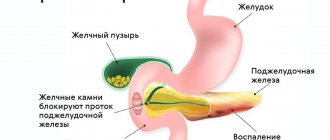
Pancreatic lipomatosis is a pathology in which normal cells are replaced by fat cells. Such changes negatively affect the performance of the organ. The situation is aggravated by the long asymptomatic period.
In fact, a person begins treatment when it is no longer possible to change something for the better using conservative methods. This article provides expert advice that will help you diagnose the disease in a timely manner and take adequate response measures.
Risk factors
Pancreatic lipomatosis is fatty pancreas.
Why some people develop lipomatosis and others do not is not known for certain.
However, ongoing statistical studies allow us to identify some risk factors, the presence of which may lead to the formation of unwanted fat cells in the pancreas.
The most common situations that provoke the development of lipomatosis are given below:
- history of acute pancreatitis;
- current chronic pancreatitis;
- frequent consumption of alcoholic beverages;
- burdened heredity;
- current diabetes mellitus or chronic hepatitis;
- obesity;
- insufficient amount of thyroid hormones.
The fact that the factors mentioned above can provoke the development of lipomatosis does not mean that those who have these conditions will necessarily develop pancreatic obesity. However, in the absence of all these factors, the disease almost never develops.
Signs of pancreatic malfunction
Due to a decrease in the percentage of healthy, normally functioning tissues in relation to the affected ones, digestion is disrupted. Protein foods and everything fatty are especially difficult to perceive. A person experiences the following symptoms:
- nausea;
- stomach ache;
- flatulence;
- heaviness, bursting feeling in the stomach;
- frequent stools containing fat and other impurities.
Due to the disease, disruptions in the production of hormones occur. As a result, complex endocrinological disorders develop. This applies to a greater extent to carbohydrate metabolism. At the same time, glucose levels increase sharply.
If this pathological course is not stopped, then over time a person will develop diabetes.
Chronic pancreatitis (CP) is an inflammatory disease accompanied by progressive morphological tissue damage and functional pancreatic insufficiency. With each attack of acute pancreatitis, the pancreatic parenchyma is irreversibly replaced by scar fibrous tissue [31]. Alcohol abuse continues to be the main cause of CP [20]. In the world, the primary annual incidence of CP is 8 new patients per 100,000 population [41], the overall incidence of CP is 26.4 per 100,000 population [52].
The main morphological characteristic of CP is the development of fibrosis and fibrocystic degeneration of the pancreas [13]. In this case, fibrous tissue gradually replaces the pancreatic parenchyma, and the severity and types of fibrous-scar changes are determined by the duration of exposure and the type of etiological factor [31].
Currently, about 90 different research methods are used to diagnose CP, but most of them do not make it possible to establish a diagnosis of CP at an early stage of the disease [8], which leads to progression of the process and the need for surgical intervention.
Operations for CP are mainly of a resection nature (resection of the head of the pancreas, trough-shaped excision of the walls of the main pancreatic duct, etc.) [56], since it has now been shown that the main complaint of patients for pain in the pancreas is associated not only with pancreatic ductal hypertension , but also with the germination of nerve pathways by fibrous tissue [37]. The severity of fibrosis during such operations is of fundamental importance, since interventions on the “soft” gland are accompanied by bleeding and postoperative complications, among which pancreatic necrosis is one of the reasons for high mortality [27, 28, 47, 54, 57]. In this regard, information about the development of fibrosis, its morphogenesis, the possibility of resorption and diagnosis acquires special significance.
Morphofibrogenesis of the pancreas
Collagen production plays a crucial role in the development of fibrosis. Currently, there are 28 types of collagen, which differ in amino acid sequence, organ and tissue affiliation, as well as the intensity of hydroxylation or glycosylation. There are type I collagen (in connective tissue, bones and other tissues), type II collagen (in cartilage, vitreous body and cornea of the eye), type III collagen (in the walls of large blood vessels), type IV collagen (in basement membranes, lens capsule ), collagen type V (in the chorion, amnion, endo- and perimysium, skin and other tissues), as well as collagen types VI-XVI - interstitial and non-fibrillar collagens [5]. The main producers of collagen are fibroblasts, as well as pancreatic stellate cells (PSC, Ito cells), which migrate to the area of necrosis and transform into myofibroblasts. Types of collagen replace each other, starting from III and ending with I, determining the phases of the fibrotic process [9], while type I collagen contains a large number of cross-links. It is characteristic of dense scar connective tissue that is poorly broken down by proteases.
