Intracavitary pH-metry as a modern method for studying gastric secretion
Doctor of Medical Sciences, Associate Professor V.V. Skvortsov, Ph.D. A.V. Tumarenko, E.M. Skvortsova, V.V. Odintsov
Almost all diseases of the upper gastrointestinal tract (GIT) are, to one degree or another, correlated with the effect of acid produced during the digestion process on the mucous membrane of the GI tract. For correct diagnosis (and sometimes treatment) of many of these diseases, it is necessary to study the acid-producing and acid-neutralizing functions of the gastrointestinal tract. The possibility of determining intragastric acidity using a special pH-metric probe was first described by McCledon in 1915.
Until the early 80s of the twentieth century, the goal of developing equipment for intragastric pH-metry was to substantiate the need for the method and create pioneering medical techniques. The first serial devices and pH probes in the USSR were designed in the city of Fryazino, Moscow Region, under the leadership of Academician of the Russian Academy of Sciences N.D. Devyatkova. In the last decade, the leading role in the development and production of domestic devices for intracavitary pH-metry belongs to the Fryazino research and production enterprise Istok-System.
Clinical problems solved using intragastric pH-metry. The advent of pH-metric instruments initiated the development of clinical studies of acid-dependent processes in the human body. pH-metry is of particular importance in the diagnosis of GERD. According to the “Recommendations for the examination and treatment of patients with gastroesophageal reflux disease” (Ivashkin V.T. et al., 2001), “The main method for diagnosing GERD is pH-metry.” However, the diagnosis of GERD, although the most recognized, is far from the only area of application of pH-metry. In modern medicine, pH measurement is used to solve the following problems.
Diagnostic tasks
Among them, the most relevant are: - topographic intragastric express pH-metry of the gastrointestinal tract - short-term (up to 3 hours) study of basal and stimulated secretion of gastrointestinal tract; — long-term (up to 24 hours or more) monitoring of gastroesophageal reflux (GER); — study of fast acid processes, such as duodenogastric reflux (DGR); — parietal intraendoscopic pH-metry; - long-term monitoring of acidity in the esophagus in order to study the role of GER in bronchopulmonary and ENT diseases: chronic cough, pneumonia, bronchial asthma, sinusitis, dysphonia, laryngitis, broncho-obstruction, contact granuloma, pharyngitis, dental erosion, malignant lesions of the pharynx, larynx, vocal cords, as well as research into the role of GER in the occurrence and development of sleep diseases; — simultaneous long-term monitoring of acidity in the gastrointestinal tract and electrical activity of the heart for the purpose of differential diagnosis of cardio- and gastrointestinal diseases and for the study of heart diseases caused by GER; simultaneous monitoring of acidity in the gastrointestinal tract and electrical activity of various parts of the gastrointestinal tract. Tasks associated with assessing the effects of pharmacological drugs: - selection of drugs and their dosages for optimal drug treatment of specific patients; — assessment of the effectiveness of drugs (or complexes of drugs) on patients suffering from certain diseases; — research related to obtaining permits for the use of certain pharmacological drugs in medical practice.
Devices for intracavitary pH-metry are in demand in solving numerous research problems, among which, in addition to the creation and testing of new methods for diagnosing and treating diseases of the gastrointestinal tract, including combined ones, we note the study of acid-dependent processes in the lower parts of the gastrointestinal tract, in the oral cavity, intravaginal and transurethral pH-metry.
Devices for intracavitary pH-metry
1. Microprocessor acidogastrometer “AGM-03” is the basic device of a series of inexpensive acidogastrometers. Designed for studying basal and stimulated acidity of the gastrointestinal tract, conducting alkaline, acid and other tests, as well as for selecting medications. A separate area of its application is topographic intraendoscopic pH-metry.
2. Computer stationary acidogastrometer “Gastroscan-5M”. Designed for short-term (including stimulated) pH-metry of the gastrointestinal tract in up to five patients simultaneously, in one, two, three or five sections of the gastrointestinal tract. The pH measurement period is 20 or 5 s. Gives tips to medical personnel on conducting the examination. Issues a conclusion about the state of the gastrointestinal tract. Saves research results in a database and performs various types of statistical processing of the research array. Allows for individual selection of drug therapy.
