment in Germany annually gives hope for recovery to thousands of patients in German clinics.
Submit a requestDoctor's profile
Specialized clinic
Doctor: Albert Eimiller. Gastroenterologist
Eimiller Gastroenterology Clinic
9.8/10
Germany, Munich
in Munich at the IZAR Medical Center
The Eimiller Gastroenterology Clinic is a department of gastroenterology headed by Dr. Albert Eimiller at the state-of-the-art medical center ISAR Klinikum, Munich.
Submit a requestDoctor's profile
Treatment of benign pancreatic tumors
- In some cases, observation tactics are chosen. If the tumor is characterized by very slow growth, is benign in its structure, does not lead to compression of blood vessels and does not affect neighboring organs, no therapeutic measures are taken, but the patient is under constant supervision by specialists.
- Surgical removal of the tumor. The scope of the upcoming operation depends on the size and location of the tumor, and includes both partial and extended (sometimes with excision of adjacent structures) resection of the pancreas.
- CyberKnife is a modern method of radiation therapy, in some cases an alternative to surgical treatment. The main advantage of this technique is the most accurate delivery of high doses of radiation directly to the tumor site. The method is unique in that it has a minimal set of side effects (compared to traditional radiation therapy or surgical treatment). The course of treatment using the CyberKnife device is only 1-5 sessions, each of which lasts no more than an hour.
how to sign up for cyber-knife treatment or pet-CT examination: - by calling the hotline - using the feedback form on the website.
call now!
Our consultants will answer all your questions, and experienced oncologists will determine the need for CyberKnife treatment.
See also: Cyber Knife treatment
Introduction
The high resolution of modern radiological diagnostic methods makes it possible to detect even small pancreatic tumors and with a high degree of probability to assume their morphological identity. Differential diagnosis of pancreatic tumors at the preoperative stage, in particular the identification of neuroendocrine neoplasia (NEN) of the pancreas, is of fundamental importance. This is due to different treatment tactics for ductal adenocarcinomas (DAC), cystic tumors, solid pseudopapillary tumors (SPPT) and NEN of the pancreas. Complex treatment of neuroendocrine tumors has undergone significant changes in recent years, which is associated with the expansion of indications for surgical treatment, including minimally invasive ones, as well as with the advent of effective biotherapy and targeted therapy. The prognosis for the course of the disease in patients with NEN is more favorable, which makes it possible to perform organ-sparing operations for small tumors and not to refuse surgical care in case of an advanced process, as well as in the presence of distant metastases [6, 20, 31, 33].
Institute of Surgery named after. A.V. Vishnevsky is one of the leading centers for the diagnosis and treatment of pancreatic tumors in the Russian Federation, and this paper outlines our view on the fundamental differential diagnostic signs of pancreatic NEN.
Material and methods
At the Institute of Surgery. A.V. Vishnevsky in 1998-2011. 140 patients with morphologically verified pancreatic NEN were treated. Differential diagnostic criteria were built taking into account the results of an examination of 211 patients with a verified diagnosis of PAC of the pancreas, 18 with SPPO, 4 with metastases of renal cell carcinoma in the pancreas. A total of 373 patients were included in the study.
Solitary NEN of the pancreas was diagnosed in 129 patients. They were localized in the head in 58 (45%), in the body in 48 (37%) and in the tail of the pancreas in 23 (18%) patients. Multiple synchronous tumors of the gland were detected in 10 patients, in 1 patient a total lesion of multicentric NEN was detected. Non-functioning NENs were present in 117 (83.6%) patients, and functioning ones - in 23. Among the latter, insulinomas were diagnosed in 15 patients, gastrinomas - in 8 patients. The sporadic nature of the disease was determined in 115 (82.1%) patients, hereditarily predisposed in the structure of multiple endocrine neoplasia (MEN-1) - in 25 patients. The distribution of patients with NEN of the pancreas in accordance with the syndromes of hormonal hyperproduction and hereditary predisposition to the development of NEN is presented in Table. 1
.
The average age of patients with solitary NEN of the pancreas was 50.6±14.5 years. Patients with multiple pancreatic NENs were younger, their average age was 33.8±11.6 years. The distribution of patients depending on gender was with a slight predominance of women (54.1%).
Laboratory diagnosis was based on determining the level of chromogranin A, carcinoembryonic antigen (CEA) and carbohydrate antigen (CA19-9) in the blood. In patients with insulin-producing NEN of the pancreas, carbohydrate metabolism was monitored.
Radiation methods for examining patients included ultrasound (US) in combination with endosonography (endo-US), spiral and multispiral computed tomography (CT) with bolus intravenous enhancement, magnetic resonance imaging (MRI) with and without contrast enhancement.
