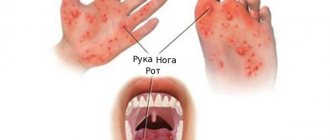
Rotovirus infection is an acute form of pathology in which the gastrointestinal tract is damaged and a clear clinical picture develops. Symptoms include dyspeptic disorders, intoxication and dehydration. The condition is common, occurring in adults and children.
How can a child become infected?
There are certain conditions under which a child may get this infection. The occurrence of the disease will be facilitated by:
- Irregular and inadequate nutrition of the child;
- Poor hygienic and sanitary conditions in which the child and his family live;
- The child eating low-quality or stale foods;
- Presence of stomach and intestinal diseases;
- The presence of immunodeficiency conditions (the child often suffers from colds or is often treated with antibiotics)
The source of infection for rotavirus disease is a person who already has this disease. It is dangerous to others from the appearance of the first symptoms until the end of the disease (5-7 days).
This infection is transmitted through the digestive system. A child can become infected while swimming in a pond where there is rotavirus in the water. Infection can occur through dirty hands, dishes, towels, napkins, toys and other items if they were used by a sick person. The disease can also occur in a baby when consuming foods that contain rotavirus - most often these are various dairy products.
Rotavirus infection most often occurs in autumn or spring. A person can get this disease several times during his life.
From the moment of infection to the appearance of vomiting, abdominal pain or diarrhea in the child, it takes from several hours to several days.
Interpretation of child tests by pediatricians online
cost of service: 500 rubles
Order
The pediatrician will interpret the tests during an online call using the Zoom or WhatsApp application.
- detailed explanation from the pediatrician.
- an alternative opinion from a competent specialist in interpreting the analyses.
- the opportunity to ask questions to the doctor regarding test results.
How the virus is transmitted
The main route of transmission of rotavirus infection is fecal-oral. Most often, it enters the body along with contaminated food or raw water. The virus tolerates low temperatures well and can remain viable in the refrigerator for a long period of time.
Infection through airborne droplets is also possible. Like classic, intestinal flu is accompanied by inflammation of the respiratory tract. When you sneeze and cough, viral particles along with tiny droplets of mucus spread through the air and infect those around you who are susceptible to the disease.
Up to contents
Symptoms
Often, cases of the disease remain unidentified because they are perceived as ordinary intestinal disorders, go away on their own or with a diet, and do not entail visiting a doctor with mandatory testing to identify the pathogen. However, in some cases, this disease can lead to severe complications in children due to large fluid losses.
At the onset of the disease, the child may develop:
- Nasal congestion;
- Runny nose;
- Cough;
- Enlarged submandibular or other groups of lymph nodes.
When the disease develops:
- Stomach ache. Since the virus affects the intestines almost along its entire length, pain can occur throughout the abdomen or in different parts of it.
- Flatulence. Since at this time the intestinal cells release a lot of fluid into the lumen, gurgling and rumbling appears in the stomach. The affected intestines cannot properly and promptly digest and absorb food.
- The baby begins to vomit. It can be 1-2 times or more often. Vomiting continues for one to two days;
- Frequent loose stools, which may be yellow water. This symptom appears simultaneously with vomiting, or a little later. Loose, frequent stools in a child can last from several days to a week. During one day, stool can be several times, and in severe cases - more than fifteen times. This is caused by the affected intestinal cells releasing a lot of water.
- Body temperature rises. This symptom may last for one or several days in a child. These days there is lethargy, decreased appetite and mood.
- Pale skin;
- Lethargy;
- In an infant, you can find retraction of the large fontanel and eyeballs. All this will be a consequence of dehydration in the child;
- With severe rotavirus infection, the child may have seizures.
Diagnostics
In order for a doctor to diagnose rotavirus infection, it is necessary to conduct a stool test for the presence of particles of the virus genome in it (this method is called “polymerase chain reaction”). In addition, various other tests are performed on blood, urine and stool. This allows you to detect infection earlier and prevent complications in the child.
Under domestic conditions, it is very difficult to diagnose this infection for an ill family member. Therefore, consultation with a doctor is necessary in any case, no matter how confident a person is in this diagnosis and methods of its treatment. There are a large number of infectious and non-infectious diseases that occur with symptoms similar to rotavirus infection.
For example, parents may mistake non-infectious diseases of the liver, stomach, intestines, and pancreas for a manifestation of rotavirus infection. With them, the sick child complains of nausea, vomiting, abdominal pain after certain (fatty, spicy, fried) foods, or indicates that all manifestations are disturbing only on one side of the body and began after severe emotional stress.
Similar signs of the disease can occur in case of poisoning with drugs or poisonous plants (more often it is children who suffer from this if they are left alone at home and can get to the home first aid kit, or walk unattended and can try different berries).