3. Daily wearable acidogastromonitor “Gastroscan-24”. The Gastroscan-24 computer device is intended for long-term (up to 24 hours or more) study of the acidity of the gastrointestinal tract. For processing, the measurement results after the end of the examination are transferred to a personal computer (PC). The device allows you to evaluate the effect of medications, select them and take them. During the study, using the keyboard equipped with the wearable unit, the patient enters data about his condition (pain, heartburn, nausea, etc.) or actions (smoking, taking medications, etc.).
4. Gastrocardiomonitor “Gastroscan-ECG”. The device is designed for simultaneous 24-hour monitoring of acidity at three points in the gastrointestinal tract and cardiac signal in three leads. Such monitoring allows for differential diagnosis of chest pain of unknown etiology based on correlation analysis of changes in acidity and cardiac signal.
5. pH-metric probes. NPP "Istok-System" serially produces a variety of pH probes that differ in design and application: a) oral, transnasal, endoscopic; b) having 1, 2, 3 or 5 measuring electrodes; c) in age versions approved by the Ministry of Health of the Russian Federation - 4 children and 1 adult (multielectrode pH probes). All pH probes provide a measurement accuracy of 0.2 pH over a range of 1.1 to 9.2 pH. Currently, gastroenterology is one of the fastest growing areas of therapy. To a large extent, this progress is due to the introduction into clinical practice of modern, high-tech methods for studying the functions of the digestive organs.
On the other hand, knowledge of the physiology of the processes of secretion, digestion, and motility in the gastrointestinal tract is expanding every year, which forces, in some cases, to rethink the methods, purpose and interpretation of the results of traditional, routinely used research methods in gastroenterology. Until relatively recently, functional diagnostics in gastroenterology was a tool in the hands of a few researchers, with the help of which they could assess the degree of dysfunction of a particular organ of the digestive tract.
Currently, a large amount of factual material has been accumulated, which allows not only to diagnose the severity of functional disorders, but also, importantly, to verify the patient’s diagnosis.
Gastrointestinal intubation for pH measurement
Contraindications. In each specific case, it is necessary to correlate the severity of the patient’s condition and the expected diagnostic value of the study. The use of microprobes reduces the number of contraindications to probing. However, the question of the appropriateness of this study must be decided individually in the following cases: diseases of the oral cavity, nose, pharynx that prevent the insertion of a probe or the patient’s breathing; diverticula, esophageal strictures; severe uncontrolled coagulopathy; bronchial asthma, cardiovascular diseases, in which stimulation of the vagus nerve is contraindicated; severe respiratory failure; recent gastric surgery; tumors and ulcers of the esophagus; varicose veins of the esophagus; mental illnesses (neuroses, psychopathy, hysteria) in the absence of mutual understanding with the patient.
Complications of intubation: bleeding from the nose or throat; injury to the nose or throat; tracheal intubation; trauma or perforation of the esophagus, stomach; vomit; syncope associated with irritation of the sensitive afferent fibers of the vagus nerve system, due to the initiation of the vasovagal reflex (an efferent discharge occurs, conducted along the motor fibers of the vagus nerve and causing cardiac arrest); bronchospasm; exacerbation of trigeminal neuralgia; patient infection.
Probing equipment for pH measurements: probe; aerosol, gel for anesthesia; vomit tray; towel; rubber gloves, portable pH meter.
Preparation for pH-metry Intubation is carried out no less than 6 hours after eating. 3-4 hours before the start of the study, smoking, drinking liquids, and chewing gum are excluded. If the evacuation of contents from the stomach is impaired on the evening before the study, the stomach is lavaged through a thick probe until clean lavage water is obtained. Before the study, it is necessary to clarify what medications the patient took on the eve of the study. Most methods require discontinuation of previous medications. The time for limiting the intake of medications depends on the duration of their effect and the method used, for example, with pH-metry, antacids and anticholinergics must be discontinued 12 hours before, H2-histamine blockers 24 hours before, and proton pump inhibitors 36 hours before.
It is important to carefully study the patient’s medical history and listen to his complaints at the time of the study. This is necessary to exclude the patient from possible contraindications for the study, allergies to medications used for anesthesia. In order to reduce neuropsychic stress and prevent complications during the study, it is necessary to explain the procedure to the patient and note its safety.
