Introduction
Currently, internists are increasingly faced with the problem of comorbidity and the need to prescribe a mass of medications.
On the one hand, comorbidity itself is an independent risk factor for death and poor prognosis for the disease and life [1]. On the other hand, inevitable polypharmacy in comorbid pathology is associated with numerous adverse consequences, which requires a careful analysis of the risks of drug-drug interactions, as well as the risk of damage to other organs and systems. Most often in clinical practice, drug-induced liver damage and damage to the mucous membranes of the digestive tract occur. The negative effects of nonsteroidal anti-inflammatory drugs (NSAIDs) and antiplatelet agents on the upper gastrointestinal tract are well known. In addition, not only NSAIDs, but also a number of other drugs, such as anticoagulants, antibiotics, chemotherapeutic agents, proton pump inhibitors, angiotensin II receptor blockers, can cause damage to the mucous membranes of the digestive tract.
The purpose of this review is to systematize and update information about intestinal lesions associated with taking the above medications.
The true prevalence of intestinal lesions associated with drug use is unknown. Data from individual epidemiological studies are quite heterogeneous and range from 3–22% of all cases of seeking medical help for intestinal symptoms [2]. Older age, the presence of concomitant diseases and the number of medications taken by the patient are the main factors associated with an increased risk of developing drug-induced intestinal damage [3].
Intestinal damage caused by medication may be accompanied by various morphological and functional changes. In most cases, clinical manifestations of drug-induced intestinal damage include bloating, abdominal pain and diarrhea that persist at night. Fever and blood in the stool may sometimes occur. The severity of symptoms can vary widely from mild to intense, simulating the picture of an acute abdomen. Physical examination findings are also quite variable and nonspecific. An important diagnostic criterion for drug-induced colitis is that drug withdrawal quickly leads to an improvement in the patient’s well-being [4].
It should be noted that some drugs, due to their pharmacological action, can cause various disorders of intestinal function without causing damage to the intestinal mucosa. Thus, the cardiac glycoside digoxin, by inhibiting Na+/K+ metabolism, can cause sodium loss at the intestinal level and the development of secretory diarrhea. Osmotic diarrhea without signs of steatorrhea can occur when using osmotic laxatives such as lactulose or macrogol (polyethylene glycol), with signs of steatorrhea when using the lipase inhibitor orlistat or the bile acid sequestrant cholestyramine. Diarrhea due to increased intestinal motor activity is caused by drugs with a prokinetic effect, which increase the activity of the migratory motor complex of the stomach and intestines (macrolides, serotonin 5-HT4 receptor agonists). On the contrary, a slowdown in intestinal motility and, as a consequence, constipation can occur as a result of the use of calcium channel blockers, anticholinergic drugs, tricyclic antidepressants, bismuth, iron, and opioid analgesics [2, 5, 6].
The pathogenetic mechanisms underlying the development of damage to the intestinal mucosa due to exposure to drugs have not been fully elucidated. It is well known that diseases of the digestive tract are formed as a result of an imbalance between protective factors and aggression towards the mucous membrane. In the last decade, new ideas have emerged about the pathophysiology of diseases of the digestive tract from the perspective of participation in their formation of damage to the mucosal barrier. An integral part and central regulator of the mucosal barrier is the microbiota [7]. It has been established that disruption of the integrity of the mucosal barrier and changes in the composition of the intestinal microbiota increase the susceptibility of the intestinal mucosa to the damaging effects of various aggressive factors, incl. medications [2, 8].
Non-steroidal anti-inflammatory drugs, antiplatelet agents and anticoagulants
Among the variety of drugs that can cause damage to the digestive tract, NSAIDs occupy a leading place.
Nonsteroidal anti-inflammatory drugs have been and remain the most popular class of pain medications. There is a wide selection of NSAIDs on the Russian pharmacological market - about 30 medicinal molecules, many of which are represented by dozens of generics. At the same time, a number of NSAIDs are classified as over-the-counter drugs, and therefore are available to the masses of people, as can be judged by the volume of their sales. Thus, according to Rosstat, in 2022 alone, 125 million packages of various NSAIDs were sold in Russia [9].
The analgesic and anti-inflammatory effect of NSAIDs is realized through inhibition of the enzyme cyclooxygenase (COX), which is involved in the breakdown of arachidonic acid to prostaglandins, prostacyclin and thromboxane. There are two known isoforms of the COX enzyme: COX-1 and COX-2. Damage to the mucous membranes of the digestive tract is explained by the blockade of COX-1 and a decrease in the synthesis of protective prostaglandins (prostaglandin E2, prostacyclin) [10]. Most NSAIDs are non-selective inhibitors of both COX-1 and COX-2. It is believed that selective COX-2 inhibitors (celecoxib, etoricoxib) cause less damage to the mucous membranes. However, it should be noted that selectivity for COX-2 is not an absolute concept and does not exclude the damaging effects of even “highly selective” drugs.
The wide range of side effects characteristic of NSAIDs, especially with their uncontrolled use, prompted experts from the Association of Rheumatologists of Russia, the Russian Society for the Study of Pain, the Russian Gastroenterological Association, the Russian Scientific Medical Society of Therapists, the Association of Traumatologists and Orthopedists of Russia, the Russian Association of Palliative Medicine to create clinical recommendations for the rational use of NSAIDs in order to reduce the incidence of potentially dangerous “class-specific” complications [9].
The most studied NSAID-induced damage to the digestive tract is damage to the mucous membrane of the stomach and duodenum. It has been shown that long-term use of NSAIDs increases the risk of developing gastric ulcers by 3–4 times and is associated with at least 40–50% of bleeding from the upper gastrointestinal tract requiring surgical or endoscopic hemostasis [9–12]. It was found that the frequency of NSAID-induced intestinal damage is comparable to the frequency of gastropathy (see table) [9]. Nevertheless, physicians' awareness of NSAID-induced entero- and colopathy remains extremely low, and in practice, if it is necessary to prescribe NSAIDs, everything comes down only to the prevention of damage to the gastroduodenal zone.
The main criteria for NSAID gastropathy are a chronological connection with taking the drug, often the absence of clinical manifestations and manifestation of ulcerative bleeding, the presence of acute multiple erosions, usually in the antrum of the stomach, foveal hyperplasia of the mucous membrane, the absence of an inflammatory shaft around the ulcerative defect, rapid healing after discontinuation of the drug [13].
Unfortunately, when taking NSAIDs, there is a risk of damage not only to the mucous membrane of the upper digestive tract, but also to the intestines, which was shown in a number of large randomized clinical trials conducted after the advent of capsule endoscopy [10, 14, 15].
The mechanisms by which NSAIDs damage the intestine are not well understood. Of course, they differ from those that are realized in the upper parts of the digestive tract. The damaging effects of NSAIDs on the intestinal mucosa may be mediated by direct toxic effects following oral administration, recurrent toxic effects due to enterohepatic recirculation of the drug, and systemic effects following intestinal absorption. It is known that NSAIDs increase intestinal permeability, causing the development of the so-called. the leaky gut phenomenon, as a result of which toxins, bacteria, bile acids, and pancreatic secretions from the intestinal lumen can penetrate under the mucous membrane and cause inflammatory reactions with the formation of erosive and ulcerative defects (see figure) [10, 13].
The participation of intestinal microbiota in the appearance of damage to the intestinal mucosa is assumed. Experimentally, administration of Lactobacillus acidophilus and Bifidobacteria adolescentis reduced NSAID-induced ileal ulceration, and the probiotic mixture VSL#3 maintained normal calprotectin levels in healthy volunteers receiving indomethacin [16]. Also, patients receiving Lactobacillus casei and low doses of aspirin had significantly smaller erosions of the small intestinal mucosa [17].
The morphological picture and severity of intestinal damage depend both on the NSAID molecule itself and on the patient’s condition: age, concomitant pathology, taking other medications [14, 15, 18].
Despite the fact that NSAID-induced intestinal lesions are not given due attention by clinicians, they can cause acute and life-threatening complications such as bleeding, perforation or intestinal obstruction [9–12].
The lack of proper awareness of doctors about intestinal damage while taking NSAIDs is primarily due to the fact that neither NSAID-entero- nor NSAID-colopathy have characteristic clinical or endoscopic signs. Most often, NSAID enteropathy is accompanied by hidden blood loss and the development of chronic iron deficiency anemia, which aggravates the course of cardiovascular diseases, bronchopulmonary pathology, and increases the risk of thromboembolic complications. Less common is NSAID enteropathy, accompanied by protein loss and hypoalbuminemia [9, 10]. Sometimes the first manifestation of NSAID enteropathy is bleeding or perforation of the small intestine [2, 9, 10].
According to video capsule endoscopy, in most cases, NSAID enteropathy is manifested by the presence of hemorrhages, erosions on the mucosa and small (up to 3–5 mm in diameter) shallow ulcers [9, 13]. The only pathognomonic sign of damage to the small (less often large) intestine associated with long-term use of NSAIDs is the formation of circular, diaphragm-like strictures as a result of a chronic inflammatory process with pronounced lymphohistiocytic infiltration and fibrosis. These strictures are most often localized in the ileum and can cause intestinal obstruction [9, 10, 19].
In NSAID-induced colopathy, the pathological process usually involves the right parts of the colon. Manifestations of NSAID-induced colopathy can range from acute inflammation and erosive and ulcerative defects to chronic colitis with the development of fibrosis and strictures [2, 3]. It has been shown that approximately 3–10% of newly diagnosed colitis is associated with the use of NSAIDs [20]. The severity of damage to the colon mucosa certainly depends on the duration of drug use, while NSAID-induced colitis can develop within a few days from the start of use [21]. It has been established that the development of erosive and ulcerative lesions of the colon is equally common with both oral and parenteral administration of NSAIDs. The use of rectal suppositories is often accompanied by damage to the rectal mucosa [2, 20, 21]. The literature even describes the formation of rectal strictures as a result of long-term use of rectal suppositories with ibuprofen [22].
A number of studies have shown that NSAIDs increase the risk of bleeding and perforation of the colon in the presence of existing diverticular disease [2, 3, 23]. In addition, NSAIDs can play a trigger role in lymphocytic and collagenous colitis, as well as provoke the onset or exacerbation of ischemic colitis and inflammatory bowel diseases (IBD) [2, 13, 18]. The latter is especially relevant for patients in whom IBD is combined with rheumatological diseases, such as ankylosing spondylitis or rheumatoid arthritis, which often require long-term anti-inflammatory therapy.
Increasing life expectancy of patients, the emergence of new interventional and surgical methods for the treatment of cardiovascular diseases (stenting, coronary artery bypass grafting, radiofrequency ablation, valve replacement, etc.) have led to an increase in the number of people who are indicated for long-term use of antiplatelet agents (acetylsalicylic acid) or anticoagulants (clopidogrel, prasugrel, ticagrelol, warfarin, rivaroxaban, apixaban, endoxaban, dabigatran), and often their combinations - double and triple antithrombotic therapy. One of the main and most dangerous side effects of these drugs is the risk of gastrointestinal bleeding [24]. At the same time, the mechanism of damage to the mucous membranes of the digestive tract while taking acetylsalicylic acid is more understandable and comparable to that during NSAID therapy, while the mechanism of the damaging effect of anticoagulants is currently not studied.
Chemotherapeutic agents
The incidence of intestinal lesions during chemotherapy reaches 50–80% in some regimens [2, 3]. The small intestine is more vulnerable to the damaging effects of chemotherapeutic agents, since it is in its mucosa that the largest number of rapidly proliferating cells is concentrated. As a rule, intestinal damage associated with the use of chemotherapeutic agents is clinically manifested by diarrhea and signs of malabsorption syndrome.
Considering the immunosuppressive mechanism of action of chemotherapeutic agents, diffuse or segmental colitis often develops during their use, requiring the administration of glucocorticosteroids [3, 25].
For colitis of this etiology, characteristic endoscopic signs are swelling, erythema, exudation and erosion. In some cases, perforation of the colon may occur against the background of ulceration. Morphological examination in the lamina propria most often reveals lymphocytic infiltrates rich in T cells, eosinophils and plasma cells, as well as neutrophilic infiltrates with cryptitis and the formation of crypt abscesses [25].
The risk of development and severity of intestinal damage during chemotherapy depend on the drug molecule, its dose and regimen. Most often, the development of drug-induced intestinal damage is observed during the use of 5-fluorouracil and irinotecan, widely used for the treatment of colorectal cancer, as well as ipilimumab in the chemotherapy of melanoma [2, 25].
Antibiotics
It is known that antibiotics have a huge impact on the qualitative and quantitative composition of the intestinal microbiota, regardless of the frequency and duration of use [26]. Numerous studies have confirmed that therapy with antibacterial drugs in 10–20% of patients is accompanied by the development of diarrhea due to a decrease in the number of saccharolytic bacteria against the background of the proliferation of opportunistic and pathogenic strains, incl. resistant to antibiotics [26, 27]. Thus, antimicrobial agents can promote the proliferation of Clostridium difficile, whose toxins have a direct damaging effect on intestinal epithelial cells, leading to the development of fulminant colitis and toxic dilatation of the colon. As a rule, Clostridium difficile infection occurs in elderly patients undergoing hospital treatment and taking proton pump inhibitors and immunosuppressive drugs in addition to antibiotics.
Most often, the development of Clostridium difficile infection is associated with the use of cephalosporins, fluoroquinolones, and nitrofurans. Clinically, the development of Clostridium difficile infection can be suspected by the appearance of abdominal pain and diarrhea, accompanied by severe symptoms of intoxication and leukocytosis, during or after antibiotic therapy [28]. Diagnosis of colitis associated with Clostridium difficile is based on characteristic endoscopic and morphological signs (erosive-hemorrhagic lesions, often localized in the rectum or sigmoid colon, the formation of fibrinous films - pseudomembranes) and the detection of Clostridium difficile toxins A and B in the feces [28, 29] .
It must be emphasized once again that some antibiotics, for example macrolides, as well as clavulanic acid in beta-lactam antibiotics, have the ability to stimulate intestinal motilin receptors and lead to diarrhea without causing damage to the mucous membrane [2, 3].
Proton pump inhibitors (PPIs)
Drugs in this group are benzimidazole derivatives that have a powerful inhibitory effect on the secretion of hydrochloric acid by parietal cells of the stomach through blockade of the H+/K+-ATPase enzyme [29].
It is important to note that PPIs, widely used for the treatment and prevention of NSAID gastropathy, on the contrary, increase the risk of damage to the underlying parts of the digestive tract. Changes in the acidity of gastric juice with long-term use of PPIs contribute to excessive bacterial colonization of the small intestine with the subsequent development of bacterial overgrowth syndrome, as well as disruption of the composition of the colon microbiota [30]. In an experiment, acid suppressive therapy with PPIs was associated with a decrease in the representation of actinobacteria in the colon and an increase in the incidence of NSAID enteropathy [31].
In addition, long-term use of PPIs can lead to changes in the composition of colonic anaerobes and the emergence of Clostridium difficile infection, which is associated with high mortality [28]. According to a meta-analysis including more than 300 thousand patients, the relative risk of Clostridium difficile infection with long-term prescription of PPIs increases by 2.79 times, and when PPIs are combined with antibiotics - up to 5 times [32].
Angiotensin II receptor blockers
In 2012, evidence first appeared that the antihypertensive drug olmesartan, which belongs to the group of angiotensin II receptor blockers, can cause intestinal lesions similar to those in celiac disease [33]. Enteropathy associated with olmesartan is clinically manifested by diarrhea, progressive weight loss, normochromic anemia and hypoalbuminemia. The morphological sign of intestinal damage of this etiology is atrophy of the villi of the small intestine.
A cell-mediated immune response is considered as a possible mechanism for this effect: olmesartan increases the level of angiotensin II, which increases the expression of transforming growth factor (TGF-β), which is responsible for activation of the immune system and damage to the intestinal mucosa [34].
Diagnosis and treatment of drug-related intestinal lesions
Clinical manifestations of drug-induced intestinal damage can often mimic other diseases of the digestive tract - celiac disease, carbohydrate intolerance, functional gastrointestinal disorders, chronic pancreatitis, inflammatory bowel diseases, malignant neoplasms. Taking this into account, drug-related intestinal lesions are essentially a diagnosis of exclusion, which requires a host of examinations.
The management of patients with drug-induced intestinal damage has not yet been determined. In the presence of pronounced clinical manifestations, a gentle diet with restriction or complete exclusion of dietary fiber, rehydration, and the prescription of antispasmodics, mesalazine, intestinal antiseptics, and probiotics are recommended [2, 3]. If possible, the potentially damaging drug should be discontinued and an alternative molecule administered. In cases where discontinuation of the drug is not possible, you should try to reduce the dose.
Conclusion
Intestinal damage associated with drug use represents a spectrum of various morphological and functional changes as a result of the use of certain pharmacologically active compounds. Most often, intestinal damage is caused by NSAIDs, antibiotics, chemotherapy drugs, PPIs, and angiotensin II receptor blockers.
The risk of developing drug-induced intestinal damage and the degree of its damage are determined by a number of factors: the patient’s age, concomitant diseases, the integrity of the intestinal barrier, the composition of the intestinal microbiota, the dose of the drug, the time of exposure, and drug-drug interactions. The lack of specific symptoms and markers makes diagnosing the disease difficult. Diagnosis should primarily be based on a thorough drug history and exclusion of alternative causes. The key diagnostic feature of drug-induced intestinal damage is the chronological relationship of symptoms with the initiation of the drug and its withdrawal.
Unfortunately, at present there are no medications for the treatment of drug-induced intestinal lesions with proven effectiveness, and the management tactics for this cohort of patients have not been determined. In this regard, it is especially relevant to carefully assess the risks of developing intestinal lesions and their prevention before starting therapy.
Source of financing
The article was prepared as part of the implementation of work under the state order of the Ministry of Health of Russia No. 056-00096-20-00.
Pseudomembranous colitis - symptoms and treatment
The pathogenesis of MVP is based on the excessive proliferation of C. diff bacteria against the background of a decrease or death of the normal microbiota of the large intestine. C. diff produces toxins A and B, which cause inflammation in the intestinal wall. The course of the disease is influenced by the state of the patient’s immune system and the pathogenic properties of bacteria, such as virulence and the presence of toxins A and B.
The main route of transmission is fecal-oral. Bacteria enter the body from contaminated surfaces, such as door handles, faucets, cistern flush handles, furniture or medical equipment. Hospital staff and infected patients can also transmit the infection.
C.diff bacteria form spores that are resistant to antibiotics and can persist for a long time in the human gastrointestinal tract, infecting others and causing relapses of the disease in the patient himself [12].
For some people, C.diff is part of the normal gastrointestinal flora, but in this case, the number of bacteria is controlled by other microorganisms and the work of the immune system.
The main damaging factors of C.diff are the toxins it produces [1]. Toxin A (enterotoxin, a 308 kDa protein molecule) affects fluid secretion, damages the intestinal lining (cell membranes) and causes an inflammatory response from the immune system.
Toxin B (cytotoxin, a protein molecule with a mass of 250 to 270 kDa) is 1000 times more cytotoxic compared to toxin A. It causes the breakdown of filament actin, a protein that maintains cell integrity. As a result, mucosal cells are destroyed and die. Toxin B is dangerous to humans, but does not lead to inflammation or damage to the intestinal mucosa in animals.
Under the influence of toxins, a specific inflammation develops in the intestinal wall with characteristic changes on the surface of the mucosa. It becomes swollen, loosens, acquires a bright red color, and the vascular pattern disappears on it. When the endoscope comes into contact with the mucous membrane, droplets of blood do not appear. There are many yellowish-white plaques with a diameter of 3–5 mm, which are tightly fixed to the mucous membrane. When taking a biopsy, they come off with difficulty and cannot be removed with the end of the endoscope. Two types of inflammatory reactions predominate: diffuse catarrhal inflammation of the mucous membrane and foci of fibrinoid necrosis - white-yellow plaques, or pseudomembranes, therefore this type of colitis is called pseudomembranous.
In biopsy samples obtained from inflammatory-changed areas of the mucosa, accumulation of immune cells in the blood and mucosa, dilation of capillaries and necrosis of integumentary epithelial cells are revealed [1].
Biopsies taken from plaques contain fibrin, fragments of necrotic covering epithelium, mucus, leukocytes, plasma cells and colonies of bacteria.
To determine the pathogenicity of clostridia, it is necessary to identify their toxins. Sowing biopsy samples on nutrient media does not give satisfactory results. The method in this case is expensive and uninformative, so C. diff toxins are determined in feces.
The inflammatory process, as a rule, involves several parts of the large intestine, but more often the rectum and sigmoid part are affected, less often the entire colon is involved. The extent of its damage is associated with the severity of the disease: the more of the large intestine is involved in the inflammatory process, the more severe the colitis and the brighter the symptoms.
Prolonged inflammation in the intestinal wall leads to the following disorders:
- fluid is not absorbed, which causes dehydration;
- blood loss from resulting erosions and ulcers leads to anemia;
- the intestinal wall becomes permeable to the toxins of the pathogen and its waste products located in the intestinal lumen, which is why general intoxication increases.
In severe cases, the peristalsis of the large intestine stops, toxic megacolon develops and multiple organ failure occurs, which can cause the patient to die.
GASTROENTEROLOGY
W. Hart
TO
Olitis may be one of the side effects of antibiotic treatment. However, treatment with immunosuppressive drugs such as gold, non-steroidal anti-inflammatory drugs and antipsychotics has also been shown to cause this disease. The severity of colitis can vary from mild diarrhea to severe fibrinous colitis. Clostridium difficile is often the main cause of this disease. D. Zehnder et al. investigated the incidence of antibiotic-associated colitis in 3 internal medicine departments at St. Gallen, Bern, Switzerland. From 1974 to 1991, they retrospectively reviewed the medical records of 31,367 patients (42,920 visits) due to adverse drug reactions. The researchers analyzed data from these patients, selecting all diagnoses of antibiotic-associated colitis: fibrinous colitis, hemorrhagic colitis, and milder forms of colitis. Of this group of patients, 38% (16,150 patients) received at least one systemically administered antibacterial drug during their hospital stay. Only 9 patients were found with one episode of colitis likely related to antibiotic use. Of these, in 5 cases, only one medication used during the hospital stay could be related. 2 patients had fibrinous colitis, 2 patients suffered from hemorrhagic colitis after antibiotic treatment and 5 had mild colitis. In 4 cases, a search was carried out for Clostridium difficile toxin, which was found in 2 patients. One patient had a culture of C. difficile. In 4 patients, a typical picture of colitis associated with the use of antibiotics was observed during endoscopy. One patient had to undergo hemicolectomy due to perforation. One patient died due to the underlying disease, the remaining 8 patients recovered. In addition, 32 additional patients were admitted to the hospital with antibiotic-induced colitis due to treatment with the same groups of drugs prior to admission to the hospital. Taking into account the characteristics of the effects of antibiotics, in 9 patients with colitis caused by antibiotics, and compared with other patients who received antibiotics during their hospital stay, the incidence of colitis depending on the group of antibiotics was estimated in 1000 patients, which was:
- for all antibacterial chemotherapeutic drugs 0.6%o;
- for all penicillins 0.6%o;
- for aminopenicillin or its analogues with or without the addition of clavulanic acid 0.6%o;
- for cephalosporins 1.4%o;
- When sulfonamides were used in combination with trimetroprim or related substances (received by 5077 patients) and fluoroquinolones (received by 1043 patients), no cases of colitis were observed;
- When using aminoglycosides (received by 1864 patients), only 1 case of antibiotic-associated colitis was observed, but there were 2 other antibiotics that could also be associated with colitis.
The incidence of diarrhea lasting at least 2 days ranged from 2 to 12% in patients receiving the same groups of medications during their hospital stay. The authors recommend discontinuing antibiotic treatment in patients with antibiotic-associated colitis and treating such patients with metronidazole or vancomycin. For patients who cannot stop antibiotic treatment, it is necessary to select an antibacterial chemotherapy agent with minimal risk of causing colitis.
Literature:
Zehnder D, KYanzi UP, Maibach R, Zoppi M, Halter F, Neftel KA, et al. Die HKufigkeit der Antibiotika-assoziierten Kolitis bei Hospitalisierten Patienten der Jahre 1974-1991 in “Comprehensive Hospital Drug Monitoring” Bern/St Gallen. Schweiz Med Wochenschr 1995;125:676-83.
INFLUENCE OF ACID SUPPRESSION ON THE EFFECTIVENESS OF TREATING HELICOBACTER PYLORI INFECTION
E.Bosch
A
Antibacterial therapy for Helicobacter pylori (H. pylory) is the main method of treating peptic ulcers. There are different ways to effectively treat: a ten-day course of a single antibiotic (amoxycillin or clarithromycin) or a combination of antibiotics (tetracycline, amoxicillin, metronidazole) with or without collodion bismuth citrate. Because the location of H. pylori under the gastric mucosa prevents the necessary access of antibiotics, another approach to facilitate access to the bacteria consists of combining two antibiotics with a proton pump inhibitor (omeprazole) or H2 receptor antagonists, which suppress acid secretion. Omeprazole appears to affect the intragastric distribution of H. pylori. Due to differences in side effects, antibiotic resistance, safety, efficacy, and patient compliance, there is controversy regarding the most effective treatment. De Boer et al. investigated the possibility of combining antibiotics with omeprazole to improve the results of triple therapy. Between June 1993 and April 1994, 108 consecutive patients with peptic ulcer disease and biopsy-proven H. pylori infection at a single center were randomly assigned to seven days of triple therapy with or without omeprazole (20 mg twice daily). Omeprazole was given from days 1 to 10; triple therapy was carried out from days 4 to 10: collodion bismuth subcitrate (120 mg 4 times a day), tetracycline (500 mg 4 times a day) and metronidazole (500 mg 3 times a day); the same triple therapy, but without omeprazole, was carried out from the 1st to the 7th day. A total of 98 (90.7%) of 108 patients were cleared of H. pylori, as determined by 10 endoscopic biopsies for urea test, histology, and 4-6 week post-treatment culture. Resistance to metronidazole pretreatment was quite rare (7.7%). The difference in effectiveness between the two groups was 14.8%; with omeprazole, 98.1% of patients were cured, without omeprazole - 83.3% of patients. Therapy was the only factor associated with cure in patients; smoking or gender did not affect treatment success. Three patients discontinued omeprazole due to nausea and vomiting. 16 and 54 patients in the omeprazole group, as well as 14 of 54 patients in the other group, had no adverse events. Vomiting occurred much less frequently in the omeprazole group. Other adverse events in the omeprazole group were headache, burning mouth, chills, fatigue, and vaginal moniliasis; the other group had more patients complaining of skin rashes, increased sweating, epigastric fullness and heartburn. It turned out that the addition of omeprazole increases the effectiveness of combating metronidazole-resistant strains, reduces side effects and leads to a high cure rate. This high treatment efficacy suggests that there is no need for a diagnostic cure test.
Literature:
De Boer W, Driessen W, Jansz A, Tytgat G. Effect of acid suppression on the efficacy of treatment for Helicobacter pylori infection. Lancet 1995;345:817-20.
ELIMINATION OF HELICOBACTER PYLORI USING OMEPRAZOLE IN COMBINATION WITH TWO ANTIBIOTICS
W. Hart
P
Orro et al. investigated the synergistic effects of omeprazole in combination with amoxicillin and metronidazole on H. pylori, recurrence of duodenal ulcers and the development of gastritis over a 1-year follow-up. Inclusion criteria for the study were at least one active, endoscopically proven duodenal ulcer and detection of H. pylori on biopsy specimens using urease testing and histology. The study was conducted over 4 weeks of treatment and 1 year of observation using a double-blind, placebo-controlled method in parallel with the main group. After endoscopy, patients were treated with omeprazole alone (20 mg/day) for 1 week. Then, 91 patients (group A) were prescribed omeprazole and metronidazole (250 mg 4 times a day) + amoxicillin (1 g 3 times a day) or omeprazole + placebo (92 patients, group B). Then treated with omeprazole alone for an additional 1 week. Endoscopy was performed upon admission and after 1 month of therapy. Cured patients underwent endoscopy at 2, 6, and 12 months after therapy or when signs of relapse appeared. In terms of demographic characteristics, both groups were similar (age, gender, smoking, alcohol consumption, duration of peptic ulcer disease). 86 patients from the two groups completed the study on day 29. The ulcer healed in 97.6% of patients in group A and in 93.0% of group B (the difference is insignificant). Histological examination aimed at identifying H. pylori was carried out 2 months after the end of treatment in 79 patients in group A and in 72 patients in group B. H. pylori was not detected in 89.8% of patients in group A and in 1.4% in group B (p < 0.001). During 12 months of observation, recurrent duodenal ulcers occurred in 4 (6%) of 63 patients in group A in whom H. pylori were eradicated, in 4 (50%) of 8 patients in group A in whom H. pylori persisted, and 52 (80%) of 65 patients in group B were persistently positive for H. pylori. A multivariate analysis conducted in 164 patients with duodenal ulcers that healed at the end of treatment showed that the risk of recurrence was 9.4 times higher in patients treated with omeprazole alone compared with those treated with omeprazole plus antibiotics. Age, gender, smoking, alcohol consumption, duration of peptic ulcer disease, and previous complications did not have a significant effect on ulcer healing or recurrence. Rapid, complete and sustained suppression of the activity of gastroduodenitis and gastric epithelial lesions was observed in the majority of patients in group A, in whom H. pylori was eliminated. Patients in group B experienced a temporary decrease in bacterial colonization and inflammation in the cavity and an exacerbation of gastritis. 11 patients in group A and 7 patients in group B had side effects. This study certainly proves that the combination of omeprazole with amoxicillin and metronidazole is a highly effective treatment for H. pylori and the prevention of ulcer recurrence.
Literature:
Porro GB, Lazzaroni M, Bargiggia S, Maconi G, Trespi E, Pereego M, et al. Omeprazole coupled with two antibiotics for Helicobacter pylori eradication and prevention of ulcer recurrence. Am J Gastroenterol 1996;91:695-700.
Antibiotics
Drug treatment of Crohn's disease and ulcerative colitis (part of the group of inflammatory bowel diseases - IBD) has two main goals:
- Achieving remission (absence of symptoms and inflammation in the affected part of the gastrointestinal tract)
- Maintaining remission (preventing exacerbations).
These goals must be achieved with the fewest side effects and the least possible risk. To achieve this, the goal of treatment is to control inflammation in the intestines – the cause of IBD symptoms.
Antibiotics are often used to treat exacerbations of IBD, although no infectious agents have been identified as the cause of these diseases. According to many researchers, antibiotics help control IBD symptoms by weakening gut bacteria and directly suppressing the gut immune system. Antibiotics may also be effective in the long-term treatment of patients with IBD (more so for patients with Crohn's disease than for patients with ulcerative colitis), especially if they have developed fistulas (channels connecting adjacent loops of the intestine). or intestine with another organ, such as the skin) or recurrent abscesses (collections of pus).
Symptoms of infections can be very similar to the symptoms of an exacerbation, so it is necessary to do a stool analysis of parasites, in particular for the presence of intestinal lamblia, and for dysbiosis, especially for the presence of the bacterium Clostridium difficile (c.diff), which occurs more often in patients with IBD. Such infections are quickly treated with antibiotics that include metronidazole or vancomycin. A new antibiotic for the treatment of c.diff is fidaxomicin.
Oral medications
Although many antibiotics are effective in treating IBD, the most commonly prescribed are:
- Metronidazole (Flagyl®)
- Ciprofloxacin (Cipro®)
- Rifaximin (Xifaxan®)
Both metronidazole and ciprofloxacin are broad-spectrum antibiotics that target a wide range of bacteria both inside and outside the gut. Rifaximin is not absorbed and therefore has a direct effect only on bacteria in the gastrointestinal tract. Metronidazole is the most actively studied antibiotic in the treatment of IBD. As the mainstay of treatment for exacerbations of Crohn's disease, this drug is stronger than placebo (dummy) and similar to sulfasalazine - especially when the disease affects the colon.
Metronidazole also reduces the risk of relapse during the first three months after ileal resection. Metronidazole is effective in the treatment of Crohn's disease affecting the perineal area, as found in 50% of patients.
Antibiotics are often prescribed to patients who develop pouchitis (pausitis) - inflammation of the reservoir formed from the ileum - during surgery to form a small intestinal reservoir with ileoanal anastomosis. In this type of surgery, after the colon is removed, an internal reservoir is created from the patient's ileum (lower small intestine), eliminating the need for an external device (a colostomy bag). Sometimes the reservoir becomes very inflamed - this inflammation is called pouchitis. Ciprofloxacin, metronidazole and rifaximin have been successfully used in the treatment of pouchitis. According to well-established studies, taking the probiotic VSL#3 after a course of treatment with rifaximin helps prevent recurrence of pouchitis.
Alternative uses
Both metronidazole and ciprofloxacin can be given intravenously if necessary and are sold in ampoules. Rifaximin is used orally only.
Side effects
Metronidazole: Common side effects include nausea, vomiting, loss of appetite, metallic taste in the mouth, diarrhea, dizziness, headaches, urine color (dark brown or red-brown). Another side effect that occurs due to long-term use of the drug is trembling in the arms and legs, which may continue even after stopping the drug.
Ciprofloxacin: Side effects include headaches, nausea, vomiting, diarrhea, abdominal pain, rash, anxiety - and are rare. There have also been cases of tendonitis (pain and inflammation of the tendons), particularly affecting the Achilles tendon (which connects the calf muscle to the heel bone) and tendon rupture.
Rifaximin: Side effects are rare but may include nausea, abdominal pain, dizziness, fatigue, headaches, muscle tension and joint pain.
Interaction with other drugs
Patients taking multiple medications (prescription or over-the-counter) at the same time should carefully study the interactions of medications with each other. These interactions may reduce the effectiveness of the drugs, increase the effect of the drug, or cause unusual side effects. Before taking any medications, you must carefully read the instructions. You should tell your doctor about all medications you are taking (including over-the-counter drugs, supplements, and alternative medications) and any medical conditions you have.
Additional Information
Metronidazole reduces the breakdown of alcohol in the body and this can lead to nausea and vomiting. Therefore, you should avoid alcohol in any form while you are taking this drug and for at least two days after your last dose.
Ciprofloxacin may interact with antacids (eg, Rolaid, Tums), reducing its effectiveness. Your doctor will likely tell you not to take these two medications at the same time. A similar interaction occurs with vitamin and mineral supplements containing calcium, iron, or zinc.
If you are pregnant, tell your doctor before taking metronidazole or ciprofloxacin. They are often prescribed to pregnant patients, but it is still recommended to consult a doctor first. Although rifaximin is poorly absorbed, it is not usually prescribed to pregnant women.
Avoid exposure to the sun during treatment with ciprofloxacin. Use sunscreen during the day and avoid tanning beds.
Antibiotics interact poorly with anticoagulants such as warfarin (Coumadin®), excessively reducing blood clotting and increasing the risk of bleeding. The dose of warfarin may need to be adjusted. You must tell your doctor who prescribes antibiotics that you are taking warfarin.
Find out as much information as possible from doctors. You can also use reliable Internet resources, websites of pharmaceutical companies.
Ulcerative colitis
- Back to the section “Proctology and coloproctology”
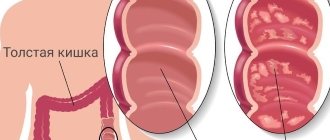
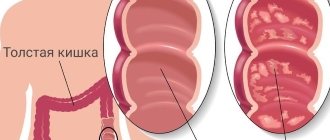
Ulcerative colitis or nonspecific ulcerative colitis (UC)
is a chronic disease of the colon, characterized by immune inflammation of its mucous membrane. In ulcerative colitis, only the large intestine is affected, and the inflammation never spreads to the small intestine.
Causes of the disease
The causes of the disease have not yet been clarified. Scientists suggest that various factors cause a disruption of the immune response, which leads to the disease.
Of the variety of causes that can cause the disease, the most common are:
- eating foods rich in carbohydrates and poor in dietary fiber;
- mental trauma and stress;
- inactivity; dysbiosis.
IMPORTANT!
If you have loose stools mixed with blood, pus and mucus, if you have severe abdominal pain (usually on the left), if you have decreased appetite, weight loss, weakness, or joint pain, you need to do it
URGENTLY!
consult
a coloproctologist
and
gastrointestinal specialist!
Symptoms
Ulcerative colitis can occur with periods of exacerbation and remission. Its severity varies from mild to moderate to severe.
Mild to medium
severity is indicated by general symptoms:
- weakness;
- malaise;
- increased temperature;
- frequent stools with blood clots;
- There are cramping pains in the abdomen.
In case of severe illness
the temperature rises to 38 degrees or higher. Wherein:
- the skin turns pale,
- anemia develops;
- tachycardia appears;
- often feel dizzy;
- weakness increases;
- there is frequent stool (more than 6 times per day) with blood;
- blood is secreted in clots; before defecation, severe cramping pain.
Types of Ulcerative Colitis
- With spastic colitis,
there is bloating, cramps, and intestinal function is disrupted. This is not a serious illness, but more of a functional disorder that can occur due to stress, nervous overexcitation and fatigue. - With chronic
intestinal colitis, inflammatory processes occur, with strophic and dystrophic changes in the mucous membrane in the colon. They may be accompanied by secretory and motor disorders. The causes of the disease are known - these are infectious diseases such as dysentery (Salmonella and Shigella), toxic substances, and exposure to drugs. - Acute colitis occurs
when the mucous membrane of the small intestine or stomach becomes inflamed. The causes may be viral infections, non-bacterial food poisoning, toxic substances. The causative agent of acute colitis is considered to be bacterial dysentery (Shigella, Salmonella). - Pseudomembranous colitis is
an acute disease of the colon and is considered a serious disease. It may develop as a result of complications of antibacterial therapy. 10-20% of patients have Clostridium difficile resistance to antibiotics, which suppresses the intestinal microflora. Timely diagnosis will allow timely discontinuation of the antibiotic causing diarrhea. - With erosive colitis
(inflammatory processes of the lining of the duodenum, which is adjacent to the stomach), it can lead to the formation of ulcers. - Ischemic colitis
(inflammation in the gastrointestinal tract) is caused by vascular damage, which leads to tissue necrosis. - Collagenous colitis
is a diffuse increase in the number of interepithelial lymphocytes, which over time leads to transformation into collagenous lymphocytic colitis. - With alcoholic colitis,
the patient develops chronic pancreatitis and lipid metabolism is disrupted, so this is a secondary disease. Alcohol, affecting the flora of the colon, makes it pathogenic. When a person stops drinking, colitis disappears. - Nonspecific ulcerative colitis
is considered diffuse inflammation of the mucous membrane of the colon and rectum (large intestine). The disease is chronic and the type of immune nature is recurrent. - The disease in the form of atonic colitis
threatens elderly and senile people. Due to constipation, which leads to hemorrhoids, the motor function of the intestine sharply decreases. 1 - In hemorrhagic colitis,
the disease is expressed by acute bloody diarrhea, which is caused by Escherichia coli. Its gram-negative strains damage the endothelium of blood vessels and are similar to dysentery. - In case of radiation damage
to the body, which is caused by ionizing radiation, intestinal damage develops. However, clinical symptoms are not always expressed in the acute form of damage to its mucous membrane. - Distal and left-sided colitis
is characterized by an inflammatory process of 30-40 centimeters of the colon. When the inflammation reaches the splenic angle, colitis is called left-sided.
Examination and diagnosis
Laboratory tests for ulcerative colitis include:
- general blood test (assessment of the degree of blood loss, severity of anemia);
- blood clotting test; blood test for bleeding.
As a key instrumental method
The study is fibrocolonoscopy, which allows you to accurately diagnose the type of colitis and assess the degree of damage to the intestinal mucosa. A flexible device is inserted through the anus into the intestinal lumen. The examinations also include a standard set of studies, which is necessary for hospitalization for inpatient treatment. Considering that during exacerbation of ulcerative colitis, colonoscopy is difficult (inflammatory changes in the intestine, danger of perforation), irrigoscopy is performed. The method is safe, but less informative. To diagnose a complication of colitis, a plain radiography without contrast agents is shown in the abdominal cavity - perforation of the colon.