In the topical diagnosis of organic hyperinsulinism for small tumors (up to 2 cm), angiography with arterial stimulated blood sampling (ASBS) was used. In accordance with the technique, blood was drawn from the right hepatic vein after arterial stimulation with a 10% calcium gluconate solution.
Results and discussion
The clinical picture of the disease in NEN of the pancreas depended on the functional state of the tumor. Functioning tumors (insulinomas and gastrinomas) had clear clinical symptoms corresponding to excessive synthesis of the hormone produced by the tumor, similar to those described in many publications.
Upon admission to the Institute of Surgery named after. A.V. Vishnevsky, 47.8% of patients with non-functioning NEN of the pancreas had no complaints. For the first time, a pancreatic tumor was detected in these patients during a preventive examination. In 8 out of 14 patients with MEN-1 syndrome, non-functioning pancreatic NENs were discovered during a targeted examination after detection of parathyroid hyperplasia/adenoma and/or pituitary adenoma. Clinical symptoms of the disease were absent in non-functioning small-sized NENs (24.5±12.8 mm). Large NEN (64.1±35.3 mm) were accompanied by symptoms characteristic of compression of adjacent organs (obstructive jaundice, etc.), a feeling of heaviness in the abdomen, and dull aching pain in the epigastric region.
The average concentration of chromogranin A in the blood of patients with NEN was 39.6±34.8 units/l and ranged from 3.5 to 107.5 units/l. The maximum value of the indicator was observed in a patient with metastatic liver disease. The majority of patients with NEN of the pancreas (G1 and G2 according to the 2010 WHO classification) are characterized by normal levels of CEA and CA 19-9. On the contrary, 80.5% of patients with AVR of the pancreas had a significant increase in the level of CA 19-9 and 42.6% had an increase in CEA. In 36.1% of patients, a synchronous increase in both markers was detected. There was no statistically significant dependence of the concentration of CEA and CA 19-9 on the degree of differentiation of pancreatic PAC. However, the level of CA 19-9 reliably reflected the extent of the tumor process. The average concentration of CA 19-9 in the blood of patients with AVR of the pancreas with and without liver metastases was 2183.3±644.8 and 52.4±48.9 units/ml. No differences in CEA concentrations in the blood were detected in these same patients.
The average glucose concentration in patients with insulin-producing NEN of the pancreas at the time of admission was 3.13±1.72 mmol/l. In patients with non-functioning NEN and PAC of the pancreas, this figure was 6.1±2.6 and 7.4±3.1 mmol/l, respectively.
During abdominal ultrasound, pancreatic NENs looked like solid (82.9%) focal formations of a round shape (64.1%) with an uneven and unclear contour (65.8%). In 7.7% of observations, the formations had multicentric growth. The structure of the NEN was more often hypoechoic (89.5%) and heterogeneous (78.9%). In 55.3% of cases, pancreatic NEN were avascular, in 31.6% - hypovascular, in 10.5% - moderately vascularized and in 2.6% - hypervascular. PAKs were visualized as hypoechoic structures, SPPOs were visualized as hypoechoic homogeneous or hypoechoic with a cystic-solid structure. During endosonography, pancreatic NENs looked like round or oval formations (77.8%) with clear (88.9%) uneven (55.6%) contours, solid (72.7%) or cystic-solid (27.3%) ) structure, visualized as alternating hyper-, hypo- and anechoic areas in the tumor structure. In 22% of patients with NEN, the pancreas had multicentric growth.
CT characteristics of pancreatic NEN depended on the size of the tumor and its functional state. Intraparenchymal (73.8%) neoplasms of round (66.7%) or irregular (28.6%) shape with clear contours (71.4%) were typical for functioning, active NEN of the pancreas (Fig. 1)
.
At the same time, the delayed phase can be effective in diagnosing poorly differentiated AVR, in which sclerotic fields intensively accumulate contrast material during the delayed phase of the study.
In 83.3% of cases, the structure of the formation was determined as soft tissue, in 16.7% - as cystic-solid. In some cases, calcifications were visualized in the tumor parenchyma. PACs of the pancreas were distinguished by a hypodense structure in the arterial phase. Rarely (10-15%) PAC has an isodense structure, but the delayed phase can help in the differential diagnosis. Non-functioning pancreatic NENs may be hypodense in the arterial phase, and this complicates their differential diagnosis with AVR and especially with SPPO of a homogeneous structure. SPPOs can be divided into three groups: 1) the homogeneous structure of SPPOs makes it difficult to differentiate from PAK and non-functioning NEN of the pancreas; 2) the cystic-solid structure of the SPBT is caused by necrosis and hemorrhages in the tumor; such a structure of the SPBT requires differential diagnosis with mucinous tumors; 3) the third form of SPPO is characterized by an increase in its size, massive calcifications, spread beyond the organ, and the presence of distant metastases, including in the liver. This form of SPPO requires differential diagnosis with a mucinous tumor and NEN with severe cystic transformation.
To determine the criteria for differential diagnosis of various morphological variants of tumor lesions of the pancreas, an analysis of the dynamics of differences in the accumulation of a contrast agent in the phase of examination of tumor tissue and gland parenchyma was carried out. Differences in the relative density of tumor tissue and pancreas in the study phases were calculated using the formula: x(t)
-
y(t)
, with
t
=
t
1,
t
2,
t
3,
t
4, where
x
is the absolute value of the relative density of tumor tissue,
y
is the absolute value of the relative density of pancreatic tissue,
t
1 - native,
t
2 - arterial,
t
3 - venous and
t
4 - delayed phases of the study.
The absolute values of the mean and standard deviation of the differences in the accumulation of the contrast agent in the study phase by tumor tissue and pancreatic parenchyma are presented in Table.
2 , from which it is clear that in the native phase of the NEN study, the pancreas is practically isodense or weakly hypodense to the gland parenchyma.
On the contrary, PAC, SPPO and metastases of renal cell carcinoma in the pancreas were visualized as hypodense neoplasms. There were no statistically significant differences in the relative density of these gland tumors. After bolus intravenous contrast, functioning pancreatic NENs became hyperdense in relation to the gland parenchyma in the arterial and venous phases of the study. The pronounced hyperdensity of renal cell carcinoma metastases in the arterial phase was leveled out in the venous and delayed phases of the study. RV PACs were markedly hypodense in the arterial and venous phases of the study. The optical density of PAK reached the density of the pancreatic parenchyma in the delayed phase or even exceeded it. SPPOs of a solid structure in all phases looked like hypodense or moderately hyperdense neoplasms. The identified differences in the relative density of tissue tumors in NEN, PAC and SPPO are statistically significant in the arterial and venous phases of contrasting (Fig. 2)
.
Figure 2. Dynamics of the average density of tumor tissue depending on the morphological variant of the tumor.
a — for neuroendocrine neoplasia (NEN) of the pancreas, ductal adenocarcinoma (DAC) of the pancreas, solid pseudopapillary tumor (SPPT); b — for neuroendocrine neoplasia of the pancreas, depending on the Grade criterion (G1 and G2, G3). The dynamics of contrast agent accumulation by pancreatic NEN tissue varied depending on the degree of malignancy of the neoplasia. G1 and G2 NEN of the pancreas are hyperdense in relation to the parenchyma in all phases of the study. The dynamics of contrast enhancement of neuroendocrine carcinomas (G3) were more consistent with those of pancreatic AVR (see Fig. 2)
.
On MRI, the majority of pancreatic NENs were identified as intraparenchymal neoplasms with clear contours (83.3% of observations). In 11.1 and 5.3% of cases, neoplasia was located extraparenchymatously or had a mixed growth pattern. 77.8% of neoplasms had a round shape. In 68.4% of cases, pancreatic NEN had a solid structure, and in 31.6%, a cystic-solid structure. In relation to the surrounding parenchyma in T1-weighted images, NENs were hypointense in 56.3% and practically isointense in 43.8% of cases; on T2-weighted images, NENs were usually hyperintense. On dynamic scanning with intravenous bolus contrast enhancement, these formations were hypervascular in relation to the gland parenchyma. Compared with PAC, the distinctive features of NEN were signal hyperintensity in the arterial and venous phases.
In our study, the sensitivity of comprehensive ultrasound was 82.8%, CT was 94.8%, MRI was 96.1%, and endosonography was 92.3%. Summary data from the literature on the informativeness of various instrumental methods in identifying tumor neoplasms of the pancreas and liver metastases in NEN of the pancreas are presented in table. 3
[3-5, 7, 8, 10, 11, 14-17, 19, 21-25, 27-30, 35].
Angiography (celiacography and upper mesentericography, return portography), supplemented by ASZK, turned out to be informative in all patients who underwent the study. As an example, we give a clinical observation.
Patient G.
, 25 years old, hospitalized at the Institute of Surgery named after. A.V. Vishnevsky with complaints of bouts of sweating, hunger, weakness, and loss of consciousness. The patient has been diagnosed since August 2011, when these complaints suddenly appeared. She was taken to the central district hospital at her place of residence in a hypoglycemic coma. After discharge, repeated loss of consciousness was noted; she was taken to the endocrinology department of the Central District Hospital at the place of residence with a glucose level of 1.3 mmol/l. An instrumental examination did not reveal any formations in the pancreas or abdominal organs. During the hospital stay, blood glucose levels ranged from 1.3 to 2.5 mmol/l.
During examination at the Institute of Surgery named after. A.V. Vishnevsky MRI and CT scan of the abdominal cavity did not reveal any convincing data on the presence of formations in the pancreas. Endo-ultrasound revealed a focal formation in the body of the pancreas, most likely NEN. There were no signs of intimate adherence of the tumor to large vessels of the upper abdominal cavity. In Fig. 3
presents a graph of changes in the concentration of insulin and C-protein in the blood during ASCH from the right hepatic vein and stimulation of various parts of the pancreas.
Figure 3. Angiogram with ASZK in a patient with organic hyperinsulinism (a) and intraoperative ultrasonogram (b).
a - graph of changes in the concentration of insulin and C-protein in blood taken from the right hepatic vein during stimulation of various parts of the pancreas. The maximum release was observed during stimulation of the body of the pancreas; b — pancreatic tumor is indicated by an arrow. A significant increase in insulin concentration was noted upon stimulation of the pancreatic body. The patient was operated on. Upon examination and palpation of the pancreas, the tumor was not detected and was detected during intraoperative ultrasound. Distal resection of the pancreas was performed using a traditional approach, preserving the spleen. Histological examination data: NEN G1 of the pancreas. Neoplasic cells express insulin. When discussing the issue of the rational scope of examination of patients with suspected NEN of the pancreas, it should be noted that there are no standards or Russian consensus on this issue, and it is impossible to extrapolate the experience of other countries to Russian reality. We are limited by the lack of routine availability of functional imaging techniques for neuroendocrine neoplasia. The European Association for the Study of Neuroendocrine Tumors (ENETS) recommends the use of ultrasound and CT for the initial diagnosis of pancreatic NEN, followed by somatostatin receptor scintigraphy and positron emission tomography combined with CT with specific tracers. Only after this is the issue of performing MRI, endosonography and other instrumental diagnostic methods resolved [9, 18]. Prospective studies have shown that somatostatin receptor scintigraphy has an informative value of 60-70% in identifying localized NEN, and in the presence of liver metastases - 92% [12, 13]. The sensitivity of somatostatin receptor scintigraphy is comparable to the total sensitivity of all traditional radiation diagnostic methods.
For all radiation research methods, including somatostatin receptor scintigraphy, an important criterion is the size of the tumor. Formations less than 1 cm are not detected in 50% of cases [2]. The greatest hopes are placed on positron emission tomography with specific tracers, such as 68Ga-DOTA,1-Nal(3)-octreotide, 68Ga-DOTANOC, 68Ga-DOTA, Tyr(3)-octreotate and 68Ga-DOTATATE [5, 15, 19, 23, 26, 34, 36]. The sensitivity of this method in visualizing NEN reaches 97% [32].
In the conditions of our country, to determine treatment tactics and subsequent monitoring of the course of the disease, we consider it advisable to perform complex ultrasound and multispiral CT as the main methods of screening and differential diagnosis of tumor diseases of the pancreas. Endosonography and MRI with bolus contrast should be used as additional methods. A great advantage of endosonography is the ability to perform a puncture with aspiration biopsy during the examination. MRI is a method that provides the possibility of subsequent long-term assessment of the tumor response to therapy in the absence of radiation exposure to the patient. For topical diagnosis of organic hyperinsulinism, ASZK is necessary. Combined X-ray diagnostics (angiography and arterially stimulated blood sampling) allows localizing a tumor or an area of β-cell hyperplasia in 96.8% of cases. A comprehensive examination of patients also requires laboratory diagnostics, which should include an assessment of the level of chromogranin A, hormones and peptides in accordance with the hyperfunctional syndrome (if any), as well as determination of the level of CEA and CA 19-9 in the blood for differential diagnosis with AVR of the pancreas [ 1].
Thus, the basis for differential diagnosis and diagnosis verification for NEN of the pancreas is the alertness and awareness of attending physicians about NEN of the pancreas. Radiation examination methods make it possible to diagnose pancreatic NEN with a high degree of probability in most cases. A CT or MRI with bolus intravenous contrast should be mandatory if a pancreatic tumor is suspected. Differential diagnosis between NEN, PAK, SPPO and metastases of renal cell carcinoma in the pancreas based on the native phase of CT, as well as on the “average phase”, when the contrast agent is administered intravenously “by hand”, is impossible. Patients with NEN of the pancreas should be under the supervision of a multidisciplinary team of doctors, including a surgeon, oncologist-chemotherapist, endocrinologist, radiology specialists and pathologist.