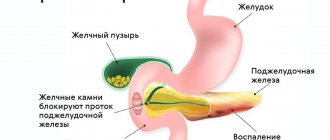
Zollinger-Ellison syndrome (ZES) is a cancer in which tumors in the gastrointestinal tract cause increased secretion of the hormone gastrin. Its excess amount is manifested by signs of paraneoplasia. In more than half of cases, the disease is malignant and requires serious regular monitoring. You can carry out this control using diagnostics in Germany, which is carried out by the Nordwest clinic.
The main symptoms of gastrinoma include tumors of the pancreas, as well as the upper part of the small intestine. The disease is quite rare and appears mainly from 30 to 60 years of age.
Symptoms
A characteristic symptom is pain in the upper abdomen, which is more often observed in men than in women. This sign of gastrinoma occurs after eating or on an empty stomach. It is similar to the pain that accompanies a regular duodenal ulcer, but is more intense, very persistent and does not disappear after antiulcer therapy is prescribed. Pain in the upper abdomen is diagnosed in half of patients with Zollinger-Ellison syndrome.
The second characteristic symptom is persistent diarrhea. It appears due to the entry of large amounts of hydrochloric acid into the small intestine, as a result of which small intestinal motility and absorption slow down. Large and watery stools contain a large amount of fat and undigested food particles. This symptom is more typical for women.
Other signs include:
1. Belching sour.
2. Constant heartburn.
3. Non-healing ulcers of the duodenum and stomach, which are manifested by bleeding.
4. Inflammation of the esophageal mucosa, accompanied by narrowing of the esophagus.
5. Mendelian symptom – pain at the site of the ulcer.
6. Weight loss due to the inability of the gastrointestinal tract to digest food normally.
When malignant tumors of the duodenum appear, the liver may become enlarged in size and similar formations may occur.
When prescribing antiulcer therapy, treatment does not produce results, since gastric and duodenal ulcers constantly recur.
Sign up for a consultation
Have you noticed one or more symptoms?
Neuroendocrine tumors: epidemiology, general provisions
Neuroendocrine tumors (NETs) are rare malignancies originating from neuroendocrine cells of the fetal intestine. A characteristic feature of NETs is the ability to synthesize hormones and biogenic amines, which determines their main clinical manifestations.
In recent decades, a steady increase in the incidence of NETs has been recorded throughout the world, which may be due to the increased availability of high-tech diagnostic methods. According to the US National Cancer Institute, the annual age-adjusted incidence rate of NETs increased 6.4 times, from 1.09 per 100 thousand in 1973 to 6.98 per 100 thousand in 2012; a total of 64,971 cases of NET were reported during this period [1]. Gastroenteropancreatic (GEP) NETs account for about 70% of all neuroendocrine neoplasms. Currently, the incidence of GEP NET is estimated at 3-5.2 per 100 thousand new cases per year, and the average prevalence rate is 5.86 per 100 thousand people per year. Men get sick somewhat more often than women: the proportion of men with detected NETs is 52% (5.35 per 100 thousand cases per year compared to 4.76 per 100 thousand in women) [2].
The most common localizations of NETs in the digestive tract, according to M. Cives and J. Strosberg (2018) [1], are the small intestine (30.8%), rectum (26.3%), colon (17.6% ), pancreas (PG) (12.1%) and appendix (5.7%).
In a study by J. Darbà and A. Marsà (2019) [3], data on patients from 192 private and 313 public clinics from different regions of Spain were analyzed. A total of 9120 cases of NET were registered between 2010 and 2015. In addition, a 2-fold increase in primary incidence was noted, while benign NETs were recorded only in 18.59% of cases, and 42.93% of patients had metastatic lesions at the time of diagnosis. The most common localizations of NETs, according to the authors, were the respiratory system, pancreas and gastrointestinal tract (GIT). Taking into account the need for extensive surgical interventions and expensive courses of chemotherapy, the cost of treatment per patient was 9092 euros, or 15,373,961 euros per year.
NETs of the pancreas account for up to 10% of all significant neoplasms of the organ. These are typically slow-growing tumors with overall 5-, 10-, and 20-year survival rates of 33%, 17%, and 10%, respectively. Pancreatic NETs can occur sporadically (75-80%) or as a manifestation of genetically determined conditions: hereditary syndrome of multiple endocrine neoplasia type 1 (MEN-1), von Hippel-Lindau syndrome, neurofibromatosis type 1, tuberous sclerosis. Pancreatic NETs are characterized by specific genetic disorders - mutations of the MEN1, DAXX and ATRX genes, as well as mTOR signaling pathway genes - TSC2, PTEN and PIK3CA. Genetic disorders in neuroendocrine pancreatic cancer are mainly represented by mutations of genes involved in the cell cycle, such as TP53, RB1 and CDKN2A (p16) [4].