Read more about food poisoning in children in our article “Recommendations from a pediatrician for food poisoning in a child.”
Online consultation with pediatrician Olga Nikolaevna Tekutyeva
Registration online
During the consultation, you will be able to voice your problem, the doctor will clarify the situation, interpret the tests, answer your questions and give the necessary recommendations.
Treatment
Rotavirus infection occurs differently in different children - in mild, moderate and severe form. If vomiting and loose stools in a child are not very frequent, then they speak of a mild form of rotavirus infection. The more often a baby has vomiting and diarrhea, the more fluid and microelements his body will lose, and the more severe this disease will be. Loss of water through stool and vomiting in a child leads to dehydration. If, with frequent vomiting and loose stools, you do not try to give a sick child a little water, this can lead to serious consequences of dehydration.
Treatment for rotavirus infection should begin as early as possible. Unfortunately, there is currently no drug that would directly destroy rotavirus. The main treatment is to restore the loss of fluid that leaves the body through vomiting and stool. The child is given oral rehydration - fed in small doses. It is best to restore fluid loss with solutions that contain sodium, potassium and glucose salts.
Where can you get infected with rotavirus?
As a rule, infection occurs in crowded places: in kindergartens, schools, offices, public transport, etc. The disease can manifest itself in isolated cases or epidemic outbreaks, most often occurring in the cold season.
The source of the spread of infection is a patient with a manifest form of intestinal influenza (clinically expressed disease) or a virus carrier. The disease is transmitted only from person to person.
Up to contents
What can parents do before the doctor arrives?
Even before the doctor arrives, parents should start giving their sick child something to drink. You can try to do this in various ways. Some children drink well during illness using a spoon. Someone will have to slowly pour liquid into their mouth from a syringe. The main thing is not to rush and do it evenly and regularly - 5-20 ml every few minutes. If the baby continues to have nausea or vomiting, you need to make the intervals between drinks a little longer, but do not stop offering the patient something to drink. In addition to saline solutions, it is useful to drink dried fruit compote and chamomile decoction.
To reduce the symptoms of the disease, sorbents and preparations containing probiotics are used. Sorbents ensure binding and removal of the pathogen from the body. For this purpose, carbon, fibrous, mineral or synthetic sorbents can be used, which effectively sorb and remove from the body food decomposition products formed during enzymatic deficiency, help accelerate epithelial regeneration, reduce flatulence and pain, and also do not affect the composition of normal intestinal flora .
In some situations, enzymes may be used in treatment if the child has difficulty digesting food due to severe damage to the intestines and pancreas.
In addition to medications, it is important to pay attention to diet during illness. To prevent nausea, food should be given to the child in small quantities and more often. For about a month, you should exclude dairy products, fresh vegetables, fruits, pasta, baked goods, pastries, sweets, and fried foods from your diet.
Food is served to the patient boiled, stewed or baked without crust. The child should have such food for about a month, and then the diet can be gradually expanded.
If you rush and do not follow a diet, recovery will slow down. Complications may arise and chronic diseases of the liver, stomach, intestines or pancreas may develop.
Prevention of rotavirus infection
In order to prevent the occurrence of the disease, it is necessary to follow simple and familiar everyday rules - wash your hands before eating, when returning home from a walk, after communicating with a sick person. These same rules must be taught to every child from a very young age. If someone in the family is sick with rotavirus infection or another infectious disease, then the patient should have separate dishes, towels, bed linen and household items, which should be soaked daily in disinfectant solutions and thoroughly washed with hot water or boiled.
The most effective way to prevent any infectious disease, including rotavirus infection, is vaccination, which can be given to children aged 1.5 to 8 months. Vaccination takes place in three stages with an interval of at least four weeks between each vaccination. It involves dropping vaccine drops into the baby's mouth. The main thing is that the child is healthy at this moment. Then, after proper vaccination, your baby will be protected from a very unpleasant rotavirus infection.
Even after suffering a rotavirus infection, there is a chance to get this disease again, since the body does not always develop lasting immunity to the disease. Therefore, it is important for parents to always follow hygiene rules and not forget to vaccinate their child.
Algorithm for the treatment of acute intestinal infections in children
In the general structure of infectious diseases, acute intestinal infections (AI) account for more than 40% of all hospitalized patients, and in the structure of infectious morbidity they occupy second place after acute respiratory viral infections (ARVI) and influenza, representing a serious problem in pediatric practice.