When studying the morphological state of a normal pancreas, fibroblasts of the connective tissue interlobular septa are characterized by weak expression of type III collagen. The basement membranes of the acini include type IV collagen. Expression of smooth muscle actin-α is observed only in the smooth muscle membranes of the vessel walls and is completely absent in the pancreatic stroma [9].
Fibrosis in CP is usually the result of necrosis of pancreatic cells, as well as a consequence of an imbalance between the synthesis and degradation of the protein extracellular matrix (EM) [49]. Pancreatic fibrosis is initiated, as already indicated, by the activation by various factors of pancreatic matrix-producing retinoid-containing stellate cells, which at the same time modify their morphological structure and acquire the ability to produce collagen fibers, leading to their excessive accumulation in the pancreatic tissue [21, 26, 45]. Pancreatic stellate cells (PSC), isolated by culture for the first time in 1998 [16, 18], are cells constantly present in the pancreas, which are located in its periacinar, periductal and perivascular spaces and make up about 4-7% of the cells of this gland normally [ 45].
The introduction of vitamin A into the culture of these cells leads to their differentiation into myofibroblast-like cells expressing muscle actin-α, collagen types I and III, laminin and fibronectin (Fig. 1 and below) [21, 24].
Rice. 1. The active form of PSC is myofibroblasts. Positive immunohistochemical reaction for α-SMA (α-smooth muscle actin - arrows). Uv. 400 [21].
In 2010, M. Erkan et al. [25] showed that pancreatic and hepatic stellate cells (key mediators of liver fibrosis) have a similar genomic transcription pattern, while PSCs differ in the production of specific type I collagen 11α1 - COLL11α1. In the study by O.V. Paklina [9] showed that as pancreatic fibrosis progresses in patients with CP, collagen types III and V accumulate in the extracellular matrix and their subsequent irreversible transformation into type I collagen, which is characteristic of cirrhotic changes, old scars and is poorly degraded by proteases. An imbalance between the production of collagens and their natural inhibitors ultimately leads to the development of pancreatic fibrosis [23, 44].
In a study by B. Muehling et al. [38] when comparing the severity of fibrosis of the head and body of the pancreas in operated patients with CP, it was revealed that in the case of alcoholic CP, the severity of fibrosis in the head of the pancreas, determined by a computer method using Adobe Photo Shop, averaged 64% with 47% in the body of the pancreas, for idiopathic CP - 40 and 32%, respectively. Moreover, in the head of the pancreas, among the components of the EM, in addition to collagen types I and III, type IV collagen (in the ductal epithelium) and laminin (in the basement membranes) are present in larger quantities compared to the body of the pancreas. The authors emphasize that this fact also indicates the role of the head of the pancreas as a “pacemaker” of chronic pancreatitis, and type IV collagen and laminin play an important role in the fibrogenesis of chronic pancreatitis [38].
After acute necrotizing pancreatitis, PGCs and their subsequent forms - activated myofibroblasts, as shown by studies by A. Zimmermann et al. [58], can participate not only in the processes of pancreatic fibrosis, but also in its remodeling through the formation of regenerative centers, tubular complexes emanating from the pancreatic lobules remaining after pancreatic necrosis [58]. This phenomenon is a compensatory response to hypoxia and damage to the pancreatic parenchyma in the form of metaplasia of acinar tissue into a more primitive ductal epithelium [9, 58].
The relationship between an episode of acute pancreatitis (AP) and the development of CP has been the subject of a number of studies over the past several decades, which have attempted to determine the possible pathogenetic mechanisms of this relationship [32]. The potential possibility of transforming AP into CP was first formulated back in 1946 by M. Comfort et al. [19] in the form of the “theory of the relationship between necrosis and fibrosis,” which currently occupies a leading position [53]. The mechanism of development of CP is realized through repeated episodes of A.P. The scientific basis for this theory is based primarily on morphological studies conducted by G. Kloppel and B. Maillet [32]. The authors showed that peripancreatic necrosis is not the cause of the development of CP, while intrapancreatic necrosis leads first to perilobular fibrosis, and subsequently to intralobular ductal changes. Currently, the theory of the development of CP as a result of relapses of AP is confirmed in most clinical and experimental studies [12]. An important role in the triggering mechanism of irreversible scar-fibrous changes is still assigned to the activation of the PGC [36].
