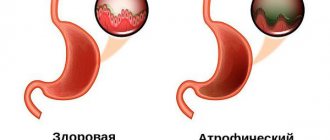
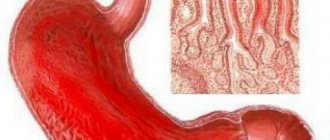
Types of diffuse gastritis
Diffuse gastritis is classified into two main types - superficial and chronic type. These types of disease differ in the degree of damage to the mucous membranes of the stomach, the risk of complications and symptoms.
Determination of the specific form of pathology occurs through a comprehensive examination of the patient. In the early stages of inflammation, it is impossible to identify the disease on your own.
Diffuse superficial gastritis
Superficial diffuse gastritis is considered a mild stage of the pathology. The inflammatory process affects only the upper layers of the mucous membranes of the stomach. The functional state of the organ is not impaired. Cells retain the ability to regenerate. Symptoms of inflammation are minimal. The pathological process is completely correctable with the help of drug therapy and adherence to a special diet.
If the antrum of the stomach is affected, pain appears 1.5–2 hours after eating, or so-called hunger pain.
Features of the disease:
- identifying the pathological process is not difficult (in some cases, the inflammatory process is determined during routine medical examinations);
- if the superficial form of gastritis is not treated, the disease can become chronic (symptoms will intensify, a functional deviation in the functioning of the digestive tract will arise);
- this type of inflammatory process does not eliminate the risk of cancer cell formation.
Chronic diffuse gastritis
In the chronic form of diffuse gastritis, inflammation spreads to the deep layers of stomach tissue. A violation of the functional state of the organ gradually develops. Digestive system disorders become persistent and progressive. Symptoms of this type of disease are accompanied by severe pain and a deterioration in the general condition of the body. Periods of exacerbation are followed by remission.
Features of the disease:
- inflammation leads to irreversible disturbances in the structure and functioning of the stomach;
- the patient experiences regular bouts of vomiting or nausea, pain in the digestive organs at night or after eating;
- Lack of timely therapy causes serious complications (peptic ulcer, risk of gastrointestinal bleeding).
Reflux gastritis
print version- home
- >
- For patients
- >
- Gastrointestinal diseases
- >
- Gastritis
- >
- Reflux gastritis
Often there is a “stub” on the lips, dry skin, anemia and weakness in the body.
This diagnosis unites a large group of patients with duodenogastric reflux (DGR), i.e. reflux of duodenal contents, containing very aggressive bile, into the stomach. GHD can be caused by taking non-steroidal anti-inflammatory drugs (see drug diseases), as a result of surgery (gastric resection).
Duodenogastric reflux is caused by insufficiency of the pyloric closure function, chronic duodenitis and increased pressure in the duodenum. This motility disorder is detected using antroduodenal manometry
.
GDR leads to damage to the gastric mucosa, mainly the antrum, by bile acids, their salts, pancreatic enzymes, lysolecithin and other components of the duodenal contents.
Diagnosis of reflux gastritis
It is very important not to make a diagnosis based on symptoms alone. It is necessary to undergo a thorough examination. You should perform a biochemical and general blood test, fibrogastroduodenoscopy (FGDS), ultrasound examination of the liver, gallbladder and pancreas, biopsy, fluoroscopy of the stomach and duodenum. GHD can be diagnosed by performing 24-hour gastric pH measurements
.
In Fig. Figure 1 shows the pH-gram of the body of the stomach of a patient with GHD, obtained using the Gastroscan-24
.
Rice. 1. Daily pH-gram of the body of the stomach, a rise in pH to alkaline values at night indicates the presence of GHD
Treatment of reflux gastritis
Treatment of reflux gastritis depends on the main causative factor and is aimed primarily at normalizing gastrointestinal motility and binding bile acids.
Since bile acids and lysolecithin have a damaging effect only in the presence of hydrochloric acid, depending on the severity of clinical manifestations, proton pump inhibitors can be used. To prevent reflux of duodenal contents into the stomach, prokinetics are prescribed.
Ursodeoxycholic acid is used to neutralize bile acids that have a damaging effect on the gastric mucosa.
Patient Materials
The GastroScan.ru website contains materials for patients on various aspects of gastroenterology:
- “Advice from doctors” in the “Patients” section of the site
- “Popular gastroenterology” in the “Literature” section
- “Popular gastroenterology” in the “Video” section
Resources for healthcare professionals regarding the diagnosis and treatment of reflux gastritis
Articles
- Minushkin O.N., Zverkov I.V., Skibina Yu.S. Some approaches to the treatment of patients with chronic (biliary) reflux gastritis // Medical alphabet. 2022. No. 19. T. No. 2. pp. 28–31.
- Lapina T.L., Kartavenko I.M., Ivashkin V.T. Pathogenetic and therapeutic significance of bile acids in reflux gastritis // RZHGGK. 2015. No. 1. pp. 86–93.
- Babak O.Ya. Bile reflux: modern views on pathogenesis and treatment // Suchasna gastroenterology. – 2003. – No. 1 (11). — P. 28-30.
- Minushkin O.N., Zverkov I.V. Chronic gastritis // Attending physician. – 2003. – No. 5. – p. 24–31.
- Galiev Sh.Z., Amirov N.B. Duodenogastric reflux as a cause of the development of reflux gastritis // Bulletin of modern clinical medicine. – 2015. – T. 8. – Issue. 2. pp. 50-61.
- Causes of chronic gastritis. Ukrainian medical portal Eurolab. — 2007.
On the website GastroScan.ru in the “Literature” section there is a subsection “Gastritis, duodenitis, erosion”, containing publications for healthcare professionals on this topic.
Video
Embutnieks Yu.V. Management of patients with biliary reflux gastritis and esophagitis
Still from video: Shumkov Yu.P.
Biliary reflux On the website in the “Video” section there is a subsection “For doctors”, containing video recordings of reports, lectures, webinars in various areas of gastroenterology for healthcare professionals.
Causes
Numerous factors can provoke the development of diffuse gastritis. The risk of severe forms of the disease arises in the presence of concomitant diseases of the digestive system. The main cause of the disease is regular irritation of the mucous membranes of the stomach. Such an effect can occur due to a violation of the diet or an overdose of potent medications.
Local immunity is weakened when the body is infected with pathogenic bacteria or viruses.
Main reasons:
- regular violation of diet;
Like most diseases, the effectiveness of treatment depends on early therapy. - the presence of a large number of fatty or spicy foods in the diet;
- complications after surgery;
- infectious infection of the digestive system;
- uncontrolled use of certain types of medications;
- disturbance of sleep and wakefulness;
- complications after food poisoning;
- constant stressful situations;
- food allergy and its complications;
- consequences of Helicobacter pylori activity;
- abuse of bad habits;
- frequent consumption of alcoholic beverages.
Superficial gastritis
30.04.2021
Superficial gastritis is an inflammation of the gastric caused by a violation of the secretory function of the stomach . At the moment, this disease is quite common and is observed in almost half of the world's population. Superficial gastritis is not a complex disease, therefore, if caught in time, it can be cured in a short time, however, if it has taken an advanced form, then treatment may be delayed, and the inflammation itself will lead to more severe forms of the disease.
Causes of superficial gastritis
One of the most likely causes of the appearance and development of superficial gastritis is the bacterium Helicobacter pylori, which affects the walls of the stomach and in an advanced stage can lead to ulcers and even stomach cancer . However, if after passing the test this reason is ruled out, there are also the following:
- disrupted diet;
- snacking on the go, poor chewing of food and dry food;
- bad habits (excessive alcohol consumption, smoking);
- eating overly spicy, peppery foods, abuse of spices;
- constant stressful situations;
- unfavorable environmental factors (work in hazardous industries, constant contact with dust, metals, etc.);
- pathological allergy ;
- frequent and uncontrolled use of medications.
Symptoms of superficial gastritis
Often, superficial gastritis may not manifest itself much. It develops gradually and sometimes it is not always possible to identify this particular disease at the initial stage. Of course, it is impossible to independently understand whether there is superficial gastritis - only a gastroenterologist after reviewing the tests . However, there are several symptoms that occur when superficial gastritis and indicate that it is time to seek help from a specialist. Among these symptoms, the most common are the following:
- bloating after eating ;
- attacks of nausea and then vomiting;
- frequent attacks of heartburn and belching ;
- pale skin, brittle hair and nails;
- coating on the tongue , most often white or gray;
- unpleasant taste in the mouth and breath bitter or sour;
- feeling of pain in the stomach at night.
Each of these symptoms may also indicate the presence of another disease, so if such signals begin to appear too often, it is better to consult a doctor .
Treatment and prevention
Treatment of superficial gastritis includes taking medications and a strict diet . Depending on the cause of the inflammation, it is necessary to first get rid of it, and then begin to treat the disease itself.
Drug treatment should only be prescribed by a specialist and includes a complex of agents that coat the stomach and relieve inflammation.
As for diet , it is also better to consult your doctor . Most often, with superficial gastritis, it is recommended to completely avoid alcohol, coffee, fatty dairy products, and too fried, salty and spicy foods. It is better to give preference in food to soups, cereals, boiled meat and fish, as well as grated vegetables that have undergone heat treatment.
Superficial gastritis is not so terrible, but the most important thing, as with any disease, is to consult a doctor to avoid possible complications.
Published in Gastroentorology Premium Clinic
Symptoms
The symptoms of diffuse gastritis differ at different stages of development of the inflammatory process. At the initial stage, the disease manifests itself with minimal discomfort in the digestive system. The patient experiences attacks of heartburn and nausea after eating. The development of inflammation causes increased symptoms.
The disease manifests itself in a significant deterioration in the general condition of the body and disruption of the functioning of the gastrointestinal tract.
Other symptoms:
- regular disturbances in the bowel movement process;
- flatulence;
- bad breath;
- attacks of nausea or vomiting;
- signs of vitamin deficiency;
- gray or yellow coating on the tongue;
- general weakness of the body;
- pain syndrome of varying intensity;
- regular attacks of heartburn.
Diagnostics
If you suspect diffuse gastritis, you should contact a gastroenterologist. After collecting anamnesis and an initial examination of the patient, the doctor prescribes a set of laboratory and instrumental procedures. The need for additional consultation with specialized specialists may arise if complications or pathologies of adjacent body systems are suspected.
Diagnostic methods:
- general analysis of urine and blood;
- examination of feces for occult blood;
- biochemical analyzes of blood and urine;
- gastroendoscopy;
- Ultrasound of the stomach;
- fibrogastroscopy;
- X-ray of the stomach;
- examination of gastric juice.
It is important to understand that only a doctor can give you the correct diagnosis.
Introduction
Gastroesophageal disease (GERD) is considered one of the most common gastroenterological diseases worldwide [1–3].
The increasing incidence of GERD in both developing and developed countries remains a global health problem. Various conditions, such as obesity, axial hernia, lower esophageal sphincter (LES) incompetence, transient relaxation of the LES, increased gastric acid production, increased intragastric pressure and prolongation of esophageal acid exposure, are risk factors for the development of GERD [4, 5]. However, the mechanisms of GERD remain incompletely understood [1, 6], and symptoms of GERD are not always associated with the presence or absence of erosive esophagitis [7]. In approximately 35% [8] and even 50% of patients, erosive esophagitis is asymptomatic [9]. The relationship between Helicobacter pylori infection and GERD remains incompletely understood. There are assumptions about both the pathogenic [10] and protective roles of H. pylori in the genesis of GERD [11], which have not been confirmed by all researchers [12]. Based on the fact that approximately 75% of the population over 40 years old has two or more diseases occurring simultaneously [13], the question arises about the functional and morphological connections between the severity of gastritis and duodenitis according to histological characteristics and the stage of GERD in comorbidity with such acid-related diseases, such as duodenal ulcer (DU) and gastric ulcer (GUD), including the period after eradication of H. pylori [10, 14].
Purpose of the study: to identify connections between pathohistological criteria for the severity of gastritis and duodenitis, and the stage of GERD, comorbid with other acid-dependent pathologies, and to additionally determine motor-secretory changes in the gastroduodenal (GD) complex to identify targets for therapeutic and preventive interventions.
Methods
The design was a case series study. The results were assessed in 305 patients aged from 18 to 69 years (224 men and 81 women, mean age 42.6±0.8 and 42.8±1.3 years, respectively). The criterion for inclusion in the study was the presence of gastroesophageal reflux (GER), which was confirmed by complaints of heartburn and/or hydrochloric acid regurgitation with a frequency of at least 1 time per week [15] in patients with DU and PU in remission and chronic pancreatitis. Exclusion criteria: age over 80 years, presence of infectious, cardiovascular, endocrine and oncological pathologies, as well as pregnancy.
The examination of patients who sought consultation from 2012 to 2022 was carried out on an outpatient basis, as well as in a hospital setting at the clinic of the Department of Surgery No. 3 of the Kuban State Medical University. In addition to general clinical, laboratory and instrumental examination methods, including the maximum pentagastrin test, all patients underwent esophagogastroduodenoscopy (EGD) with biopsy samples taken from the duodenum, the antrum of the stomach (AG) and the body (fundus) of the stomach for histological, morphometric studies and determination of H. pylori infection, as well as endoscopic manometry using the method of an open, constantly perfused catheter. During endoscopy, based on the 2nd revision of the Los Angeles classification, the following grades of the condition of esophageal mucosa were determined: N – no changes; M – minimal changes; A – one or more erosions <5 mm; B – one or more erosions >5 mm; C – more than one erosion extending beyond two folds; D – erosions are located along the entire circumference of the esophagus [16]. Changes in esophageal mucosa of N–M degrees were classified as non-erosive peptic ulcer disease (NEPD); reflux esophagitis grades A–D – to GERD. The morphological state of the HD SM was assessed in sections stained with hematoxylin and eosin with separate assessment (0–3 degrees of severity in points) of inflammatory (mononuclear) infiltration, intestinal (or gastric) metaplasia and glandular atrophy. H. pylori infection was determined in Giemsa-stained preparations, distinguishing 3 degrees of infestation: 1) weak, up to 20 microbial bodies per field of view (f/o); 2) average, 20–50 microbial bodies in the p/z; 3) strong, more than 50 microbial bodies in the area. Endoscopic manometry (registration of cavity pressure - P in the duodenum, pyloric canal, ASF, body of the stomach, in the LES and esophagus) was performed using the method of an open, constantly perfused catheter (after aspiration of air from the stomach), successively pulling it at a speed of no more than 1 cm/s from duodenum into the esophagus with delays in the segments of maximum activity of the pylorus and LES. The secretory activity of the stomach was studied by probe in the interdigestive period (basal acid production - BA) and in response to maximum stimulation (peak acid production - PAC) with pentagastrin (6 μg/kg, s.c.).
To carry out differential inter-etiological diagnosis of morphological and functional HD changes, depending on the stage of GERD, separate subgroups were formed, comparable by gender and age: patients with PUD and PUD, as well as with comorbid GERD of mixed origin. Written informed consent was obtained from study participants, as well as consent to the processing of personal data.
Statistical processing was carried out using MS Excel 10 and Wizard-Statistics (USA) programs with a preliminary assessment of the correctness of the mathematical distribution in the corresponding samples (Kolmogorov test). If there was a normal distribution in the compared groups, the Student's test (t-test) was used with data presented as the mean value (X), standard error of the mean (m) and indications of a statistically significant level of p<0.05. In samples with incorrect distribution, the χ2 test, Kruskall–Wallis, and Mann–Whitney tests were used. Analysis of correlation dependencies was carried out with the calculation of correlation coefficients according to Pearson or Spearman, and analysis of variance was carried out using the one-way ANOVA method.
results
The structure of the study cohort included 305 patients who had the following comorbid diseases (with varying degrees of esophagitis): 62 – GERD and PUD (A – 42, B – 7, C – 13), 12 – GERD and PUD (A – 8, B – 2, C – 2) and 6 – GERD and chronic pancreatitis (A – 3, B – 3); among patients with N- and M-degrees of NERD, there were 180 patients with DU (N – 111; M – 69), 16 with PU (N – 9; M – 7), 6 with a combination of DU and PU (N – 3; M – 3) and 23 – chronic pancreatitis (N – 9; M – 14) (Fig. 1).
It should be noted that the parallelism we identified between the degrees of inflammation (in points) of the HD SO according to endoscopic data and the results of histological reports was confirmed by a direct correlation (Pearson coefficient and t-test, respectively) between the following diagnostic criteria:
a) the severity of duodenitis according to endoscopic data and such histological criteria as infiltration (p<0.001; p<0.001), gastric metaplasia (p<0.001, p<0.001) and duodenal atrophy (p<0.001, p<0.001); b) the severity of antral gastritis according to endoscopic data and according to histological data such as infiltration (p=0.006, p=0.019) and atrophy of the AO (p<0.001, p<0.001); c) the degree of gastritis of the body of the stomach according to endoscopic data and the severity of inflammatory infiltration (p<0.001, p<0.001), intestinal metaplasia (p<0.001) and mucus atrophy (p<0.001).
Further, it turned out that in patients with varying degrees of damage to the esophageal mucosa (N – 138, M – 87, A – 56, B – 8 and C – 16 people), the degree of H. pylori infection in the mucous membrane of the duodenum, AOF and body of the stomach was not statistically detected significant differences. It should be noted that most studies have not identified a causal relationship or direct correlation between H. pylori infection and GERD [18, 19].
Our analysis showed that, compared with patients with N-grade NERD, only patients with C-grade GERD had statistically significant differences in severity:
- gastritis of the body of the stomach;
- antral gastritis by endoscopic signs;
- endoscopic duodenitis;
- histological data due to the predominance of gastric metaplasia and gastric atrophy (Fig. 2).
Thus, with severe reflux esophagitis grade C, inflammatory changes manifested themselves in the duodenal bulb to a more pronounced extent than in the AO and the body of the stomach. According to our data, only in GERD grade C did the interdigestive BV value reach statistically significant differences compared to the BV values in patients with M- and N-grades of NERD and A- and B-grades of GERD (Fig. 3).
We found an inverse correlation between the BV value and H. pylori infection of the gastric body (t-test; p=0.021). At the same time, H. pylori infection in the gastric corpus mucosa, compared with its absence, was accompanied by a smaller BVV value (Fig. 4). However, when comparing comorbid groups of patients - GERD and PUD (group 1) and GERD and PUD (group 2), it turned out that despite a significant increase in BV in patients of group 1 (8.34±0.43 vs. 3 .47±0.54 mmol/h; p<0.001), degree of reflux esophagitis (0.48±0.03 versus 0.56±0.07 points) and Rnps (15.4±0.72 versus 14, 7±1.79 mmHg) were statistically indistinguishable.
According to our data, within the group with comorbid GERD, when comparing the subgroups of patients with DU and PU, it turned out that with PU, pathohistological changes in the gastric corpus mucosa are more pronounced, and with DU, the duodenal mucosa is more pronounced (Fig. 5).
A comparative analysis revealed a statistically significant direct correlation (Pearson coefficient and χ² test, respectively) between the degree of esophagitis (in points) according to esophagoscopy and the severity (in points) of the following HD morphological changes:
- duodenitis (according to endoscopic [p<0.001] and histological indices [p=0.018], mainly due to the severity of duodenal atrophy; p=0.001);
- antral gastritis (according to endoscopic [p=0.019] and histological indices, χ²-criterion; p=0.005, due to more pronounced inflammatory infiltration χ²; p=0.046);
- gastritis of the body of the stomach according to the endoscopic index (Pearson coefficient [p<0.001], χ²-test, p<0.001, and index of inflammatory infiltration of CO, χ²-test; p=0.021).
Moreover, we found a statistically significant direct correlation confirming the associative relationships between various histological changes in the SM in each of the organs of the HD complex:
- In the duodenum, between HP infection, on the one hand, and gastric metaplasia and inflammatory infiltration of the mucus, on the other (Pearson coefficient; p=0.001; t-test; p=0.001); between gastric metaplasia and SO atrophy (Pearson coefficient, p<0.001; t-test, p<0.001; Mann-Whitney, p<0.001), as well as between gastric metaplasia and SO infiltration (Pearson coefficient, p<0.001, t-test , p<0.001, Mann-Whitney, p<0.001), between gastric atrophy and CO infiltration (Pearson coefficient, p<0.001; ANOVA, p<0.001, Kruskall-Wallis, p<0.001);
- In the AO between intestinal metaplasia and atrophy of the mucous membrane (Pearson's coefficient, p=0.016), as well as between atrophy and inflammatory infiltration of the mucous membrane (Pearson's coefficient, p<0.001; ANOVA, p<0.001; Kruskall-Wallis, p<0.001);
- In the FOG, there was a direct correlation between intestinal metaplasia and mucous atrophy (Pearson coefficient, p<0.001; t-test, p<0.001; Mann–Whitney, p<0.001), with each of which there was a direct correlation, respectively, with mucus infiltration (Pearson coefficient, p<0.001; t-test, p=0.044) and (Pearson coefficient, p<0.001; t-test, p<0.001; Mann-Whitney; p<0.001).
Further, it turned out that in 145 patients with GERD comorbid with acid-related diseases, we did not identify statistically significant differences in the absolute values of the pressure gradient ΔRNPS-FOG with different degrees of damage to the esophagus. However, in different parts of the esophagogastroduodenal zone, a synergistic unidirectional increase in cavity pressure was detected, confirmed by a direct positive correlation between RDC and RAF (Pearson coefficient, p<0.001; ANOVA, p=0.001; Kruskall–Wallis test, p<0.001), RAF and RAF ( Pearson's coefficient, p<0.001; ANOVA, p<0.001; Kruskall–Wallis test, p<0.001), RFJ and RNPS (Pearson's coefficient, p<0.001; Kruskall–Wallis test, p<0.001). Moreover, a statistically significant direct correlation was determined between the increase in P in the distal parts of the HD zone and P in the LES (RAOJ-RNPS - Pearson coefficient, p<0.001; Kruskall-Wallis test, p<0.043 and RDK-RNPS -Pearson coefficient, p<0.001; ANOVA, p<0.013; Kruskall–Wallis test, p<0.004).
At the same time, a direct correlation was noted between the pressure in the LES and the degrees of inflammatory cell infiltration (Kolmogorov test, p=0.002; Pearson coefficient, p<0.002; Spearman coefficient, p<0.001; Kruskall–Wallis test, p=0.001), as well as atrophy of the duodenum mucus (Kolmogorov test, p=0.011; Pearson coefficient, p<0.001; ANOVA, p=0.039, Kruskall–Wallis test, p<0.001).
Discussion
GERD is a polyetiological disease with extremely complex pathophysiological multifactorial interactions. Our data on the prevailing frequency of patients with NERD (73.7%) compared with GERD (26.3%) coincide with the results of authoritative studies (67.9 and 32.8%, respectively) [17]. Based on the parallelism we identified in the assessments (in points) between the degrees of inflammation of the gastrointestinal tract according to endoscopic and histological criteria, we can assume that the clinical use of endoscopic scoring of the severity of inflammation of the gastrointestinal tract in patients with different stages of GERD, which precedes a more accurate scoring histological diagnosis, is correct.
It should be noted that in most studies, like ours, no causal relationship or direct correlation was identified between H. pylori infection and the stage of GERD [18, 19]. Of interest are our data on more pronounced inflammatory changes in the duodenal bulb compared to the AO and the body of the stomach in grade C reflux esophagitis compared with N-grade NERD. The latter can be explained by increased secretion of HCl with periods of acidification of the duodenal bulb. Based on the literature data, it can be assumed that in patients with severe GERD, a consistent, potentially protective physiological homeostasis is disrupted, which consists in the fact that the esophagus can signal the stomach to change the secretion of HCl and bicarbonates [20]. This mechanism is confirmed by the fact that in healthy people, infusion of HCl into the esophagus reduces gastric acid production [20, 21].
It is interesting that in our work, the presence of H. pylori infection in the gastric corpus mucosa, compared with its absence, was accompanied by a lower BV value, which could minimize the risk of developing GERD. Our data do not contradict scientific studies, which state that H. pylori in the body of the stomach produces proteins that transiently inhibit HCl secretion [23], and the result of successful eradication may be an increase in HCl secretion [22] and the transient occurrence of “mild forms of GERD” [24 ], i.e. NERB. According to authoritative studies, H. pylori infection in patients with peptic ulcer is accompanied by gastritis of the body of the stomach with a decrease in HCl secretion, and in patients with peptic ulcer it is manifested mainly by antral gastritis, which provokes an increase in the secretion of Hcl [22]. Moreover, mild or moderate antral gastritis (35.2%) was often detected in GERD, and its severity correlated with the stage of GERD (p < 0.01) [17]. However, these studies did not study comorbid GERD, in which, according to our data, with syntropy with PUD, gastritis of the gastric body is more pronounced, and with syntropy with PUD, duodenitis is more pronounced.
As for the stage of comorbid GERD, according to our data, the degree of esophagitis was associated with the severity of inflammatory changes in each of the sections of the gastrointestinal tract, which must be taken into account when treating GERD. Moreover, a statistically significant positive correlation was determined between individual inflammatory substrates in each of the HD segments. Histological studies have shown that after eradication of H. pylori, a significant decrease in atrophy and intestinal metaplasia of the gastric mucosa was confirmed after 10 years [24, 25]. This targets long-term observation and preventive therapy for relevant pathological changes.
The synergistic unidirectional increase in cavity pressure in all parts of the esophagogastroduodenal zone that we identified is consistent with literature data according to which long-term contractility of the esophagus can provoke symptoms of GERD [7]. Moreover, the direct correlation found in the study between the pressure in the LES and the degrees of inflammatory infiltration and atrophy of the duodenal mucosa suggests that duodenitis with atrophy of the duodenum, and not just chronic atrophic gastritis [7], can provoke impaired gastroduodenal motility and hypersensitivity of the esophageal mucus.
In general, the results obtained indicate possible targets for therapeutic interventions in various parts of the HD complex.
Conclusion
Among patients with GERD with comorbid acid-related diseases, those with NERD predominated. Significant endoscopic and histological changes in the mucous membrane of the duodenum, arterial fluid and body of the stomach accompanying esophagitis, as well as increased interdigestive acid secretion, were recorded only in the C-stage of GERD compared to the M- and N-degrees of changes in the esophageal mucosa. At the same time, the presence of combined metaplasia and atrophy in the duodenum, ASF and in the body of the stomach both in patients with H. pylori infection and without H. pylori infection was accompanied by disturbances in HCl secretion, HD luminal pressure and LES tone not only in GERD, but also in NERB. Thus, syntropic pathohistological changes in each of the sections of the gastrointestinal tract had a certain association with the severity of GERD, comorbid with acid-related diseases. Based on the specific comorbid situation, patient-oriented treatment of patients with GERD and NERD should include probable cytoprotective therapy [4], aimed at minimizing proven reversible atrophy and accompanying intestinal metaplasia of the gastric mucosa [25], which is essentially oncopreventive.
Treatment methods
Therapy for diffuse gastritis includes taking medications and following a dietary nutrition program. In the initial stages of development, inflammation responds well to treatment. The risk of complications is virtually eliminated. Severe forms of the disease require long-term therapy. As a supplement, you can use folk remedies or physiotherapeutic procedures.
Medicines
Self-medication for gastritis is prohibited. The drug therapy regimen is prescribed individually. The goal of taking medications is to restore the functional state of the digestive tract, relieve existing symptoms, eliminate the cause of the inflammatory process and generally strengthen the immune system. If the disease is bacterial in nature, the patient is prescribed antibiotics.
Examples of medicines:
- antibiotics to destroy pathogenic microorganisms (Tetracycline, Azithromycin, Clarithromycin);
- proton pump inhibitors (Omez, Omeprazole);
- antacids (Rennie, Maalox, Almagel);
- antispasmodics for pain relief (Spazmolgon, No-Shpa);
- enzyme agents (Mezim, Panzinorm, Festal);
- immunostimulating drugs (Imudon).
- See our article “The best folk remedy for gastritis!” To get rid of it QUICKLY and FOREVER...
- How to get rid of gases in the intestines and why do they occur?
- Activated carbon cleaning - is it worth starting?
ethnoscience
Traditional medicine recipes can increase the effectiveness of basic therapy. Natural ingredients improve the functioning of the digestive system and strengthen the immune system. Some folk remedies have the ability to stop inflammatory processes. When choosing recipes, you should take into account the individual characteristics of the body. They may contain products that can cause food intolerance or an allergic reaction.
Examples of folk remedies:
- gruel of grated apple with honey (the skin of the apple is first removed, the pulp can be crushed in a blender or grated, the product should be consumed a few teaspoons thirty minutes before breakfast);
Traditional medicine methods are not recommended to be combined with the use of medications to avoid serious consequences. - herbal mixture (St. John's wort, plantain, mint and calamus root mixed in equal proportions, pour a tablespoon of the mixture with a glass of boiling water, leave for at least fifteen minutes, take several times a day before eating);
- aloe juice with honey (combine the ingredients in equal proportions, take a teaspoon before main meals);
- flax seeds (pour a teaspoon of the ingredient with a small amount of boiling water, after swelling of the seeds you should get a slimy paste, the product is used before each meal).
Diet
Diffuse gastritis requires mandatory adherence to a diet. The main reason for the exacerbation of the inflammatory process is dietary errors. When preparing dishes, it is allowed to use the method of steaming, stewing or cooking.
During the period of exacerbation of the disease, all ingredients must be thoroughly crushed. Meals should be fractional and balanced.
Nutrition principles:
- exclusion from the diet of too fatty, salty, spicy foods;
- It is prohibited to consume alcoholic and carbonated drinks, coffee, strong tea;
- You should not introduce into your diet foods that can irritate the mucous membranes of the digestive organs;
- the menu should include fermented milk products with a reduced fat content;
- meals are provided up to six times a day in small portions;
- the menu should be compiled only on the basis of the list of permitted products.
Approaches to the diagnosis and treatment of gastritis associated with bile reflux
The wide distribution of reflux gastritis in clinical practice in the absence of a single term [2] (synonyms: gastritis type C, alkaline gastritis, reactive gastropathy), as well as an understanding of the mechanisms of formation, standards of diagnosis and therapy make it relevant to analyze and highlight the existing ideas about this variant of gastritis .
Gastritis associated with biliary reflux (Latin refluo - to flow backwards) is rarely primary in nature [2, 3], mainly developing secondary to anatomical changes associated with surgery: gastric resection, gastroenterostomy, enterostomy, vagotomy, cholecystectomy. Failure of the sphincter apparatus, antroduodenal discoordination (impaired coordination between the antral, pyloric parts of the stomach and the duodenum), as well as resection of part of the stomach, leading to the elimination of the natural anti-reflux barrier, are the cause of the formation of recurrent reflux of duodenal contents containing bile into the stomach with damage to it mucous membrane.
Any diagnostic process begins with a survey and medical history. In the presence of symptoms of dyspepsia, which are not specific in nature and in some cases of reflux gastritis may be absent, as well as in diseases and conditions that have a high risk of biliary reflux (cholelithiasis, conditions after gastric resection, vagotomy, etc.), It is necessary to conduct an endoscopic examination with a biopsy of the gastric mucosa to determine the presence, degree and stage of gastritis, as well as Helicobacter pylori (HP) infection.
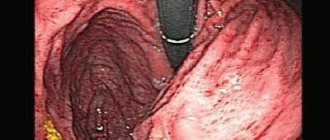
The endoscopic picture of reflux gastritis is characterized by hyperemia and swelling of the mucous membrane, which spread circularly from the pylorus in the proximal direction. At the same time, bile stains are often found on it, there is a visible reflux of bile from the duodenum into the stomach or a high content of bile in the lumen of the stomach.
There is no “gold standard” for diagnosing duodenogastric reflux yet. As a method of functional diagnostics, 24-hour pH-metry, fluoroscopy and ultrasound examination of the stomach are used. The main method for diagnosing biliary reflux is currently the pH-monitoring system Bilitec 2000, which quite qualitatively assesses bile reflux and pH in the stomach. Barrett MW et al [4] showed the high sensitivity of this device for monitoring bile reflux in vivo, but somewhat lower in vitro, with a false-negative rate of about 23%. In acidic conditions, the amount of bilirubin absorption determined by this device decreases, which can lead to an increase in false negative results and, accordingly, a decrease in false positive results. Although this method cannot be classified as the “gold standard” for duodenogastric reflux, it is the most convenient and usable for dynamic monitoring of bile reflux [5].
Why is reflux of duodenal contents into the stomach dangerous, what structural changes occur in the gastric mucosa under the influence of duodenal refluxate?
It is known that bile acids, which have detergent properties, promote the solubilization of lipids in the membranes of the surface epithelium, while their negative effect on the epithelium depends on their concentration, conjugation and pH of the environment, as well as on the length of time during which the mucous membrane is exposed to bile [6]. At low pH values, only taurine conjugates damage the mucous membrane, and at high pH values (for example, in the gastric stump after surgery), it is unconjugated bile acids that have a negative effect. In addition to the bile acids themselves, lysolecithin, formed under the influence of pancreatic phospholipase A from lecithin, has a damaging effect, with an increase in the reverse diffusion of hydrogen ions, as well as an increase in the release of histamine and gastrin. In recent years, the possible interaction of bile acids with a certain subtype of muscarinic receptors localized on chief cells has been discussed, as a result of which active inflammation, gland atrophy, intestinal metaplasia and focal hyperplasia develop in the gastric mucosa [7].
In addition, bile reflux, changing the chemical composition of the surface of the mucous membrane, can potentiate the action of other pathogenic factors: infections of HP and gastric juice [8, 9]. Chen SL et al [10], assessing the effect of biliary reflux on the severity of damage to the gastric mucosa in 49 patients with dyspepsia and chronic gastritis, did not establish a relationship between the duration of duodenal reflux and pH in the gastric cavity, which indicates the presence of complex feedback mechanisms, including regulation of the secretory and evacuation functions of the stomach. At the same time, the degree of changes in the mucous membrane, as well as colonization with HP infection, correlated with the duration of biliary reflux. Moreover, the data obtained clearly demonstrated the relationship between biliary reflux and the severity of damage to the gastric mucosa. Thus, severe reflux correlated with active inflammation (r = 0.3949, P < 0.05), chronic inflammation (r = 0.8938, P = 0.0001), mucosal atrophy (r = 0.4619, P < 0.005 ) and HP colonization in the body of the stomach (r = 0.8938, P = 0.0001). In this work, as in some others [11], the cumulative pathogenic effect of HP infection and biliary reflux on the gastric mucosa was demonstrated. One can assume a complex mechanism for this effect. Thus, the absorption of bile refluxant on the surface of the gastric mucosa has a direct damaging effect, and also enhances the effect of pepsin and hydrochloric acid; damage to the mucous membrane, in turn, contributes to the colonization of HP from the antrum to the body of the stomach. In the absence of infection of HP due to the reflux of alkaline contents from the duodenum and changes in pH, bacterial contamination by the microflora of the underlying parts of the digestive tract is possible, which also leads to more pronounced damage to the gastric mucosa.
The characteristic morphological signs of this variant of gastric damage include the phenomenon of foveal hyperplasia, proliferation of smooth muscle cells in the lamina propria, as well as changes in the production of mucins by mucocytes, which play an important role in the protection of the mucous membrane [12] (Fig.). Modification of secreted mucins, detected immunohistochemically, leads to reactive/regenerative changes similar to the manifestations of gastropathy caused by nonsteroidal anti-inflammatory drugs (NSAID gastropathy) [13]: the expression of membrane mucin (MUC1), which is involved in the processes of adhesion and polarity, and secretory mucins (MUC5AC and MUC6). The detected changes differ from HP-associated gastritis, in which the expression of MUC5AC on the surface epithelium decreases, and the MUC6 mucin spreads to the surface of mucocytes [14].
In prognostic terms, another aspect of changes in the gastric mucosa associated with biliary reflux is also extremely important. Persistence of inflammation with hyperproduction of free radicals, impaired microcirculation and, as a consequence, impaired cellular renewal processes leads to a more frequent occurrence of atrophy and intestinal metaplasia of the gastric mucosa compared to a similar age population. It is appropriate here to recall the higher risk of developing intestinal cancer in the gastric stump, which has been demonstrated in both experimental and epidemiological studies [15, 16].
Of course, the presence and severity of the inflammatory infiltrate, as well as atrophy of the gastric mucosa, do not determine whether the patient has clinical symptoms of dyspepsia resulting from disorders of gastric secretion, gastroduodenal motility, and visceral sensitivity. Why, then, is it so important for a clinician to adequately interpret the pathologist’s findings? The answer lies in the goals of the gastritis classification system - to assess the condition of the mucous membrane, characterize the inflammatory process for prognosis and personalize the patient’s observation program, primarily to prevent the progression of inflammatory changes and the development of atrophy, intestinal metaplasia, dys/neoplasia.
A thorough analysis of the dyspepsia syndrome, data from endoscopic, biopsy and functional studies, carried out by the clinician, creates the basis for the formation of treatment goals for the patient:
- relieve manifestations of dyspepsia (if any) to improve the patient’s quality of life;
- reduce changes in the mucous membrane to prevent the formation of atrophy with intestinal metaplasia, and if they are present, stop the progression of changes in the mucous membrane to prevent stomach cancer.
An important component of the treatment program should be the modification of the patient’s lifestyle and diet. Timely intake of food, the absence of excess animal fats and sweet carbonated drinks in the diet, cessation of smoking and alcohol intake contribute to positive changes in the course of gastritis with dyspepsia syndrome.
If HP infection is present, eradication therapy should be planned to eliminate an additional factor that damages the gastric mucosa and increases the risk of recurrence of dyspepsia syndrome.
Since motor disorders occupy a key position in the formation of reflux gastritis, it is advisable to prescribe prokinetics (Ganaton, Trimedat) as one of the means of pathogenetic therapy. However, only by prescribing drugs of this group it is impossible to achieve all the goals of therapy for reflux gastritis.
The next group of pathogenetic therapy agents, rational for a number of patients with cholelithiasis, functional disorders of the biliary tract, and concomitant chronic diffuse liver diseases, are ursodeoxycholic acid preparations (Ursosan, Ursofalk, Urdoxa, Ursodex), which are prescribed in a daily dose of 10–13 mcg /kg of weight in courses of 6-8 weeks, and if necessary - for a longer period.
The classic conservative approach to the treatment of gastritis associated with biliary reflux is the administration of antacids and bismuth salts. In one well-designed study, the use of sucralfate and rabeprazole in patients with biliary reflux after cholecystectomy demonstrated its effectiveness on both clinical manifestations and the severity of macroscopic signs of inflammation diagnosed by endoscopic examination [17].
Thus, the appointment of a course of therapy with prokinetics, antisecretory agents and mucocytoprotectors makes it possible to relieve the symptoms of dyspepsia. It is also possible to reduce changes in the mucous membrane in order to prevent the development of atrophy with intestinal metaplasia, and if they are present, to stop the progression of changes in the mucous membrane for the prevention of stomach cancer as a result of including muco/cytoprotectors in therapy.
The group of muco/cytoprotectors includes several drugs (table), among which the therapeutic capabilities of bismuth salts, including De-Nol, should be separately highlighted. Numerous studies have shown that bismuth preparations form a protective layer on the affected areas of the mucous membrane, protecting it from the effects of aggressive factors, stimulate the secretion of mucus and bicarbonate, inhibit the activity of pepsin, protect epithelial growth factors from decay, promoting the regeneration of epithelial cells, improve microcirculation, stimulate secretion of gastroprotective prostaglandins. Along with the cytoprotective effects listed above, two factors should be mentioned separately. This is the bactericidal effect of De-Nol against Helicobacter and the ability of De-Nol to bind bile acids [26]. Helicobacter eradication using a four-component regimen, including De-Nol, significantly reduces the severity of duodenogastric reflux, which was convincingly shown in a study by SD Ladas et al. [27]. Neutralization of bile acids and their salts is also extremely important in terms of eliminating one of the leading pathogenetic factors of reflux gastritis. Considering the above, the use of De-Nol for gastritis caused by duodenogastric reflux is absolutely justified from a pathogenetic point of view.
Under conditions of contamination of the gastric mucosa with non-NR microorganisms [18], including Helicobacter spp. [19], Proteus mirabilis, Citrobacter freundii, Klebsiella pneumoniae, Enterobacter cloacae, Staphylococcus aureus, with an additional stimulus for the persistence of the inflammatory infiltrate, activation of lipid peroxidation processes and the production of nitrosamines by bacteria, which have pro-carcinogenic potential [20], it is also important for the clinician to use the effects of salts bismuth. Firstly, not being an antibiotic, De-Nol suppresses the activity of bacterial flora without the risk of developing resistance, which has been successfully used for many years in standard eradication therapy. Secondly, in conditions of persistent inflammation, the properties of De-Nol that affect the activity of the inflammatory process become necessary, namely a decrease in the content of pro-inflammatory cytokines and the presence of an antioxidant effect. It should be noted that these properties of the drug are successfully used in the treatment of other diseases, including intestines. Thirdly, patients with reflux gastritis, especially with atrophy, especially need mucocytoprotective therapy with increased production of mucus, prostaglandins, and improved microcirculation in the gastric mucosa [25].
Finally, attempts are being made to correct the negative impact of reflux using surgical methods [21] (entero-enteroanastomosis according to Brown, Roux-en-Y), especially after gastrectomy as a “reserve” if all other methods of therapy have been ineffective.
Thus, gastritis associated with bile reflux, despite the lack of diagnostic and treatment standards, can be detected in a timely manner using an integrated approach and there are quite reliable, safe and effective drugs for its treatment.
Literature
- Kawiorski W., Herman RM, Legutko J. Current diagnosis of gastroduodenal reflux and biliary gastritis // Przegl Lek. 2001; 58:90–94.
- Hermans D., Sokal EM, Collard JM, Romagnoli R., Buts JP Primary duodenogastric reflux in children and adolescents // Eur J Pediatr. 2003, Sep; 162(9):598–602. Epub 2003, Jun 26.
- Lin JK, Hu PJ, Li CJ, Zeng ZR, Zhang XG A study of diagnosis of primary biliary reflux gastritis // Zhonghua Neike Zazhi. 2003; 42:81–83.
- Barrett MW, Myers JC, Watson DI, Jamieson GG Detection of bile reflux: in vivo validation of the Bilitec fibreoptic system // Dis Esophagus. 2000; 13:44–45.
- Barrett MW, Myers JC, Watson DI, Jamieson GG Dietary interference with the use of Bilitec to assess bile reflux // Dis Esophagus. 1999; 12:60–64.
- Stathopoulos P., Zundt B., Spelsberg FW, Kolligs L., Diebold J., Goke B., Jungst D. Relation of gallbladder function and Helicobacter pylori infection to gastric mucosa inflammation in patients with symptomatic cholecystolithiasis // Digestion. 2006; 73 (2–3): 69–74.
- Raufman JP, Cheng K., Zimniak P. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications // Dig Dis Sci. 2003; 48:1431–1444.
- Chen SL, Mo JZ, Chen XY, Xiao SD The influence of bile reflux, gastric acid and Helicobacter pylori infection on gastric mucosal injury: Severity and localization // Weichang Bingxue. 2002; 7: 280–285x.
- Johannesson KA, Hammar E., Stael von Holstein C. Mucosal changes in the gastric remnant: long-term effects of bile reflux diversion and Helicobacter pylori infection // Eur. J Gastroenterol Hepatol. 2003; 15:35–40.
- Chen SL, Mo JZ, Cao ZJ, Chen XY, Xiao SD Effects of bile reflux on gastric mucosal lesions in patients with dyspepsia or chronic gastritis // World J Gastroenterol. 2005. May 14; 11(18):2834–2847.
- Abe H., Murakami K., Satoh S., Sato R., Kodama M., Arita T., Fujioka T. Influence of bile reflux and Helicobacter pylori infection on gastritis in the remnant gastric mucosa after distal gastrectomy // J Gastroenterol. 2005, Jun; 40(6):563–569.
- Mino-Kenudson M., Tomita S., Lauwers GY Mucin expression in reactive gastropathy: an immunohistochemical analysis // Arch Pathol Lab Med. 2007, Jan; 131(1):86–90.
- Mino-Kenudson M., Tomita S., Lauwers GY Mucin expression in reactive gastropathy: an immunohistochemical analysis // Arch Pathol Lab Med. 2007, Jan; 131(1):86–90.
- Corfield AP, Myerscough N., Longman R., Sylvester P., Arul S., Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease // Gut. 2000. 47: 589–594.
- Ma Z., Wang Z., Zhang J. Carcinogenicity of duodenogastric reflux juice in patients undergoing gastrectomy // Zhonghua Wai Ke Za Zhi. 2001, Oct; 39(10):764–766.
- Oba M., Miwa K., Fujimura T., Harada S., Sasaki S., Hattori T. Chemoprevention of glandular stomach carcinogenesis through duodenogastric reflux in rats by a COX-2 inhibitor // Int J Cancer. 2008, Oct 1; 123(7):1491–1498.
- Santarelli L., Gabrielli M., Candelli M., Cremonini F., Nista EC, Cammarota G., Gasbarrini G., Gasbarrini A. Post-cholecystectomy alkaline reactive gastritis: a randomized trial comparing sucralfate versus rabeprazole or no treatment // Eur J Gastroenterol Hepatol. 2003, Sep; 15(9):975–979.
- Takako Osaki, Katsuhiro Mabe, Tomoko Hanawa, Shigeru Kamiya. Urease-positive bacteria in the stomach induce a false-positive reaction in a urea breath test for diagnosis of Helicobacter pylori infection // Journal of Medical Microbiology. 2008, 57, 814–819.
- Cinthia G. Goldman and Hazel M. Mitchell. Helicobacter spp. other than Helicobacter pylori 2010 Blackwell Publishing Ltd, Helicobacter 15(Suppl. 1): 69–75.
- Correa P. A human model of gastric carcinogenesis // Cancer Res. 1988; 48:3554–3560.
- Madura JA Primary bile reflux gastritis: diagnosis and surgical treatment // Am J Surg. 2003, Sep; 186(3):269–273.
- Slomiany V. L., Nishikawa H., BHski J., Slomiany A. Colloidal bismuth subcitrate inhibits peptic degradation of gastric mucosa_epidermal grown factor in vitro // Amer. J. Gastroenterol. 1990. V. 85. P. 390–393.
- Lambert JR, McLean. Pharmacology of colloidal bismuth subcitrate (De-Nol) and use in non ulcer dyspepsia / Helicobacter pylori in peptic ulceration and gastritis; eds. B. J. Marshall, R. W. McCallum, R. L. Guerrant. Oxford, London, Edinburgh, Melbourne: Blackwell Scientific Publications, 1991, pp. 201–209.
- Kononov A.V. Cytoprotection of the gastric mucosa: molecular cellular mechanisms // Russian Journal of Gastroenterology, Hepatology and Coloproctology. 2006. No. 3. pp. 12–16.
- O'Connor A., Gisbert JP, McNamara, O'Morain C. Treatment Of Helicobacter pylori Infection 2011 // Helicobacter. 2011. Sep. 16:53–58.
- Wagstaff AJ, Benfield P, Monk JP Colloidal bismuth subcitrate. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in peptic ulcer disease // Drugs. 1988; 36: 132–157.ha
- Ladas SD, Katsogridakis J., Malamou H. et al. Helicobacter pylori may induce bile reflux: link between H. pylori and bile induced injury to gastric epithelium // Gut. 1996; 38: 15–18.
M. F. Osipenko*, Doctor of Medical Sciences, Professor M. A. Livzan**, Doctor of Medical Sciences
* State Educational Institution of Higher Professional Education "Novosibirsk State Medical University of the Ministry of Health and Social Development of Russia", Novosibirsk ** State Educational Institution of Higher Professional Education "Omsk State Medical Academy of the Ministry of Health and Social Development of Russia", Omsk
Contact information for authors for correspondence
Complications
In the absence of adequate therapy, diffuse gastritis takes on a chronic and then subatrophic form. The consequence of the development of inflammation is the formation of erosions, ulcers, and disruption of the process of hydrochloric acid production. The movement of food through the esophagus becomes difficult. Such conditions cause an increase in the scale of tissue damage, and additional pathological processes develop.
Other complications:
- reflux gastritis;
- peptic ulcer;
- precancerous condition;
- formation of malignant tumors;
- gastrointestinal bleeding.
Video on the topic: Gastritis - causes and types of treatment.
Duodeno-gastric reflux
Several factors are important in the development of duodenogastric reflux: insufficiency of the pyloric part of the stomach with gaping of the pylorus, impaired motility of the stomach and duodenum, increased pressure in the initial parts of the small intestine, and the aggressive effect of the contents of the duodenum on the gastric mucosa.
Bile acids and pancreatic enzymes damage the protective barrier of the gastric mucosa; provoke reverse diffusion of hydrogen ions into the deep layers of the stomach wall (this leads to increased acidity); stimulate the production of gastrin by the antral glands and damage the lipid membranes of cells, increasing their sensitivity to the components of gastric juice. In addition, due to retrograde reflux of duodenal contents, the pressure in the gastric cavity increases.
The reflux of duodenal contents into the stomach often accompanies diseases such as chronic gastritis, gastric and duodenal ulcers, stomach cancer, impaired tone of the sphincter of Oddi, and duodenostasis. The condition often occurs in patients who have undergone surgery to remove the gallbladder or suturing a duodenal ulcer. Impaired motility of the stomach and initial parts of the small intestine is the root cause of reflux in functional diseases of the gastrointestinal tract, and in organic pathology, impaired motility is secondary.
Discoordination of motility leads to impaired evacuation of the contents of the stomach and duodenum, which leads to gastro- and duodenostasis, reverse peristalsis, and reflux of duodenal masses into the stomach cavity. Dysmotor disorders can be observed in various parts of the digestive tract, combined with pylorus pathology: normal gastric tone, accompanied by pylorospasm and duodenostasis, or gastric hypotension in combination with pylorus gaping, duodenal hypertension.
Previously, it was believed that the condition is a protective reaction to the inflammatory process in the stomach and the increased acidity of gastric juice entering the duodenum: supposedly, duodenal juice, when it enters the stomach, alkalizes its contents, which prevents further damage to the duodenal mucosa.
However, today it has been proven that bile acids contained in duodenal juice not only damage the mucous barrier of the stomach, but also provoke reverse diffusion of hydrogen ions into the submucosal layer, stimulate the secretion of gastrin by the antral glands, which leads to an even greater increase in acidity in the stomach. Thus, the ulcerogenic effect of duodeno-gastric reflux was substantiated and the theory of its protective nature was refuted.
Prevention and prognosis
If diffuse superficial gastritis is detected, the prognosis for the patient will be favorable. Adequate therapy eliminates the risk of complications, restores tissue regeneration processes, and eliminates all abnormalities in the functioning of the digestive system. With a chronic type of pathology, favorable prognoses are possible if the treatment schedule is followed. Long periods of remission will not affect the patient's quality of life.
Prevention measures:
- adherence to a fractional, balanced diet;
- exclusion of harmful foods and drinks from the diet;
- refusal to abuse bad habits;
- maintaining a healthy and active lifestyle;
- regular examination by a gastroenterologist.