According to periodic reports from Rospotrebnadzor on infectious and parasitic diseases registered in the Russian Federation, in the first half of 2015, the incidence rate per 100 thousand population of acute intestinal infections of established etiology was 98.3 people, and for the child population - 511.8. For AEI of unknown etiology, this figure for the first 6 months of 2015 was 170.0, and for children - 556.6 per 100 thousand population. High incidence rates (from 13.06 to 44.98 per 100 thousand population) were registered in the Nenets, Khanty-Mansiysk, Yamalo-Nenets Autonomous Okrugs, Tomsk, Tambov, Lipetsk, Irkutsk, Novosibirsk, Amur, Orenburg, Kemerovo, Kaliningrad regions, republics of Komi, Mordovia, Sakha [1].
The algorithm for choosing therapeutic tactics for acute intestinal infections begins with establishing the etiopathogenetic group of diarrhea. The most optimal way is to determine the etiology of the disease using express diagnostic methods (for example, tests for diagnosing viral acute intestinal infections SD BIOLINE Rotavirus, RIDA Quick Rotavirus R-Biopharm AG, Cito Test Rota and others), allowing you to quickly identify the pathogen and select a further treatment algorithm .
Unfortunately, in routine clinical practice, the etiology of acute intestinal infections in most cases remains unidentified and therapeutic tactics are determined based on the etiopathogenetic group of diarrhea, the diagnosis of which is carried out on the basis of clinical and epidemiological data. Thus, watery diarrhea in most cases is caused by viral agents and requires the use of antiviral drugs as etiotropic therapy, while invasive diarrhea is caused by bacterial agents, which implies antibacterial therapy if there are appropriate indications.
Clinical differential diagnosis of DCI is based on the clinical features of the leading syndromes (Table 1).
Epidemiological data on the etiological structure of acute intestinal infections are currently characterized by the predominance of viral agents over bacterial ones and the presence of combined forms in 26.0 ± 1.6% of patients of viral-bacterial and viral-viral etiology.
Among viral agents in children during primary infection, the first place is occupied by rotavirus infection (87.6 ± 1.4% among intestinal monoinfections of viral etiology), among bacterial agents - salmonella, and, as a consequence, the most common form of combined forms is the combined form of rotavirus infection and salmonellosis (9.2% ± 1.1% in the overall structure of deciphered OKI). Among viral acute infections, the most significant etiological factors are rotavirus and norovirus infections, which determines this combination as the most common not only when infected with two viral agents at the same time, but also when infected with a large number of pathogens (4.8 ± 0.8% in the overall structure of deciphered OKI).
The epidemiological history of the disease is assessed according to the following scheme (Table 2). It is necessary for the doctor to make assumptions about the etiology of the disease. Thus, food and water transmission routes are more typical for bacterial acute infections, while contact and household transmission are more typical for viral agents. In the autumn-winter period, there is an increase in the incidence of viral acute intestinal infections, and in the summer - bacterial ones.
When conducting clinical and epidemiological analysis of a patient, it is necessary to take into account the age structure of ACI. For children of all ages, rotavirus infection is recorded significantly more often, while it accounts for 83% of all patients with established rotavirus infection in patients under 3 years of age (p < 0.01) (Fig.). Norovirus infection is characterized by the largest number of patients aged 3 to 7 years - 43.6 ± 6.7%.
According to the form of severity, OCI is divided into mild, moderate and severe. Establishing the severity of the disease is carried out through an integral analysis of clinical data:
1) the prevalence of damage to the gastrointestinal tract (GIT) and other organs; 2) intensity of manifestation of the main clinical symptoms of the disease; 3) intensity of manifestation of the patient’s main complaints [2] (Table 3).
The form of severity can be determined visually: the more points are noted in block 1 and the greater the total number of points in blocks 2 and 3, the more severe the form of the disease is observed in the patient.
However, it is more preferable to calculate the integral index of clinical symptoms, which is carried out according to the formula:
where indicator A is the sum of positive values for each item in block 1; B and C are the sum of positive values for each item in blocks 2 and 3, respectively.
Values of this indicator ranging from 1% to 35% refer to a mild form of the disease, from 36% to 70% to a moderate form of the disease, and 71% or more to a severe form of the disease.
The severity of acute intestinal infection in children is largely determined depending on the volume of fluid loss by the patient, and the correct assessment of the degree of dehydration in a child with ACI is of particular importance.
To diagnose dehydration, the “gold” standard is to assess the patient’s body weight dynamics. Thus, degree I exicosis corresponds to a loss of up to 5% of body weight, which is up to 50 ml/kg of fluid, degree II exicosis is a loss of 6–10% of body weight (60–100 ml/kg), and degree III exicosis is a loss of more than 10%. body weight (110–150 ml/kg). Dehydration, characterized by a loss of body weight of more than 20%, is incompatible with life [3].
However, for pediatric practice, the use of a method for assessing body weight loss is not always acceptable. In this case, clinical assessment of the symptoms of dehydration comes first.
The MH Gorelick trait scale is quite widely used abroad:
- change in the general condition (type) of the patient;
- presence of tears;
- capillary reperfusion > 2 seconds;
- sunken eyes;
- decreased diuresis;
- condition (dryness, turgor) of the skin and mucous membranes;
- basic hemodynamic parameters (pulse rate and filling);
- breathing disorders.
Assessing the form of dehydration on this scale involves counting the number of signs a patient has:
- mild (<5%) dehydration ≤ 2 signs;
- moderate (6–9%) dehydration 3–5 signs;
- severe (> 10%) dehydration - 6–7 signs.
However, the significance of each of the symptoms of dehydration in clinical practice may not always be high enough, especially with grade I exicosis (Table 4).
The scheme of the recommended clinical and laboratory examination for acute intestinal infections is presented in table. 5.
Therapeutic tactics for acute intestinal infections in a particular patient are based on knowledge or assumption (based on clinical characteristics, epidemiological history data) about the etiology of the disease: bacterial or viral infection. In addition, it is necessary to take into account the age of the patient, the characteristics of his premorbid background and the period of the disease.
The scheme of therapeutic tactics for acute intestinal infections depending on the type of diarrhea and the period of the disease is given in table. 6.
Etiotropic therapy is a key element and has three main directions:
- elimination of the infectious agent (antibacterial, antiviral, antiparasitic drugs, bacteriophages);
- pathogen binding (specific antibodies and serums, sorbents);
- excretion (sorbents) [3, 7].
All patients, regardless of the etiology and severity of the disease, should be prescribed sorbents (carbon, synthetic, mineral, fibrous) as one of the important aspects of etiotropic therapy. Currently, there is a fairly large number of drugs on the Russian pharmaceutical market that have sorption properties to varying degrees [8]. The administration of enterosorbents is indicated as early as possible in the course of the disease - before identification of the pathogen, which makes it possible to achieve a “terminating” effect on the course of acute intestinal infections. The use of enterosorbents in the late stages of the disease (after 5–7 days), especially with invasive acute intestinal infections, has less effect on diarrhea syndrome, but has a pronounced detoxification and enteroprotective effect. Important positive aspects of the use of enterosorbents include the lack of influence of these drugs on the composition of the obligate intestinal microbiota. The course of treatment with enterosorbents is usually 5–7 days. The criterion for early drug withdrawal is stable normalization of stool or its retention within 2 days [7].
Antiviral drugs are recommended for viral acute intestinal infections. Antiviral drugs recommended for acute intestinal infections and proven effective in clinical studies: affinity purified antibodies to human interferon gamma, interferon alpha-2b in combination with taurine, umifenovir [9–12].
The issues of antibacterial therapy for acute intestinal infections remain among the most pressing for the practicing physician. Unfortunately, most doctors approach the issue of prescribing antibiotics in a formulaic manner, without taking into account the etiology of the disease, recommending them even for viral acute intestinal infections, and without knowledge of data on the sensitivity and resistance of the main bacterial pathogens.
Indications for the prescription of antibacterial drugs are divided into absolute, basic and additional (Table 7) [7, 13].
Absolute indications for prescribing antibacterial therapy have absolute force - antibacterial therapy is indicated for all patients for whom they are established. The presence of main indications in combination with one of the additional points is an indication for antibiotic therapy. The presence of additional indications alone is not an indication for antibiotic therapy.
Antibacterial agents recommended for acute intestinal infections are divided into two types: intestinal antiseptics and drugs intended for systemic action. The first group can be recommended for use in outpatient clinics, where the most justified tactics for initial treatment of acute intestinal infections is the use of nitrofurans (nifuroxazide, nifurantel). Quinolones (nalidixic acid, ciprofloxacin) have proven themselves in the treatment of salmonellosis. Cephalosporins are recommended for systemic antibacterial therapy for moderate and severe acute intestinal infections in a hospital setting. It is possible to prescribe tetracyclines, metronidazole, aminoglycosides, chloramphenicol.
If a diagnosis of campylobacteriosis is made, the most optimal for initial etiotropic therapy are macrolides (erythromycin, azithromycin, clarithromycin).
The duration of the course of antibiotic therapy in the acute phase of localized acute intestinal infections is determined by the clinical situation and, as a rule, is at least 5–7 days. The generally accepted indications for changing the drug are clinical ineffectiveness of the drug within 3 days [7, 14].
It should be emphasized that in recent years, most pathogens of invasive acute intestinal infections have resistance to furazolidone. Salmonella remain highly sensitive to fluoroquinolones (for example, ciprofloxacin - 96.7% of strains are sensitive, but 23.3% are moderately resistant and 17.2% are resistant to pefloxacin), but their use in pediatric practice is limited; nalidixic acid (53.1%), amikacin (61.1%), netilmicin (63.9%), some cephalosporins II (cefoxitin, cefuroxime) - 86.7–57.9%, III (ceftriaxone, cefotaxime, ceftazidime ) - 84.4%, 85.0%, 81.7% and IV generation (cefepime) - 91.3% of sensitive strains.
An obligatory component of antibacterial therapy from the moment of its prescription and during the convalescence period is the administration of probiotics.
Among the pathogenetic methods of therapy, the most important are rehydration agents (oral, parenteral), drugs that affect dehydration processes (gelatin tanate), and probiotics.
Oral rehydration is a necessary component of therapy, included in the list of therapeutic measures recommended by the World Health Organization, and is prescribed to all patients with ACI. For oral rehydration, the most justified is the use of ready-made solutions that are balanced in electrolyte composition and osmolarity (75 mEq/L sodium and 75 mEq/L glucose and osmolarity 245 mOsm/L).
Oral rehydration is carried out in two stages.
Stage 1 - primary rehydration is the replenishment of losses that occurred before seeking medical help, and is calculated for 6 hours. A total amount of liquid of 50-80 ml/kg is prescribed for 6 hours.
Stage 2 - maintenance rehydration, the task of which is to replenish current fluid losses during acute intestinal infections. 80–100 ml/kg of liquid is prescribed per day. The duration of the second stage of oral rehydration continues until recovery or the appearance of indications for parenteral correction of dehydration.
It must be taken into account that correction of dehydration is impossible without the use of salt-free solutions, among which preference should be given to drinking water (not mineral!), it is possible to use pectin-containing infusions (apple compote without sugar, carrot-rice infusion). The ratio of glucose-salt solutions and drinking water should be 1:1 for watery diarrhea, 2:1 for severe vomiting, 1:2 for invasive diarrhea [3, 15].
Severe forms of acute intestinal infections, lack of effect from oral rehydration or the presence of profuse vomiting, edema, development of functional (acute) renal failure are indications for parenteral rehydration, which can be carried out using one of the modern domestic solutions - 1.5% solution of meglumine sodium succinate , which has proven its effectiveness in intensive therapy of these conditions [16].
The use of antidiarrheals (loperamide) in acute intestinal infections is not pathogenetically justified, since the mechanism of action of these drugs involves a decrease in gastrointestinal motility (increased motility is the body’s protective reaction in acute infectious intestinal lesions) and can contribute to the aggravation of intoxication syndrome in acute intestinal infections.
OKI of any severity cause significant changes in the microbiocenosis of the gastrointestinal tract - for example, with Sonne's dysentery in 67.8–85.1% of patients, with salmonellosis - in 95.1%, yersiniosis - in 94.9%, rotavirus infection - in 37, 2–62.8% of patients [17–20].
Probiotics should be prescribed as part of complex initial therapy, regardless of the etiology of the disease, as early as possible. These drugs are also indicated for all patients during the period of convalescence in order to restore microbiocenosis parameters. Their use in acute intestinal infections in children is not only pathogenetically substantiated, but also refers to the highest level of evidence A - in accordance with the principles of evidence-based medicine [21].
The modern view of probiotic therapy implies a strain-specific approach, which means the establishment in clinical studies of the therapeutic effects characteristic of certain genetically certified strains and their further use, taking into account the strain-specific properties of probiotics in various clinical situations [22].
With regard to acute intestinal infections in children, the working group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition, ESPGHAN in 2014, based on an analysis of published systematic reviews and the results of randomized clinical trials, including placebo-controlled, published a memorandum in which it recommended (despite the low level of evidence according to experts) several probiotic strains in the treatment of acute intestinal infections: Lactobacillus GG, Saccharomyces boulardii, Lactobacillus reuteri strain DSM 17938 (original strain ATCC 55730), as well as This group of probiotics included the thermally inactivated strain of Lactobacillus acidophilus LB, which formally cannot be classified as probiotics as living microorganisms with specified beneficial properties, but it has shown its effectiveness in acute infectious gastroenteritis [23].
Currently, probiotic strains Bifidobacterium lactis BB-12, Escherichia coli Nissle 1917, Lactobacillus acidophilus, Bacillus clausii are classified as a group of microorganisms for which there is insufficient data on the effectiveness of their use in the acute period of ACI. However, previous studies have shown the presence of clinically significant positive properties, the effectiveness and safety of their use in acute intestinal infections, post-infectious bacterial overgrowth syndrome and the prevention of gastrointestinal microbiocenosis disorders against the background of antibacterial therapy. Thus, the range of strains that can be recommended in the treatment of acute intestinal infections requires further study.
In this regard, the most promising probiotic strains are microorganisms characterized by a high ability of adhesion, resistance to the action of aggressive environments of the human gastrointestinal tract (hydrochloric acid, bile) and belonging to the donor category.
Among such probiotic strains, microorganisms of the genus Bifidobacterium can be distinguished. Bifidobacteria belong to the dominant species in the microbiocenosis of the human gastrointestinal tract - their proportion in the composition of microbiocenoses ranges from 85% to 98%. This genus is characterized by a high ability for adhesion, a leading role in ensuring colonization resistance of the body, regulating the metabolism of fats, proteins and minerals, and the synthesis of biologically active substances, including vitamins. The most studied strains are Bifidobacterium longum and Bifidobacterium animalis lactis.
One of the lines of probiotic preparations that can be recommended for complex therapy of acute intestinal infections in children is the probiotic preparations Bifiform.
Bifiform Baby contains Bifidobacterium BB-12 1 × 108 CFU and Streptococcus thermophilus TH-4 1 × 107 CFU.
Preclinical studies of Bifidobacterium lactis BB-12, which is a component of the natural intestinal biofilm of healthy humans, have demonstrated its ability to exhibit high levels of adhesion to surfaces with mucin (polycarbonate well plates were used), without mucin, and cell culture films (Caco-2, HT29?MTX) [ 14], including against the background of rotavirus infection and after it [24, 25].
This strain has shown antagonistic activity to a whole range of pathogenic pathogens (Bacillus cereus, Clostridium difficile, Clostridium perfringens Type A, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella enterica subsp enterica serovar Typhimurium, Salmonella enterica subsp enterica serovar Typhi, Shigella flexneri, Shigella sonnei, Campylobacter jejuni and Candida albicans) [26, 27], which makes its use preferable for acute intestinal infections of bacterial etiology.
Bifidobacterium lactis BB-12 is resistant to the action of aggressive environments in the human body - hydrochloric acid and bile, due to the synthesis of a pH-dependent ATPase, which regulates the acid-base balance inside the bacterium [28] and the presence of bile salt hydrolase, which allows the bacterium to remain active in the presence of bile [29].
Patients who require treatment with antibacterial drugs deserve special attention. Changes in the gastrointestinal microbiota caused by the course of the infectious process can be aggravated by the action of antibiotics. Therefore, this category of patients needs to include probiotic drugs aimed at maintaining microbiocenosis in the complex therapy of acute intestinal infections. Bifidobacterium lactis BB-12 is resistant to antibiotics such as gentamicin, streptomycin, polymyxin B, nalidixic acid, kanamycin, neomycin, cycloserine, tetracycline, which makes it the strain of choice when prescribing these antibacterial agents to patients, for example, for acute intestinal infections (salmonellosis, shigellosis) [30].
Placebo-controlled studies have shown that in addition to therapeutic properties, the Bifidobacterium lactis BB-12 strain also has preventive properties. In particular, its use reduces the risk of developing gastrointestinal infections, including rotavirus, associated with the provision of medical care [31, 32].
It should be noted that the high safety profile of this strain was approved by regulatory authorities in Europe - in 2008, the European Food Safety Authority (EFSA) awarded it the status of Qualified Presumption of Safety (unconditional safety) - and in the USA, where it is recognized by the Food and Drug Administration (FDA) as Generally Regarded As Safe (GRAS).
Streptococcus thermophilus, which is part of Bifiform Baby, has demonstrated an antagonistic effect against pathogens of acute intestinal infections in studies; in particular, its effectiveness in the prevention of traveler's diarrhea has been shown.
This strain has been shown to have a symbiotic relationship with Lactobacillus bulgaricus.
Bifiform Baby is intended for children from the first days of life to 2 years. The daily dose (the mark on the pipette corresponds to 1 dose) is 0.5 g ~ 0.5 ml. Used once a day during meals. It is most optimal to use it during antibacterial therapy for acute intestinal infections, during the period of convalescence, as well as for preventive purposes (for example, when going on vacation with a child, visiting public events, a swimming pool).
Bifiform capsules contain Bifidobacterium longum, which is also a donor strain and is characterized by pronounced antagonistic activity against pathogenic and opportunistic microorganisms. The inclusion in the preparation of apathogenic Enterococcus faecium, which is not one of those not recommended for use in pediatric practice [23], but normally colonizes the small intestine, makes it possible to have a positive effect on the condition and digestive functions of not only the large but also the small intestine, especially in the presence of fermentation dyspepsia and flatulence phenomena.
The drug is indicated for children over 2 years of age. For acute diarrhea, the drug is taken 1 capsule 4 times a day until stool normalizes. Then the drug should be continued at a dose of 2-3 capsules per day until the symptoms disappear completely. To normalize the intestinal microbiota and support the immune system, the drug is prescribed in a dose of 2-3 capsules per day for 10-21 days. Children from 2 years old: 1 capsule 2-3 times a day.
Symptomatic therapy includes treatment of febrile conditions. Antipyretics are not indicated for all patients, since an increase in temperature is an adaptive response of the body to an infection, creating optimal conditions for immune restructuring of the body. The prescription of this category of drugs is indicated for all patients with hyperthermia, and in the presence of severe concomitant pathology - with a fever of more than 38.5 ° C.
The development of secondary pancreatic insufficiency and exacerbation of chronic pancreatic pathology is often observed during the period of repair and convalescence of acute intestinal infections. It should be noted that with norovirus infection, damage to the pancreas is observed more often than with acute intestinal infections of other etiologies. In such cases, the administration of enzyme preparations is indicated, preferably in minimicrosphere form. It should be noted that in the acute period of AII, enzyme preparations are not indicated. The most optimal period for their prescription, if indicated, is 5–6 days; the criterion for prescription is the appearance of appetite in the patient.
To relieve persistent vomiting, you can use prokinetics and antiemetic drugs: metoclopramide, domperidone, promethazine, 0.25% novocaine - 1 spoon (teaspoon, dessert spoon, table spoon according to age) [3, 7].
Criteria for assessing the effectiveness of treatment:
- clinical (relief of intoxication syndrome, normalization of temperature, relief of vomiting, diarrhea and other symptoms);
- clinical and laboratory tests (persistent normalization of hemogram, coprocytogram, negative results during bacteriological and PCR examinations).
Due to the fact that sanitation from the pathogen, complete repair of the intestine and restoration of its impaired functions occur much later than the clinical manifestations of the disease disappear, it is advisable to carry out dynamic monitoring of patients who have suffered acute intestinal infections.
Thus, acute intestinal infections require special approaches from the doctor to diagnosis, management tactics and therapy. When supervising patients with acute intestinal infections, it should be taken into account that even mild forms lead to significant changes in the gastrointestinal microbiota in children, which requires the use of probiotic preparations not only in the acute period of the disease, but also in the period of convalescence.
Literature
- Information on infectious and parasitic diseases (Form 1) for January–June 2015. www.rospotrebnadzor.ru (https://www.rospotrebnadzor.ru/activities/statistical-materials/statictic_details.php? ELEMENT_ID=3919). Login 08/10/2015.
- Gorelov A.V., Ploskireva A.A. et al. Application No. 2012156024 for the invention “Method for assessing the severity of acute intestinal infections in children,” 2014.
- Gorelov A.V., Milyutina L.N., Usenko D.V. Tactics of complex therapy of acute intestinal infections in children // Issues of modern pediatrics. 2003; 2:82–85.
- Gorelick MH, Shaw KN, Murphy KO Vaildity and reliability of clinical signs in the diagnosis of dehydration in children // Pediatrics. 1997; 99(5), E6.
- Kimberly Pringle at al. Comparing the accuracy of the three popular clinical dehydration scales in children with diarrhea // Int J Emerg Med. 2011; 4: 58. Published online 2011 Sep 9.
- Adam C. Levine at al. Prediction of Severe Disease in Children with Diarrhea in a Resource-Limited Setting // PLoS One. 2013; 8(12):e82386. Published online 2013 Dec 3.
- Gorelov A.V., Milyutina L.N., Usenko D.V. Clinical guidelines for the diagnosis and treatment of acute intestinal infections in children. M., 2006. 49 p.
- Shcherbakov P. L., Petukhov V. A. Comparative effectiveness of enterosorbents for diarrhea in children // Issues of modern pediatrics. 2005; 4 (4): 86–90.
- Gorelov A.V., Ploskireva A.A., Tkhakushinova N.H. Clinical and virological assessment of the effectiveness of an interferon inducer containing antibodies to interferon gamma in a release-active form in the treatment of acute viral intestinal infections // Infectious diseases. 2012; 10(3):3.
- Levin D. Yu., Kadura A. A. Efficacy of using the drug arbidol for rotavirus infection in children // Bulletin of medical Internet conferences. 2013; 3 (2): 188.
- Lityaeva L. A., Kovaleva O. V., Yakubovich I. S. Features of acute intestinal infections in children in the Orenburg region // Epidemiology and infectious diseases. Current issues. 2013; 6:39–42.