Among the factors of pancreatic damage that are triggers in the mechanism of direct and indirect activation of the pancreas with subsequent hyperproduction of essential oils and the development of pancreatic fibrosis, ethanol and its metabolites (acetaldehydes), oxidative stress, parenchymal and ductal pancreatic hypertension, hyperglycemia, cyclooxygenase-2, activated G- peptide receptor-2 and infectious factor [17, 48].
A number of studies have shown that the progressive destruction of pancreatic tissue and its replacement by fibrosis fields are accompanied by increasing functional failure of the pancreas [15]. This is confirmed by a long-term study conducted by R. Ammann et al. [13]. The authors found a significant ( p
<0.001) direct correlation between the severity of pancreatic fibrosis and its functional exo- and endocrine insufficiency. Among the identified features in the first 6 years from the onset of the disease, the predominance of necrosis foci compared with fibrosis fields in 49% of patients and the presence of pancreatic pseudocysts in 90% of patients were noted. The authors distinguish different types of fibrosis - perilobular and intralobular, focal and diffuse forms. At the same time, the severity of pancreatic fibrosis increased in direct proportion to the duration of the disease.
The formation of fibrosis is largely associated with bone marrow stem cells migrating to the area of damage to the pancreas. These cells are highly active, secreting fibronectin, collagen, and hyaluronic acid into the intercellular spaces. Along with this, they also express extracellular proteases that remodel the structure of the primary matrix formed during the first phase of healing of pancreatic necrosis (inflammation). Subsequently, mesenchymal cells—stem cells, fibroblasts, myofibroblasts—secrete collagen (mainly type III) and remodel granulation tissue into a collagen matrix [4]. At this fibrotic stage, repair of areas of necrosis of the pancreas stops and restoration of the structure and function of the pancreas does not occur. Each attack of AP creates a new field of fibrosis in the pancreas or, if fibrosis does not develop, a pancreatic pseudocyst.
Inhibitors of pancreatic morphofibrogenesis
The main inhibitors of morphofibrogenesis are matrix metalloproteinases (MMPs). The MMP family consists of 20 enzymes capable of breaking down almost all components of the extracellular matrix of connective tissue. They play an important role in many normal physiological processes, such as embryonic development, morphogenesis, reproduction and tissue remodeling, as well as in various pathological processes: arthritis, malignant growth and cardiovascular diseases. The amount of newly synthesized MMPs is regulated mainly at the transcriptional level, and the proteolytic activity of existing MMPs is controlled both by the activation of proenzymes and by the inhibition of active enzymes by endogenous inhibitors, α2-macroglobulin and tissue inhibitors of metalloproteinases (TIMPs). Based on their specificity, MMPs can be divided into collagenases (MMP-1, MMP-8 and MMP-13), gelatinases (MMP-2 and MMP-9) and stromelysins (MMP-3 and MMP-10). MMPs are secreted by various cells (fibroblasts, macrophages, smooth muscle cells of the vascular wall, neutrophils, chondrocytes, osteoblasts, etc.) and hydrolyze all components of the extracellular matrix: all collagens and procollagens, proteoglycans, elastin, fibronectin, laminin, as well as adhesive and other proteins of the connective tissue tissue [5, 40]. Expression of gelatinase (MMP-9) is observed not only in fibroblasts, endothelial cells, macrophages, but also in the epithelium of the pancreatic ducts and nerve trunks. Normally, collagenase MMP-1 and gelatinase MMP-9, as well as their inhibitor TIMP-1, are characterized by weak expression. Expression of proteases is observed in the nuclei and cytoplasm of acinar cells, fibroblasts, endothelium, macrophages, nerve trunk cells and the cytoplasm of single cells of the pancreatic ductal epithelium. Expression of tissue inhibitors of MMPs is observed only in acinar and endocrine cells. It is precisely this pattern of expression of matrix proteases and their tissue inhibitors under normal conditions that probably reflects the balance between them, in which collagen does not accumulate in the extracellular matrix.