Intubation technique
Immediately before the test, place the probe in warm water for a while to minimize temperature changes in the catheter and increase its elasticity. Place the patient in a chair. If the probe is inserted through the nose, ask the patient to breathe deeply with the mouth closed, alternately pinching one of the nostrils, to assess nasal breathing. Intubation is carried out through the nasal passage with the most effective nasal breathing. Test the gag reflex by touching the uvula or pharynx. Patients with a weak or absent gag reflex are at greatest risk for pulmonary aspiration. If there is no allergy to the drug, anesthesia of the nasal passage or pharynx is performed with an aerosol anesthetic (lidocaine, etc.). However, some patients tolerate repeated examinations well even without local anesthesia. After the onset of anesthesia, the probe is slowly and carefully inserted into the nasal passage, or into the mouth, and then into the patient’s throat. When inserting a probe into the nasopharynx, the patient is recommended to tilt his head forward so that the chin touches the chest. Tilt of the head forward leads to the closure of the trachea by the epiglottis and facilitates the passage of the probe into the esophagus. At the moment of insertion of the probe, the patient breathes deeply and makes swallowing movements. The appearance of a cough indicates that the probe is not installed correctly. If there is hypertonicity of the lower esophageal sphincter (achalasia cardia), the probe may twist in the distal part of the esophagus. In this case, it is necessary to remove the probe and slowly insert it again. The probe advances to the desired depth. During the study, it is necessary to monitor the patient's reaction, since gastric intubation and fear can lead to acute vasomotor reactions, including loss of consciousness. The probe is secured with a patch on the cheek and behind the ear.
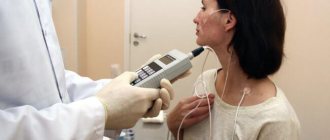
Before starting the study, it is necessary to allow the patient to get used to the probe. In most studies, saliva is spat into a special tray. To conduct the study, equipment is used for long-term monitoring of acidity, consisting of a compact wearable pH recording unit, to which is attached a pH-metric probe, inserted transnasally into the patient’s stomach, and a computer with software. The panel of the wearable unit has special buttons, by pressing which the patient registers in the device’s memory the time of occurrence and duration of pain, dyspeptic symptoms, food intake and other events. Intracavitary pH is determined at set time intervals from 1 to 60 s in various devices.
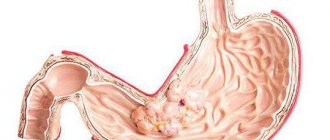
pH is measured on an empty stomach in the body and antrum (6-7), normally in the antrum after administration of histamine pH = 4-7. The conditional norm for stimulating secretion with extractive substances is considered to be pH = 1.7-1.5 in the body of the stomach, and with maximum stimulation with histamine - 1.2-1.1. If the pH remains above 1.8 during histamine stimulation, then the likelihood of atrophy of the gastric mucosa is significant.
To assess intragastric acidity, the following types of intragastric pH measurements are used: Topographic express pH measurements. Intragastric pH monitoring. Topographic transendoscopic pH-metry. Topographic intragastric express pH-metry A probe is inserted into the patient's esophagus on an empty stomach up to the lower esophageal sphincter. The length of insertion of the probe is approximately determined by the distance from the patient’s earlobe to the xiphoid process or from the upper lip to the navel. Then, as the probe is further introduced, pH measurements are taken every centimeter. A total of 20 pH measurements are taken for no more than 3 minutes. The probe is fixed and left in the stomach for 10 minutes. After 10 minutes, the probe is removed, and the pH is also measured every centimeter. For the study, acidogastrometers AGM-01, AGM-03 and Gastroscan-5M are used. The pH values upon insertion of the probe determine the level of fasting acid formation. pH values when removing the probe allow you to assess the level of basal acid formation. When performing pH measurements, it is important to consider in which part of the stomach the sensor is located.
In the antrum of the stomach, in most cases the pH is higher due to the neutralization of hydrochloric acid by the alkaline secretion of the glands. At the same time, the degree of severity of the acid-neutralizing function of the stomach is determined by the difference in pH between the antrum and body of the stomach. If the pH difference is 2.1 or more, compensated alkalization is diagnosed, 1.0-2.0 - subcompensated, 1.0 or less - decompensated alkalization in the antrum. There are research schemes when the pH is recorded for 45 minutes under basal conditions, and then over the next 45 minutes after stimulation of secretion. To stimulate gastric acid formation, the same medications are used as for multi-stage gastric intubation (histamine dihydrochloride, histamine phosphate, gastrin (2 mcg/kg), pentagastrin (pentavlon) at a dose of 6 mcg/kg, etc.