- Gorelov A.V., Feklisova L.V., Ploskireva A.A. et al. New opportunities in the treatment of acute intestinal infections in children // Infectious diseases. 2012; 10 (1); 42–49.
- Guarino A., Albano F., Ashkenazi S., Gendrel D., Hoekstra JH, Shamir R., Szajewska H. European Society for Pediatric Gastroenterology, Hepatology and Nutrition/European Society for Pediatric Infectious Diseases Evidence-based Guidelines for the Management of Acute Gastroenteritis in Children in Europe // J Pediat Gastroenterol Nutr. 2008. Vol. 46, Suppl. 2, 81–122.
- Kadzhaeva E. N., Usenko D. V., Gorelov A. V., Ardatskaya M. D. Modern nitrofurans in the treatment of intestinal infections in children // Farmateka. 2007; 13: 79–82.
- Maleev V.V., Gorelov A.V., Usenko D.V., Kuleshov K.I. Current problems, results and prospects for studying acute intestinal infections // Epidemiology and infectious diseases. 2014. No. 1. P. 4.
- Ploskireva A. A., Gorelov A. V., Zhuchkova S. N. et al. Modern approaches to intensive care of acute intestinal infections in children // Infectious diseases. 2012; 10 (1): 50–55.
- Zhelezova L.I. Clinical and laboratory features of microecological disorders of the colon mucosa during acute intestinal infections in children. 2006.
- Kramar L.V., Rodionova N.V., Arova A.A. Microecological features of the intestinal biocenosis of children of the first year of life with acute intestinal infections // Fundamental Research. 2014. No. 2. pp. 90–93.
- Bitieva R. L. Evaluation of new approaches to the diagnosis and treatment of rotavirus infection in children. Author's abstract. dis. ...cand. honey. Sci. M., 2007.
- Allen SJ, Martinez EG, Gregorio GV et al. Probiotics for treating acute infectious diarrhea // Cochrane Database Syst Rev. 2010; CD003048.
- Kligler B., Cohrssen A. Probiotics // Am Fam Physician. 2008 Nov 1; 78(9):1073–1078.
- Szajewska H. Advances and limitations of evidence-based medicine - impact for probiotics // Ann Nutr Metab 2010; 57 (suppl): 6–9. Rijkers GT, Bengmark S, Enck P et al. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research // J Nutr. 2010; 140:S671–S676.
- Szajewska H., Guarino A., Hojsak I., Indrio F., Kolacek S., Shamir R., Vandenplas Y., Weizman Z. Use of probiotics for the management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics // J Pediatr Gastroenterol Nutr. 2014 Apr; 58(4):531–539.
- Laparra JM, Sanz Y. Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium // Lett Appl Microbiol. 2009 Dec; 49(6):695–701.
- Juntunen M., Kirjavainen PV, Ouwehand AC, Salminen SJ, Isolauri E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection // Clin Diagn Lab Immunol. Mar 2001; 8 (2): 293–296.
- Martins FS, Silva AA, Vieira AT, Barbosa FH, Arantes RM, Teixeira MM, Nicoli JR Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties // Arch. Microbiol. 2009, 191, 623–630.
- Collado MC, Meriluoto J., Salminen S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus // Lett. Appl. Microbiol. 2007, 45, 454–460.
- Matsumoto M., Ohishi H., Benno Y. H+-ATPase activity in Bifidobacterium with special reference to acid tolerance // Int J Food Microbiol. 2004 May 15; 93 (1): 109–13.
- Garrigues C., Stuer-Lauridsen B., Johansen E. Characterization of Bifidobacterium animalissubsp. lactis BB-12 and other probiotic bacteria using genomics, transcriptomics and proteomics // Aust. J. Dairy Technol. 2005, 60, 84–92.
- Amund OD, Ouoba LI, Sutherland JP, Ghoddusi HB Assessing the effects of exposure to environmental stress on some functional properties of Bifidobacteriumanimalis ssp. Lactis // Benefit Microbes. 2014 Dec; 5 (4): 461–469.
- Saavedra JM, Bauman NA, Oung I., Perman JA, Yolken RH Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus // Lancet. 1994, 344, 1046–1049.
- Chatterjee S., Kar P., Das T., Ray S., Ganguly S., Rajendiran C., Mitra M. Randomized placebo-controlled double blind multicentric trial on efficacy and safety of Lactobacillus acidophilus LA-5® and Bifidobacterium BB- 12® for prevention of antibiotic-associated diarrhoea // JAPI. 2013, 61, 708–712.
A. A. Ploskireva1, Candidate of Medical Sciences A. V. Gorelov, Doctor of Medical Sciences, Professor
Federal Budgetary Institution Central Research Institute of Epidemiology of Rospotrebnadzor, Moscow
1 Contact information