Great importance in the resorption of fibrosis is currently attached to stem cells, in particular mesenchymal stromal cells of the bone marrow. In the process of in
vitro
, it is possible to obtain a line of stem cells, specifically organ-oriented, which, upon transplantation, turns into functionally active cells of the organ. In this pathway, stem cells suppress the main cellular sources that express collagen and matrix proteins, and also secrete MMPs that resorb collagen fibers. There are experimental and isolated clinical observations indicating the possibility of resorption of connective tissue in cirrhosis of the liver and some other organs [7].
Laboratory diagnosis of fibrosis
The search for various laboratory criteria for the early diagnosis of pancreatic fibrosis led to the study of a family of plasma metalloproteinases, namely MMP-9 (gelatinase B, involved in the biodegradation of collagen types I, III and IV in the extracellular matrix). A. Venkateshwari et al. [55], studying 112 patients with CP, showed a significant increase in the level of MMP-9, which leads to activation of pancreatic stellate cells, as well as to excessive destruction of type IV collagen, which is normally involved in intercellular connections.
When studying the levels of MMP-1 (collagenase) and MMP-3 (stromelysin), as well as transforming growth factor β1 (TGF-β1) in 71 patients with CP compared with the control group (100 healthy ones), a significant increase in the level of MMP-1 and TGF was revealed -β1 ( p
<0.05), while the increase in MMP-3 levels was insignificant (
p
= 0.37) [34]. Thus, an increase in the expression of TGF-β1, which is involved in the hyperproduction of EM, promoting the migration of PGCs and their activators - monocytes to the fibrogenesis zone, as well as an increase in the level of MMP-1 and MMP-9, which are responsible for the degradation of various components of EM, indicate high activity of the fibroplastic process with an application point in the pancreas. This is one of the criteria for laboratory diagnosis of chronic pancreatitis [11, 23, 34, 55]. In a study by K. Adrych [11], an increase in TGF-β1 was noted along with an increase in the level of laminin and hyaluronic acid in the blood serum of patients with CP - markers of the proliferation activity of the extracellular matrix of P.Zh. However, in these works there are no clinical and morphological comparisons of the identified laboratory changes in blood plasma and the severity of pancreatic fibrosis, both histological and instrumental, so at present it is too early to consider the presence of so-called laboratory markers of the severity of pancreatic fibrosis as proven.
In the studies of M.I. Voevoda et al. [1-3] showed a correlation between the optical properties of blood serum, determined by the methods of spectral ellipsometry and infrared Fourier spectroscopy, depending on its bioorganic composition, with the histologically determined severity of liver fibrosis. In chronic pancreatitis, such research has only just begun, but it can reveal the severity and nature of collagen types by analyzing changes in the polarization state of the light beam resulting from reflection of the beam from the test sample [6]. Fourier transform infrared spectroscopy makes it possible to determine both the qualitative and quantitative elemental composition of the substances under study [1–3].
Instrumental diagnosis of fibrosis
It is known that pathological changes alter the elasticity and density of tissues [51]. Often structures visualized by ultrasound have the same echogenicity, but differ significantly in mechanical properties [43]. In 1991, J. Ophir et al. [42] proposed an ultrasonic compression elastography method based on measuring tissue stiffness and viscosity. In this case, as a rule, tissue compaction is noted when they are scar-fibrous or tumor-affected, and their stiffness can be described using Young’s modulus, which is the ratio of the force of pressure produced to the amount of stretching [22, 33]. Dense tissues are more resistant to compression than soft tissues and will therefore have a higher Young's modulus [46]. However, the depth of the organs, the constitution and severity of the subcutaneous tissue, as well as the presence of free fluid and other physiological barriers make direct compression unregulated and therefore non-standardized. And here, an important source of force acting on tissue is the energy of the acoustic pulse, which is directly proportional to the absorption of acoustic intensity and inversely proportional to the speed of sound (acoustic pulse-wave elastometry) [43]. In this case, the speed of propagation of the shear wave in the tissue (shear wave velocity - SWV) is determined, measured quantitatively by ultrasonic elastometry in meters per second.
