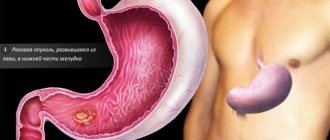
Gastric cancer (GC) is a global health problem. Despite advances in treatment, due to frequent recurrence and metastasis of tumors, the overall prognosis is unfavorable [1]. It is necessary to search for new therapeutic approaches to improve the clinical outcomes of GC. The cancer stem cell (CSC) model has been proposed to explain tumor heterogeneity, which determines the high relapse rate and resistance to systemic therapy [2]. CSCs have been identified in many solid malignant tumors, including cancer. Targeting the active minority of CSCs may increase the effectiveness of therapy [3].
Tumor heterogeneity
Most tumors are monoclonal in origin, but at the time of detection they consist of genetically, epigenetically and phenotypically heterogeneous clones. Two main concepts attempt to explain this heterogeneity: the cancer stem cell hypothesis and the clonal (stochastic) evolution model (Fig. 1).
The clonal evolution model assumes that each tumor cell has the ability to initiate tumor formation. Progression is directed by rare stochastic events occurring in all cells. Cells with mutations that confer a growth advantage will predominate over other tumor cells and may form a new clone containing cells of different phenotypes that have different proliferative potential [4–6].
The CSC theory, formulated in 1997 [7], is based on the assertion that in the structure of tumor cell populations there is a clear hierarchy, at the top of which there is a population of transformed cells that have properties similar to normal stem cells. CSCs are called tumor-initiating cells (TIC) or tumor-propagating cells (TPC) [8,9]. According to the concept, the RSC population:
- Makes up a small proportion of the total tumor cell population;
- Expresses a specific set of surface markers;
- Selectively supports the ability for tumorigenesis in contrast to other populations of tumor cells;
- Supports the growth of a heterogeneous mass containing a full complement of partially (or fully) differentiated cancer cells;
- Forms a separate pool of cells, identified by biological and physicochemical methods (at least two pools of cells in the tumor: CSCs and their derivatives - cells differentiated to varying degrees);
- Shows the ability, like normal stem cells, for unlimited self-renewal and differentiation in many directions [9];
- Shows high resistance to standard therapy [2].
Rice. 1 | Models of tumor heterogeneity. A - evolutionary stochastic model; B – hierarchical model of cancer stem cells [5].
Self-renewal of the gastric epithelium and gastric stem cells (GCs)
The structure of the human gastric mucosa is histologically heterogeneous. Fundal and antral gastric units are fundamentally distinguished [10]. Continuous cellular renewal of the gastric epithelium occurs due to the proliferation and differentiation of multipotent SCJ, first discovered in the isthmus region of the glands. These undifferentiated cells have two defining characteristics. Firstly, they are able to maintain their population for a long time (self-renewal). Second, adult stem cells are the source of all cell lineages of gastric units due to asymmetric divisions leading to different types of progenitor cells that proliferate (transient amplifying cells) and differentiate into mature cells (multipotency) [11]. The following signaling pathways are involved in the regulation of SGC: Wnt/[beta]-catenin (activation of self-maintenance and inhibition of differentiation), PI3K/Akt kinase (cell growth, survival and proliferation), transforming growth factor [beta] (TGF-[beta]) ( inhibitory effect on cell proliferation) and insulin-like growth factor 1. The stem cell niche forms a complex dynamic system with the nervous system and the vascular bed [12].
The descendants of the stem cell spread in two directions (to the lumen and to the base of the gland) and form three main cell lineages with 11 cell types (Fig. 2):
- Surface (accessory) mucocytes: surface mucocyte precursors, surface pre-mucocytes, mature surface mucocytes (AAA lectin and TFF1);
- Chief cells: cervical mucocyte precursors, cervical pre-mucocytes, mature cervical mucocytes (GSII lectin and TFF2), zymogenic cell precursors and mature zymogenic cells (intrinsic factor [GIF], pepsinogen, Mist1);
- Parietal cells: parietal cell precursors, pre-parietal cells, parietal cells (H/K-ATPase and VEGF).
The rate of renewal of parietal and zymogenic cells is lower than the rest [13].
Rice. 2 | Schematic representation of the human gastric unit and its bidirectional self-renewal from multipotent stem cells. The stages of differentiation of the main cell lines and some of the characteristic secreted products are presented (suggested by the author of the article based on research [10,13,14,22]).
There are also 5 main types of endocrine cells: G cells (gastrin-producing), D cells (somatostatin-producing), enterochromaffin (EC) cells (serotonin-producing), EC-like cells (histamine-producing) and X/A cells (ghrelin-producing). As a result of the asymmetric division of multipotent SGC, an endocrine progenitor (marker Ascl1) is formed, which, under the influence of various transcription factors, is committed to a specific cell line. Bipotent D- and G-cell progenitor cells express the markers NGN3 and PAX6. NKX6-3, PDX1 and ARX are required for its differentiation into G cells, PAX4 is required for the production of D cells. Interestingly, in the body of the stomach, EC cells can be formed from nonepithelial mast cells [14].
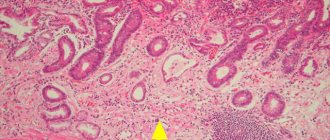
Molecular markers of SQOL have been discovered only recently. The first biomarker was Villin. It is an epithelial cell-specific calcium-regulated protein that modulates the reorganization of actin microfilaments. In contrast to actively proliferating isthmus stem cells, villin-positive SSCs (V-SCSCs) are in a quiescent state. They are located in the lower third of the antral glands [10].
Another population of SGCs express the G protein-coupling receptor Lgr5 (Gpr49). Lgr5+ SGCs (L-SCGs) are found at the base of the fundic and antral glands in the embryonic stomach, but are limited to the antrum postnatally. By analogy with V-SCF, L-SCF are also multipotent, but have high proliferative activity [15]. The coexistence of active and quiescent stem cells has been found in several tissue types. It is believed that active stem cells are responsible for the physiological renewal of tissue, and quiescent ones serve as a reserve, primarily in case of injury [16]. Of particular interest is the subpopulation of fully committed zymogenic cells at the base of the fundic glands, which are multipotent. These cells express a member of the Troy tumor necrosis factor receptor superfamily. Troy+ cells (T-SC) proliferate slowly and are activated after cytotoxic drug-induced tissue damage [10].
The SGC population expressing the Sox2 marker (S-SCG) is scattered in the isthmus of the fundic and antral glands, as well as in their lower parts. They are multipotent and have the potential for self-renewal. Interestingly, S-SCs are derived from fetal Sox2+ progenitor cells. S-SCFs are most similar in characteristics to the SCGCs originally discovered in the proliferative zone of the isthmus [17].
Recently, a new population of stem cells (at position +4) in the mouse antral gland with expression of the gastrin receptor CCK2 (C-GC) was isolated. They are located slightly above the typical location of L-SGC. C-SCF are capable of undergoing conversion due to a hormonal trigger (treatment with progastrin, but not amidated gastrin, leads to the transition of C-SCF to L-SCF) [18].
An important problem is the relationship between the monoclonal origin of the gastric unit and the fact that the stem cell population is heterogeneous. The most likely model seems to be a competitive model, which assumes that a particular stem cell achieves clonal dominance [19]. SGCs exhibit unique plasticity, moving in different directions between multipotency and committed progenitor stages. A probable inducer of transdifferentiation is Notch signaling [20].
Structure
Canaliculus
And the canal
this is an adaptation found on gastric parietal cells. This is a deep fold or small channel that serves to increase surface area, such as secretion. The parietal cell membrane is dynamic; the number of tubules increases and decreases in accordance with secretory needs. This is achieved by fusion of tubular precursors, or “vesicle tubules,” with the membrane to increase surface area and reciprocal tubular endocytosis (reformation of tubular vesicles) to decrease it.
RSC in the stomach
Since CSCs are formed from regional stem cells [19], it is natural to assume a connection between SGC and GC. Two main approaches have been used to detect putative CSC markers (Table 1). The first used genetic manipulation and tracing of CSC cell populations in mouse models. The other is based on the study of mouse cells from human GC xenografts [21,22].
Table 1 | Review of studies of immunohistochemical markers of CSC in GC.
| Marker | Functions | Statistically significant clinicopathological associations | Source |
| CD44v9 transmembrane (TM) glycoprotein | integrator of extracellular and intracellular signals, regulates cell proliferation, migration and differentiation, EMT | low five-year survival rate, metastasis to lymph nodes, more often associated with intestinal-type GC | Hirata 2013 [23] |
| CD133/Prom1 (prominin-1) glycoprotein containing five TM domains | binds to membrane cholesterol, modulates the structure of plasma membrane extensions and local membrane domains | metastasis, active invasion, low five-year survival rate | Hashimoto 2014 [24] |
| ALDH1 aldehyde dehydrogenase 1 | oxidizes intracellular aldehydes and converts retinol into a proliferation modulator - retinoic acid, hyperregulation enhances proliferation, resistance to alkylating agents and protection against oxidative stress | metastasis, deep invasion, more often associated with diffuse type GC | Nishikawa 2013 [25] |
Also, stem cell markers OCT-4, SOX2, NANOG were recommended for identification of CSCs in GC [26].
V-SGC and the tumor suppressor Klf4. V-SCF are concentrated within the lesser curvature of the antrum [10], a common location for gastric cancer [12]. Transformation of V-SCOL can lead to GC. The transcription factor Klf4 (Kruppel-like factor 4) belongs to the Klf family of “zinc fingers” and is actively expressed in the intestine; it is one of the four transcription factors of the “Yamanaka cocktail”, sufficient for the transformation of fibroblasts into induced pluripotent stem cells [27]. A decrease in Klf4 expression is associated with GC and tumor progression, which allows us to consider it as a tumor suppressor [12]. Deletion of Klf4 in murine gastric mucocytes using the Foxa3-Cre transgene expressing Cre recombinase (cyclic recombinase) results in widespread hypertrophy and precancerous metaplasia in the antrum and fundus within 6 months [28]. In this model, tumor initiation is significantly accelerated by administration of the chemical mutagen N-nitroso-N-methylurea (NMM). However, gastric adenomas do not progress to adenocarcinomas even in the presence of NMM, which indicates the need for additional genetic mutations for cancer progression. However, these studies established the role of V-SCG transformation in the occurrence of GC, as well as the suppressor activity of Klf4 [29].
Gastric RSC of bone marrow origin
Multipotent mesenchymal stem cells (MMSCs) from the bone marrow, under conditions of chronic inflammation, migrate to the gastric mucosa, where they actively interact with the microenvironment. Under conditions of an “unhealthy” microenvironment (excess of reactive oxygen species (ROS) and chronic inflammation), MMSC can provoke the appearance of adenocarcinoma [30].
H. pylori (HP) and dysregulation of gastric epithelial self-renewal
H. pylori-induced chronic gastritis is the most important risk factor for non-cardiac GC [12]. HP infection leads to characteristic proinflammatory signals: activation of nuclear transcription factors NF-kB and AP-1, release of interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF[alpha]). HP are able to colonize the antral glands, which leads to a change in the dynamics of cell self-renewal due to the proliferation of L-SCZ. It is assumed that HP infection is associated with DNA damage in L-SCF [31].
A distinctive feature of carcinomas (epithelial tumors) is a change in cell phenotype to acquire invasive properties. For example, a change in cell polarity: from apical-basolateral (in epithelial cells) to a flat type (mesenchymal phenotype). This process is known as epithelial-mesenchymal transition (EMT) [26]. Tumor cells exposed to EMT enter a stem-like state (Bmi1 expression). HP infection initiates EMT with the help of the transcription factor ZEB1 and microRNA miR-200. The classic marker of the epithelial phenotype, E-cadherin/CDH1, is not expressed in mesenchymal cells, which contributes to the loss of cell-cell contacts and invasive growth. Numerous studies have established a connection between CDH1 mutations and the development of hereditary diffuse type GC [32].
Autoantibodies to stomach cells secreting hydrochloric acid and internal Castle factor, the appearance of which during an autoimmune process has pathogenetic significance in atrophy of the gastric mucosa, impaired absorption of vitamin B12 and the development of pernicious anemia.
Synonyms Russian
APKZH.
English synonyms
Gastric Parietal Cell Antibodies; GPA; Antiparietal cell antibody; APCA.
Research method
Indirect immunofluorescence reaction.
What biomaterial can be used for research?
Venous blood.
How to properly prepare for research?
- Do not smoke for 30 minutes before the test.
General information about the study
Autoimmune gastritis is a variant of atrophic gastritis (chronic gastritis type A). Its cause is the production of antibodies by the own immune system to the parietal cells of the gastric mucosa. The disease may go undiagnosed for a long time due to its subclinical course, absence of complaints and obvious objective changes in digestion. Autoimmune gastritis can occur independently or in combination with autoimmune processes in the thyroid gland, alopecia and vitiligo.
The appearance of antibodies to parietal cells in autoimmune gastritis leads to the destruction of this cell population, chronic inflammation, and progressive atrophy of the mucosa with intestinal metaplasia. Parietal (lining) cells of the stomach are located mainly in the glands of the mucous membrane of the fundus of the stomach. Their main function is the secretion of hydrochloric acid, which is an important component of human digestion, and intrinsic factor Castle, necessary for the absorption of vitamin B12 from food.
Antigens for ARGC are the surface of the parietal cell, mitochondria and the beta subunit of H+/K+-ATPase, which provides the function of the proton pump necessary for the secretion of hydrochloric acid into the gastric cavity. Damage to the parietal cells causes a decrease in the secretion of hydrochloric acid (hypochlorhydria) or its complete absence (achlorhydria, achylia), which leads to impaired absorption of many nutrients (malabsorption). With a deficiency of intrinsic factor in the intestine, the absorption of vitamin B12 is impaired, without which the full formation of red blood cells in the bone marrow does not occur, and B12-deficiency (pernicious, megaloblastic) anemia develops.
Antibodies to gastric parietal cells are present in 90% of people with pernicious anemia and in 30% of cases in their close relatives. For this pathology, APLC are highly specific, but greater sensitivity is characteristic of antibodies to intrinsic Castle factor, which is found in 50% of patients with pernicious anemia.
The antibody titer does not correlate with the severity of the atrophic process in the stomach, therefore it is not used to monitor the course of the disease.
What is the research used for?
- For the diagnosis of megaloblastic (pernicious) anemia of autoimmune origin;
- to diagnose the causes of vitamin B12 deficiency;
- for the diagnosis of autoimmune gastritis.
When is the study scheduled?
- If hyperchromic hyporegenerative anemia is detected based on the results of a clinical blood test;
- with clinical signs of pernicious (megaloblastic) anemia (pallor, general weakness, numbness or tingling sensation in the extremities, neuropathy, bright red, “varnished” tongue).
What do the results mean?
Reference values:
Reasons for the increase:
- autoimmune gastritis and pernicious anemia (in 90% of patients);
- autoimmune gastritis without pernicious anemia
- pathology of the thyroid gland (with Hashimoto's thyroiditis, it is detected in 30% of patients);
- diabetes;
- stomach cancer;
- stomach ulcer;
- myasthenia gravis;
- Addison's disease;
- Iron-deficiency anemia;
- vitiligo;
- focal alopecia.
What can influence the result?
False-positive results can be obtained with elevated levels of immune complexes and heterophilic antibodies in the blood.