The main disadvantage of the method is the inability to estimate the volume of gastric contents and, as a result, acid production. However, the Neller alkaline test helps to indirectly assess acid production. The alkaline test involves introducing a solution of 0.5 g of baking soda (NaHCO3) into 30 ml of boiled water through the pH probe channel. It is carried out 20 minutes after stabilization of the pH under basal conditions or 45 minutes after the introduction of stimulants. This technique allows you to get an idea not only of the concentration of hydrogen ions in the lumen of the stomach, but also of the amount of gastric juice, i.e. hydrochloric acid products. The indicator of this test is the alkaline time - the interval between the increase in pH after the introduction of the solution until it returns to the original level. Normally, in the body of the stomach it ranges from 15 to 30 minutes. A decrease in alkaline time of less than 15 minutes indicates an increase in the flow rate of hydrochloric acid, an increase of more than 30 minutes indicates a suppression of acid formation.
The test is carried out under basal and stimulated conditions. If acid formation is high, an atropine test is performed. It makes it possible to differentiate the neuroreflex mechanism of basal acid production from the humoral one. The test is carried out both under basal conditions and when secretion is stimulated. In this case, 1 ml of a 0.1% solution of atropine sulfate is injected subcutaneously and the pH in the body of the stomach is recorded for an hour. The results of the atropine test under basal conditions are assessed by the degree of pH increase in the body of the stomach. When the pH increases by more than 2 units. - the effect is strong, from 1.1 to 2.0 - medium, from 0.5 to 1.0 - weak, up to 0.5 - negative. When assessing the test results, it is necessary to take into account that anticholinergics primarily reduce the volume of acid production, having little effect on the concentration of hydrochloric acid in gastric secretions.
Intragastric long-term pH monitoring
The method allows you to: evaluate the daily rhythm and intensity of hydrochloric acid secretion; assess the speed of onset and duration of the effect of antisecretory drugs; correlate the occurrence of symptoms of an acid-dependent disease with fluctuations in intragastric pH; differentiate chest pain of cardiac and non-cardiac origin. To conduct the study, equipment is used for long-term monitoring of acidity, consisting of a compact wearable pH recording unit, to which is attached a pH-metric probe, inserted transnasally into the patient’s stomach, and a computer with software.
The panel of the wearable unit has special buttons, by pressing which the patient registers in the device’s memory the time of occurrence and duration of pain, dyspeptic symptoms, food intake and other events. The pH-metric probe has several electrodes and allows you to simultaneously record pH from 2-3 parts of the stomach. pH is determined at set time intervals from 1 to 60 s in various devices. Not long ago, clinical trials of the acidogastrometric automated complex AGM-24 MP (Gastroscan-24) produced by Istok-System (Fryazino, Moscow Region) were completed at the Federal Gastroenterological Center. The AGM-24 MP device is designed for continuous recording of pH values in the esophagus, stomach and duodenum for 24 hours with a reading interval every 20 seconds. The study is carried out using a pH probe inserted transnasally, connected to a secondary converter (AGM-24 MP acidogastrometer), with subsequent transfer of the data array for processing on a PC. At the end of the examination, information is provided in text and graphic modes about the dynamics of pH during the study. The obtained results are saved in the database.
The AGM-24 MP acidogastrometer is a secondary converter that works with a pH probe. The acidogastrometer is made in a portable version (weight about 500 g) with autonomous power supply from batteries. This allows pH values to be recorded every 20 seconds for 24 hours. The device is equipped with a symbolic-digital indicator that allows you to control its operation. The presence of 15 keys allows you to both set the operating mode of the device (calibration, data reading, start and end of work), and mark various time intervals and events during the examination of the patient (eating, medications, pain, heartburn, etc.). The pH probes used in this study consist of 2 mm diameter antimony measuring electrodes mounted in a 2.0 mm diameter polymer tube and an external silver chloride reference electrode. The probes have three main types: with one (G1), with two (G2) and three (G3) measuring electrodes.
For patients of different age groups, the transducers are made with interelectrode distances of 50, 70, 90, 110 and 120 mm. The software included in the Gastroscan-24 system is designed to run on an IBM PC-compatible PC. The software package includes files with a database, files for conducting examinations, files with instructions for operating the complex, and demo files. This program allows you to analyze the data obtained over various time intervals and save research data in a database.
Also, NPP Istok-System produces equipment for conducting traditional studies of gastric secretion within two to five hours (“Gastroscan-5M”, “Gastroscan-5”, “Gastroscan”, “AGM-03”), which can be used in work functional diagnostic rooms, gastroenterological and surgical departments of hospitals, clinics and diagnostic centers, as well as in scientific research. These devices are equipped with pH probes, which have from one to five pH sensors with a diameter of 7 to 1.8 mm, which makes it possible to measure pH through the instrumental channel of the fiberscope.
Topographic transendoscopic pH-metry
Allows visual control of the pH measurement site and functionally complements endoscopic examination. The method is based on an analysis of the functional state of zones of acid formation and neutralization of secretions during endoscopic examination. The study is carried out by measuring pH values through a pH-metric probe placed in the endoscopic channel of the endoscope. For the study, acidogastrometers AGM-01, AGM-03, and Gastrotest MK-90 are used. Before the study, it is advisable to pass a pH-metric probe with a measuring electrode through the biopsy channel of the endoscope to the level of its outlet at the distal end. This will prevent possible contact of the electrode with thick mucus of gastric contents that enters the biopsy channel of the endoscope during suction, which can change the readings of the pH meter. If pH measurement is performed during an endoscopic examination, before inserting the pH probe, the biopsy channel of the endoscope should be washed with 20 ml of sterile distilled water, injecting it into the lumen of the channel with a syringe. To reduce irritation of the mucous membrane, endoscopic examination should be performed with minimal insufflation of the stomach with air.
Determination of pH should be carried out under visual control with moderate pressure of the electrode on the mucous membrane (until a slight funnel-shaped “crater” appears around the electrode). Contact of the electrode with the mucous membrane is carried out for 5-10 s, the measurement results are recorded and read from the acidogastrometer indicator. If there is no contact, the pH values will be incorrect and may be below 0.8. pH values can vary depending on the pressure of the probe on the mucous membrane and the angle of attack of the probe to the surface of the gastric mucosa. For more reliable readings, it is recommended to measure pH three times at each control point and calculate the average result. Long-term monitoring of pH in the upper parts of the digestive tract allows us to identify gastroesophageal and duodenogastric reflux, determine the acid-forming function of the stomach after surgical interventions on the stomach, and also provides the opportunity for individual selection of the dose and regimen of taking an antisecretory drug and monitoring the treatment. In the process of studying the profile of 24-hour intragastric acidity in healthy people, a daily circadian rhythm of hydrochloric acid production was revealed and its significant changes in patients with chronic gastritis (antral, gastric body, pangastritis), duodenitis (anthropyloroduodenitis), peptic ulcer, reflux esophagitis.
Based on this study, it is possible in most cases to differentiate autoimmune gastritis (up to achlorhydria as a result of atrophy of parietal cells) from Helicobacter gastritis. The latter never manifests itself as achlorhydria, since in this form of gastritis the atrophic process is focal in nature, and in antral gastritis, antropylorobulbitis, peptic ulcer with localization of the ulcer in the outlet of the stomach and in the duodenal bulb, the acid-producing function is usually increased, especially at night. In case of reflux esophagitis and insufficiency of the lower esophageal sphincter, using this method, reflux of acidic gastric contents is detected, and it is also possible to study the frequency and duration of refluxes. It is extremely important that this method allows one to evaluate the effect of various drugs on the intraesophageal and intragastric environment depending on the dose, route of administration and time of administration.
Literature
1. Beatty A.D. Diagnostic tests in gastroenterology: Translated from English - M.: Medicine, 1995. - 224 p.
2. Gastroenterology. — P/ed. J. Barona, F. G. Moody. - M., Medicine. -1988. —TI
3. Grozdova T.Yu., Chernenkov Yu.V. Current issues in pediatric gastroenterology “Gastric acid formation (research methods, clinical significance, correction of therapy)” - Educational and methodological manual. - Saratov, 1998. - 44 p.
4. Gastrointestinal hormones and pathology of the digestive system: trans. from English — P/ed. M. Grossman. - M.: Medicine, 1981. - 272 p.
5. Laboratory research methods in the clinic: Handbook / Menshikov V.V., Delectorskaya L.N., Zolotnitskaya R.P. and etc.; Ed. Menshikova V.V. - M.: 1987.- 368 p.
6. Leya Yu.Ya. Modern assessment of gastric acid formation // Clinical. medicine. - 1996. - T. 74, N 3. - P. 13 -16.
7. Leya Yu. A., Birgale E. L., Linar E. Yu. Normal gastric acid formation according to intragastric pH-metry. //Ter. archive. - 1984. - No. 2. - P. 40-42.
8. On the unification of methods for studying the secretory (acid-forming) function of the stomach: methodological. recommendations for students, interns and general practitioners. — Ed. V.V. Nedogody. - Volgograd, 1986.
9. Okhlobystin A.V. Use of intragastric pH-metry in clinical practice / guidelines for doctors. - Moscow, 1996. - 32 p.
10. Sablin O.A., Bogdanov I.V. The use of intraesophageal rheography for the diagnosis of gastroesophageal reflux disease // Russian Journal of Gastroenterology, Hepatology, Coloproctology. - 1998. - T. 8, N 5. - P. 9-10.
11. Sablin O.A., Grinevich V.B., Uspensky Yu.P., Ratnikov V.A. Functional diagnostics in gastroenterology: educational and methodological manual. - St. Petersburg, 2002.
12. Trifonov M.M. Intracavitary pH-metry is the “gold standard” in gastroenterology, pulmonology and other areas of medicine. Practical Medicine. - 2003. - No. 3.
13. Udaltsov B.B. Intragastric electrometric studies in gastroduodenal diseases: Dis. ...cand. honey. nauk.- L., 1981. - 172 p.
14. Zimmerman Ya.S., Verzhbitsky F.R. New criteria for assessing the acid-forming function of the stomach using intragastric pH-metry // New methods of diagnosis and treatment in gastroenterology. - Perm, 1983. - pp. 3-7.
15. Chernov V.N., Chebotarev A.N., Donskov A.M. Gastroenterology (research methods, instruments, automated systems and choice of treatment method). - Rostov n/d, Publishing house Rost. University, 1997.- 464 p.
16. Yakovenko A.V. Modern methods of studying gastric secretion // Attending physician. - 1999. - No. 6.
17. Baron JH The clinical use of gastic function tests. — Scand. J. Gastroenterol., Suppl.. -1970, 6. - P. 9-46.
18. Kay AW Effect of large doses of histamine on gastric secretion of HCl; an augmented histamine test. — Br. Med. J. - 1953. - R. 77-80.
19. Stendal C. Practical guide to gastrointestinal function testing. - Tennessee: Blackwell Science, 1997. - 281 rub.
The article was published on the website https://www.gastroscan.ru
Volume of gastric juice production
About 2 liters of gastric juice are produced in the stomach of an adult per day. Basal (that is, at rest, not stimulated by food, chemical stimulants, etc.) secretion in men is (25-30% less in women):
- gastric juice - 80-100 ml/h;
- hydrochloric acid - 2.5-5.0 mmol/h;
- pepsin - 20-35 mg/hour.
The maximum production of hydrochloric acid in men is 22-29 mmol/h, in women - 16-21 mmol/h.
To conduct these studies, the workplace must be equipped with the following elements:
- Set of test tubes, pipettes.
- Glass capillary tubes.
- Scalpel.
- Millimeter ruler.
- Magnifier.
- Water bath.
- Thermostat.
- Stopwatch.
- Fixanal hydrochloric acid.
- Dimethylamidoazobenzene.
- Phenol.
- Ferric chloride.
- Sodium hydroxide.
- Pepsin.
- Calcium chloride.
- Copper sulfate.
- Potassium iodide.
- Crystalline iodine.
- Glacial acetic acid.
- Pyramidon.
- Ether.
- Glycerol.
- Fresh chicken egg.
- Raw low-fat milk.
- Litmus or universal indicator paper.
- Methyl orange.
Determination of uropepsin
The method for determining uropepsin is based on determining the curdling time of milk casein at pH 4.9. The following reagents are required for the study:
- 2 N solution of hydrochloric acid: 82.4 ml of hydrochloric acid (specific gravity 1.19) and 300-350 ml of distilled water are poured into a half-liter volumetric flask, the contents of the flask are mixed and added to the mark with distilled water.
- 0.2% aqueous solution of methyl orange: 0.2 g of methyl orange is placed in a 100 ml cylinder, dissolved in 50-60 ml of distilled water, and then topped up with water to the mark.
- Acetate buffer: 4.2 g of sodium hydroxide is placed in a cylinder with a capacity of 100 ml and dissolved in 50-60 ml of distilled water, 9.2 ml of glacial acetic acid is added and water is added to the mark.
- Milk-acetate mixture: Raw low-fat milk and acetate buffer are mixed in equal volumes. The mixture will keep in the refrigerator for 4-5 days.
- Standard solution of pepsin in hydrochloric acid: 100 ml of 3% hydrochloric acid solution and 2 g of pepsin.
For research, a portion of urine excreted during the second morning urination is delivered. The amount of urine is recorded. The directions should indicate the time in hours between the first and second urination of the morning.
Determination technique. Accurately measure 3.8 ml of urine into a test tube, add 0.2 ml of 2 N hydrochloric acid solution and 1-2 drops of 0.2% aqueous solution of methyl orange. At pH 3, the contents of the test tube turn red. To convert uropepsinogen into uropepsin, the test tube is placed in a thermostat at 37° for one hour. After one hour, 0.1 ml is poured into two test tubes, and in cases where a small amount of uronepsin is expected, 0.3-0.4 ml of acidified urine, 1 ml of acetate buffer solution pH 4.9 and up to 2 ml are added distilled water. All solutions are also heated to 37° before adding milk. Add 0.5 ml of a mixture of milk and acetate buffer to the test tubes. A stopwatch is used to measure the time the milk is added and the flakes fall out. The reaction should occur at 37°.
The rate of milk curdling is determined using a standard pepsin solution: 5 ml of milk-acetate mixture is quickly added to 0.1 ml of pepsin solution. Milk is considered suitable for the reaction if curdling occurs in 40-45 seconds.
Determination of volatile fatty acids
Technique for determination of volatile fatty acids. Organoleptically volatile fatty acids (butyric, acetic acid) are determined by their characteristic odor (acetic acid smells like vinegar, butyric acid smells like rancid oil).
They are chemically determined as follows: 3-5 ml of the gastric contents being tested are heated in a test tube, after which a strip of moistened blue litmus paper is added to it. In the presence of volatile fatty acids, litmus paper turns red.
For a more accurate determination of volatile fatty acids, an ether extract is prepared from gastric contents (5 ml of ether is added to 1-2 ml of gastric contents, the mixture is shaken, and then allowed to settle). 2 ml of distilled water is poured into a separate test tube and a drop of 1% ferric chloride solution is added. The prepared ethereal extract is layered onto this mixture. In the presence of butyric acid, an orange ring is formed at the liquid interface, and in the presence of acetic acid, a dark red ring is formed.
Determination of blood pigment
To detect blood pigment, the following reagents are required:
- 50% acetic acid solution: add 50 ml of distilled water to 50 ml of glacial acetic acid.
- 5% solution of pyramidon in 96° ethyl alcohol.
- 3% hydrogen peroxide solution.
Technique for determining blood pigment (Thevenon and Rolland test). To 1 ml of gastric contents add 16 drops of a 50% acetic acid solution. Then, carefully, along the wall, layer a mixture of 1 ml of a 5% pyramidon solution and 12 drops of a 3% hydrogen peroxide solution. In the presence of blood pigment, a blue-violet ring is formed at the interface of the liquids.
Note. In the presence of blood-stained mucous patches or small blood clots selected for microscopic examination, the reaction to blood pigment with the total mass of gastric contents may give a negative result. In such cases, the presence of blood in patches of mucus or clots is noted.
Determination of pepsin
Quantitative determination of pepsin using the Metta method. Prepare thin-walled glass tubes with a clearance of 0.5 to 1 mm and about 2 cm in length. They are washed and dried, after which they are filled with filtered egg whites (the whites from several eggs are mixed and filtered through a cloth; if there are air bubbles in the whites, they are placed in a desiccator for several hours). The tubes are filled by sucking the protein, after which one end of the tube is sealed. In this way, fill all the prepared tubes, which are kept in a horizontal position in water at a temperature of 85° until the protein completely coagulates.
After the water has cooled, the tube with the coagulated protein is removed, washed with water and stored in glycerin or water saturated with chloroform.
To determine the activity of pepsin, the gastric juice to be tested is poured into a test tube, four prepared tubes with coagulated protein (Metta stick) are placed in it and placed in a thermostat at a temperature of 37-38°. In the absence of hydrochloric acid, gastric juice is first acidified.
After 24 hours, the tubes are removed and the length of the digested protein column on each side of the tubes is measured using a millimeter ruler and a magnifying glass. This is how pepsin activity is determined. The average of the eight measurements is calculated. There is no direct proportionality between the amount of pepsin and the length of the dissolved column. According to the Schutz-Borisov rule, the amount of enzyme is proportional to the square of the length of the digested protein column. If on average 1.5 mm of protein column was digested, then the peptic activity of gastric juice is 1.5 X 1.5 = 2.25. Normally, after 2 hours, the arithmetic mean of eight measurements is 1.2-1.5 mm.
Determination of lactic acid
A small amount of lactic acid is found in normal gastric contents taken after a bread-and-water breakfast, since bread contains lactic acid. Therefore, it is recommended to determine lactic acid in gastric contents taken on an empty stomach in the absence of free hydrochloric acid. To determine lactic acid, the Ufelman reaction is used.
Technique for determining lactic acid. The principle of the reaction is based on the fact that ferric iron salts give a yellow-green color with lactic acid, due to the formation of lactic iron. The reaction requires Ufelman's reagent, which consists of the following solutions: 1) 2% solution of phenol (carbolic acid). Place 2 g of phenol in a 100 ml volumetric flask and add distilled water to the mark; 2) 10% ferric chloride solution (10 g of ferric chloride is placed in a 100-ml volumetric flask and topped up with distilled water to the mark).
The reagent is prepared immediately before the reaction. Place 10 ml of a 2% phenol solution in a test tube, add dropwise a 10% ferric chloride solution until a grayish-violet color appears.
Setting up a reaction. Filtered gastric contents are added dropwise to 5 ml of Ufelman's reagent. In the presence of lactic acid, the grayish-violet color turns into greenish-yellow. The free hydrochloric acid present in the gastric contents discolors the Ufelman reagent. Therefore, in the presence of free hydrochloric acid in the gastric contents, the Ufelman reaction is carried out with an ether extract.
The ether extract is prepared as follows: 5 ml of filtered gastric contents are placed in a small separating funnel, 20 ml of ether is added and shaken thoroughly. At the moment when the contents are divided into layers, the lower aqueous layer is poured into one test tube from the separating funnel, and the ethereal layer into the other. To the resulting ethereal extract add 20 ml of distilled water and 2 drops of a 10% ferric chloride solution. In the presence of lactic acid, the aqueous layer acquires a greenish or greenish-yellow color. The intensity of the color depends on the amount of lactic acid in the gastric contents.
Determination of hydrochloric acid deficiency
Hydrochloric acid deficiency is determined in gastric contents, in which free hydrochloric acid is absent. The following reagents are required for this reaction:
- 0.1 N hydrochloric acid solution (prepared from hydrochloric acid fixanal);
- 0.5% alcohol solution of dimethylamidoazobenzene.
Determination technique. To 5 or 10 ml of filtered gastric contents, add 1-2 drops of a 0.5% solution of dimethylamidoazobenzene and titrate with a 0.1 N solution of hydrochloric acid until a red color appears. The initial level of 0.1 N hydrochloric acid solution and the level at the end of the titration are recorded. Make a calculation.
Calculation example. The initial level is 0.1 N hydrochloric acid solution at division “0”. To titrate 5 ml of gastric contents, 2 ml of 0.1 N hydrochloric acid solution was used. Consequently, 20 times more is consumed per 100 ml: 2X20 = 40 ml of 0.1 N hydrochloric acid solution.
Answer: the hydrochloric acid deficiency is 40. When secretion completely stops, the hydrochloric acid deficiency is about 20.