In ultrasound elastography, tissue density/elasticity is displayed as a color code superimposed on the normal B-mode image, with the densest structures coded in blue, the softest structures in red or vice versa depending on the choice of color scale, and in the gray scale more soft structures are visualized closer to white, dense structures - to black (Fig. 2 and 3). Elastography can also be performed with endoscopic ultrasound; it is a method for qualitative diagnosis of chronic pancreatitis, its differential diagnosis with pancreatic tumors, and can be combined with elastometry. The sensitivity and specificity of these techniques is 90–92% [29, 30, 35].
Rice. 2. Elastograms of a normal pancreas. a — B-mode, green arrow — body of normal pancreas; b - color scale, white arrow - “soft” body of the pancreas (green); c — gray scale, black arrow — “soft” body of the pancreas (white color) [35].
Rice. 3. Elastography for chronic calcific pancreatitis. a — B-mode, green arrow — the body of the pancreas has a diffusely heterogeneous echostructure with calcifications; b — color scale, black arrow — “dense” body of the pancreas (red color) [35].
Among the instrumental methods for determining tissue density, MR elastography has been used since 1995, which includes visualization of the propagation of the transverse component of vibrations of the acoustic frequency range in tissues, which makes it possible to calculate quantitative values for the mechanical parameters of tissue [39].
Among the radiological methods for diagnosing pancreatic fibrosis, the determination of the CT-density ratio in different phases of bolus contrast is used. At the same time, Y. Hashimoto et al. [28] determined the L/E ratio - “late” (hepatic parenchymal phase at 60-70 seconds from the moment of contrast agent administration) and “early” (pancreatic parenchymal phase - at 40-45 seconds) phases of bolus contrast minus from each indicator in Hounsfield units the density of each section of the pancreas in the native phase before contrast. The authors showed a significant positive correlation of the L/E ratio with the histological degree of fibrosis.
Assessment of the perfusion properties of pancreatic tissue in chronic pancreatitis in a study by Russian author N.I. Yashina [10] indirectly showed that with an increase in the severity of pancreatic fibrosis, the coefficient of change in density in the arterial phase decreases, and in the delayed phase of the study it increases, however, in this study, a statistically significant correlation analysis of histological and CT data was not carried out.
Predictors of pancreatic fibrosis
There are various predictors of the presence of fibrotic changes in the pancreas, indirectly indicating the severity of its fibrosis. Thus, one of the markers of the severity of pancreatic fibrosis can be the presence of calcifications in its parenchyma. R. Ammann et al. [13] and G. Kloppel et al. [31] identified them in 88.4% of patients with severe fibrosis on a 12-point scale (Fibrosis Scale - FS) more than 7 points, in 44.5% of patients with moderate fibrosis less than 6 points. Y. Hashimoto et al. [28] established a significant correlation between the severity of pancreatic fibrosis and dilation of the main pancreatic duct ( p
<0.001), the presence of diabetes mellitus (
p
<0.05), as well as manual sensations of pancreatic density by the surgeon during surgery (
p
<0.001).
To study the severity of exocrine insufficiency, which is one of the predictors of pancreatic fibrosis [14], in addition to generally accepted laboratory tests, perfusion computed tomography is used [50]. Arikawa Shunji et al. (2012) examined 4 parameters—perfusion (PF), peak enhancement intensity (PEI), time to peak (TTP), and blood flow volume (BV). In patients with CP with laboratory proven exocrine insufficiency, a significant decrease in PF, PEI, BV was noted with a significant increase in TTP ( p
<0.05) compared to healthy patients [50]. A correlation with the severity of fibrosis was not traced, and the data presented, apparently, can only indirectly indicate the presence of fibrosis in the studied group of patients with CP.
Thus, the presence of pancreatic fibrosis (PG) and the degree of its severity are important, since they largely determine the possibility of surgery. Among the promising areas of research, it is worth noting the determination of the activity of pancreatic stellate cells, their activation factors (TGF-β1), the study of collagen types, the level of metalloproteinases, chemo- and cytokines in blood serum, as well as its optical properties. Great prospects are associated with the use of stem cells, their ability for directed differentiation, impact on stellate cells and fibroblasts in order to reduce collagen production and resorption of fibrosis of the pancreas. To obtain criteria for early instrumental diagnosis of pancreatic fibrosis, it is of great importance to compare the data of pancreatic elastometry (both ultrasound and magnetic resonance), biphasic computed tomography of the pancreas with histological data on the severity of fibrosis. The presence of reliable and early information about the severity of pancreatic fibrosis will make it possible to predict and influence the outcome of treatment of chronic pancreatitis, and, if surgical intervention is necessary, to prevent surgical and postoperative complications as much as possible.