Most pancreatic NETs are hormonally inactive; clinical symptoms of such formations begin to appear with local spread of the tumor process (symptoms of compression/stenosis) or with the development of metastatic liver damage. Tumors associated with clinical syndromes caused by abnormal hormone production are considered functioning (syndromic) NETs. These include insulinomas, gastrinomas, glucagonomas, VIPomas and other rarer neoplasms [5].
Diagnostics
Before making a diagnosis, the endocrinologist performs the following actions:
1. Collects anamnesis of the life and illness of the patient, as well as his family.
2. Makes an objective examination.
Instrumental studies allow:
1. Determine the level of gastrin: in laboratory conditions it is possible to detect the content of this hormone in the blood. In Zollinger-Ellison syndrome, gastrin reaches 1000 pg/ml, while in a normal ulcer its amount is about 100 pg/ml.
2. Test stomach acid.
3. Test the patient for the presence of Helicobacter pylori.
4. Examine the abdominal cavity using the ultrasound method.
5. Perform selective abdominal angiography.
6. Test secretin.
7. Obtain a mandatory consultation with a gastroenterologist and surgeon.
The most informative studies are:
1. Ultrasound examination.
2. Computed tomography.
3. Selective abdominal angiography, in which blood is taken from the pancreatic veins to further determine the gastrin content in it.
Clinical observation
Patient K., 36 years old, was hospitalized on August 27, 2019 in the gastroenterology department of the Peter the Great Clinic at the Department of Propaedeutics of Internal Medicine, Gastroenterology and Dietetics named after. CM. Ryss Federal State Budgetary Educational Institution of Higher Education Northwestern State Medical University named after. I.I. Mechnikov of the Ministry of Health of Russia with complaints of moderate intensity pain in the epigastric region before eating, a feeling of fullness in the epigastric region, a feeling of rapid satiety; episodes of heartburn, belching of air, periodically (2 times a month during the previous year) loose stools (up to 2-3 times a day, type 5-6 on the Bristol scale, without pathological impurities), loss of body weight by 10 kg For 3 months, while restricting simple carbohydrates (as recommended by a doctor), she periodically notes low-intensity pain in the lumbar spine.
From the anamnesis it is known that for the first time pain in the epigastric region and dyspeptic symptoms arose in June 2022 while taking non-steroidal anti-inflammatory drugs (NSAIDs) - 2 courses in March and June 2022 for intense pain caused by sequestration of a herniated lumbar intervertebral disc spine. Before planned surgical treatment, on June 24, 2019, the patient underwent esophagogastroduodenoscopy (EGD), which revealed acute erosions of the gastric mucosa. The condition was regarded as NSAID-induced gastropathy, NSAIDs were discontinued, treatment was prescribed: bismuth tripotassium dicitrate (480 mg/day), rebamipide (300 mg/day), PPI in a standard daily dosage, itopride hydrochloride (150 mg/day). After 2.5 weeks of taking the drugs, against the background of high patient compliance, no significant clinical and endoscopic response was achieved: epigastric pain syndrome persisted, and during control endoscopic examination, acute erosions in the body and fundus of the stomach persisted. The patient was recommended to continue taking bismuth tripotassium dicitrate, rebamipide, and PPI. Repeated endoscopy was performed on 08/05/19 (6 weeks after the start of therapy): against the background of persistent erosions in the body and fundus, the appearance of flat SM defects measuring 0.4–0.5 cm, dark brown in color, was observed in the antrum of the stomach. Continued use of PPIs and cytoprotectors. Due to the lack of clinical response against the background of long-term adequate therapy, the patient was hospitalized to clarify the diagnostic idea.
During further examination, pathology of the parathyroid glands was excluded; radiography of the esophagus, stomach and duodenum revealed no pathology. When assessing tumor markers: CA 72-4, CEA, CA 19-9 - within the reference values. In clinical and biochemical blood tests, general urine analysis, coprogram - without clinically significant deviations from the norm. Ultrasound of the abdominal organs did not reveal any organic pathology.
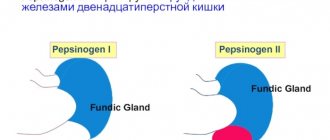
2 weeks after stopping the PPI (therapy with cytoprotectors and antacids was prescribed), the patient was tested to determine H. pylori infection (13C urease breath test) - H. pylori status was negative. When performing a complex study "Gastropanel", a 5-fold increase in the basal level of gastrin-17 (G-17b) to 33.4 pmol/l (normal 1-7 pmol/l), an increase in the level of pepsinogen I (PGI) to 196 mcg/l was revealed. l (normal 30-160 µg/l), pepsinogen II (PGII) up to 33.2 µg/l (normal 3-15 µg/l), PGI/PG II ratio, total gastrin level - within the reference values.
After consulting a psychotherapist (chronic psycho-emotional stress: leadership position, maternal cancer, difficult relationships with children in the family), the patient was prescribed antidepressant therapy (amitriptyline 25 mg/day) to level out the psycho-emotional component as a possible cause of the torpid course of the disease.
In the control endoscopy No. 4 (08/30/19), while taking PPI in a double dose, negative dynamics were again recorded: an increase in the area of erosions and hemorrhages of the gastric mucosa, involvement of the esophageal mucosa in the pathological process (erosive esophagitis grade A according to LA) and duodenum (multiple erosions under fibrin interspersed with hematin measuring 2-5 mm, Fig. 1) .
Rice. 1. Multiple erosions of the duodenum under fibrin interspersed with hematin.
A morphological examination of biopsy specimens (in order to clarify the diagnosis and exclude oncopathology, including MALT lymphoma) revealed fragments of gastric mucosa with hyperplasia of the integumentary epithelium, areas of erosion, regenerative changes in the epithelium of the glands without foci of intestinal metaplasia, with a decrease in the number of glands, edema, and small foci hemorrhages in the submucosal layer, congestion of blood vessels, with diffuse weak infiltration of lymphocytes, areas of sclerosis. No H. pylori was detected by Romanowsky-Giemsa staining. When assessing the histological picture of the duodenum, signs of hyperplasia of mucus-producing cells in the integumentary epithelium of the glands, diffuse lymphocytic infiltration, vascular congestion and areas of sclerosis were revealed. Morphologist's conclusion: Chronic gastritis stage II, grade I according to OLGA, H. pylori status negative.
Upon discharge, the diagnosis was formulated: Chronic gastritis stage II, grade I according to OLGA, H. pylori status negative. Multiple erosions of the stomach and duodenum, hypergastrinemia of unspecified origin (Zollinger-Ellison syndrome?). Erosive esophagitis, stage A (according to LA). Asthenic neurosis.
The patient was recommended to continue taking PPIs in a double dose, cytoprotectors, antidepressants, as well as further examination.
In November 2022, the patient underwent EUS (11/07/19), and a submucosal formation of the descending branch of the duodenum was detected (neuroendocrine tumor?). A scan of the identified submucosal formation of the descending branch of the duodenum was performed (Fig. 2) : the formation is homogeneous, hypoechoic, coming from the 2nd echo layer (deep layers of the CO). Size 7´5 mm. The contours are blurry and uneven. The submucosal layer under the formation can be traced and is clearly thinned. The muscle layer is intact. Elastography revealed a formation of increased density. Paragastrically in the area of the 4th echo group there are multiple lymph nodes with a diameter of up to 6 mm, round. Along the hepatoduodenal ligament there are lymph nodes up to 12 mm, with a preserved cortico-medullary structure. Conclusion: The EUS picture most likely corresponds to the NET of the descending branch of the duodenum, T1N0MX. Paragastric lymphadenopathy. Lymphadenopathy along the hepatoduodenal ligament.
Rice. 2. Submucosal formation of the descending branch of the duodenum.
Histological examination of duodenal biopsies (11/08/19, N.N. Petrov National Medical Research Center): fragments of duodenal mucosa with preserved villi, moderate chronic inflammatory infiltration, without activity, with focal hyperplasia of neuroendocrine cells. Histological examination of gastric biopsies: chronic diffuse gastritis with a weak inflammatory component, without activity, no H. pylori contamination.
According to the results of MSCT with contrast (11/16/19, N.N. Petrov National Medical Research Center), a hypervascular formation with a diameter of 5 mm in the duodenum was revealed. No other hypervascular formations were found in the scanning area.
In December 2022, the patient underwent electrosurgical tumor removal using the EPR method. A morphological study of the surgical material revealed a highly differentiated NET G1 of the duodenum (7 mm in the greatest dimension, 0-1 mitosis per 2 mm2 of tumor), localized in the lamina propria and muscularis lamina mucosa with invasion into the submucosal layer to a depth of 2300 µm. Lymphovascular invasion was not detected. According to immunohistochemical study: CK 8, CgA, Synaptophysin - positive, Ki-67 - 2%.
The postoperative period proceeded without complications. After discharge, the patient’s complaints resolved, and PPI therapy was continued for 4 weeks. After 4 weeks, endoscopic control was performed: the condition after full-wall endoscopic removal of the NET D2 portion of the duodenum (G1 gastrinoma). No endoscopic data were obtained for postoperative duodenal stenosis. Erythematous antral gastropathy was revealed.
Treatment
There are two types:
1. Surgical – consists of surgical removal of malignant tumors – resection.
2. Conservative – involves treatment with a proton pump inhibitor (omeprazole, pantoprazole, lansoprazole), which reduces the secretion of hydrochloric acid. These are drugs that eliminate stomach and duodenal ulcers.
You can get a doctor’s consultation at our endocrinology center in Samara by submitting an online application from our website or by calling. Be healthy!