Compression of surrounding tissues
Abdominal pain is a sign of gastrointestinal dysfunction.
Fat cells can develop to fill a large space. As a result, adipose tissue grows, which takes up much more space than the healthy cells that precede it.
If the fat cells are distributed evenly throughout the pancreas, then this will not cause problems. The situation is worse when cells are collected in groups.
Then they start talking about lipoma - a benign tumor. There is nothing too scary about it, because it will not give metastases, which means it will not cause harm to neighboring organs.
Trouble will occur when the tumor develops to such a size that it begins to put pressure on the vessels, pancreatic ducts, and nerve endings. Such exposure will lead to pain, nausea, flatulence and other unpleasant symptoms.
Watch the video about the risk of developing diabetes and obesity:
How to recognize the disease?
First, the doctor collects a thorough medical history and asks about all the diseases that the patient previously had. To confirm the diagnosis on an outpatient basis, an ultrasound of the abdominal organs and coprogram is sufficient. The first study allows you to clearly examine the gland. It usually becomes smaller. On ultrasound you can see areas of compaction, cysts and other complications of necrosis. Pancreas tumors are also well detected by this method. A coprogram is a complex stool analysis that examines the presence of undigested fiber and fats in the discharge. This may indicate impaired organ function.
For more accurate and specific diagnosis, CT, MRI and biopsy are used.
Nutrition for lipomatosis
There is an opinion in everyday life that reducing the amount of fat in the diet will help stop lipomatosis. This is a completely false statement.
Even completely stopping fat intake will not stop the degeneration of healthy cells into fat cells. The development of lipomatosis has nothing to do with nutrition. However, it is still better to exclude fats. This will have a beneficial effect on the body:
- relief of pancreas;
- getting rid of extra pounds.
Reducing the amount of incoming fat helps alleviate the condition; many signs of the disease recede and become less pronounced. In the absence of external manifestations of the disease, we can talk about preserved pancreatic functionality.
This means that all the ducts are functioning normally, they are not compressed by adipose tissue. With normal functioning of the pancreas, limiting fats in food will help you lose weight, but will not affect the further development of the disease.
Symptoms of the disease
Steatosis develops over a long period of time, months or even years. It all depends on a person’s lifestyle. At first there are no signs of the disease, but gradually the problem grows and the following arise:
- nausea,
- weight loss,
- heartburn,
- vomit,
- intolerance to fatty foods, alcohol and nicotine,
- pressure under the ribs
- diarrhea,
- light-colored stool, which may contain particles of undigested food,
- flatulence,
- sudden allergies to certain foods,
- skin itching,
- loss of appetite,
- weakness.
Cherepenko Lyudmila Vikentievna
doctor - therapist • doctor - cardiologist
When symptoms begin to appear, the disease is already in an advanced stage and requires prompt treatment. You can get an initial remote consultation from our specialists. They will collect complaints, draw up a clinical picture and tell you which treatment is right for you.
Online consultation
Treatment of lipomatosis
Ibuprofen is a drug to relieve abdominal pain.
It is almost impossible to get rid of lipomatosis. The current treatment method goes in 3 directions:
- Lifestyle changes.
- Use of medications.
- Surgical intervention.
Changing your lifestyle in the right direction will help improve the situation as a whole. With persistence and perseverance, you can achieve a lot.
The condition of patients seeking recovery improves even without additional medications. The basics of getting rid of pathology are giving up alcohol and other bad habits, normalizing your diet, and getting rid of excess weight.
To achieve good results, you need to be more active. The second important factor is diet. Following the nutritional principles outlined below will help get rid of many problems. The principles are:
- Fractional meals. The desired number of meals is at least 5, optimal is 6.
- Limit fat. Avoiding sweet, fatty foods.
- A general reduction in the calorie content of dishes, the desire to reduce the daily volume of incoming calories.
It is almost impossible to resolve the situation with medications. Taking medications only helps to get rid of unpleasant symptoms. To eliminate the severe consequences of pancreatic obesity, the following drugs are taken: