Anal fissure is one of the most common diseases of the anus. About 15% of all patients turn to a proctologist with a similar problem - and only hemorrhoids manage to exceed this figure, with which about 40% of visitors come to the doctor.
Previously, the medical doctor had already described in detail what an anal fissure is, what factors provoke its occurrence, what symptoms it manifests, and also listed modern methods of treating ruptures in the mucous membrane of the anal canal. At the same time, many questions that acutely concern patients with such a delicate disease remain unanswered.
To correct this unfortunate omission, seven of the most frequently asked questions about anal fissures will be discussed in detail below.
How to distinguish an anal fissure from hemorrhoids?
It is extremely difficult to do this without an examination by a specialist, because the symptoms of both diseases are similar: pain and bleeding during and/or after defecation, as well as itching and/or discomfort in the anus.
The presence of external hemorrhoids in a patient does not simplify the task of preliminary diagnosis, since these are not mutually exclusive problems: in some cases, patients have both hemorrhoids and anal fissure.
Conditional pre-medical differentiation will help to compare the distinctive symptoms characteristic of the diseases in question:
| Symptoms | Anal fissure | Haemorrhoids |
| Pain | Acute, occurs at the beginning of the act of defecation and quickly subsides after its completion. | Most often dull, pressing and constant, slightly dependent on the nature of defecation. |
| Blood color | Always bright scarlet. | Scarlet, dark red or dark cherry. |
| Bleeding volumes | More often it is scanty - a few drops, up to 1 teaspoon. Rarely - abundant. | May vary in intensity. |
| Nature of bleeding | Only after defecation. | It does not always directly depend on going to the toilet. |
| Itching or discomfort | May be observed. | May be observed. |
| Anal spasm | Often present. | Absent. |
| Mucus discharge | None. | May be observed. |
Is it possible to get rid of an anal fissure forever?
Proctology considers anal fissures as a recurrent pathology, but this statement is not unconditional.
If a rupture of the mucous membrane of the anal canal occurred as a result of mechanical impact (anal sex, diagnostic procedures, etc.), then the likelihood of relapse is regarded as minimal - provided that the patient undergoes the full course of appropriate therapy.
The prognosis is significantly worse if the main cause of the fissure is degeneration of the mucous membrane of the anal canal, which has arisen as a result of neglected internal hemorrhoids, untreated atherosclerosis, etc. In this case, the rupture may occur again if the patient does not follow the recommendations for the prevention of anal fissures: bowel movement regulation , avoidance of physical activity, treatment of the underlying disease.
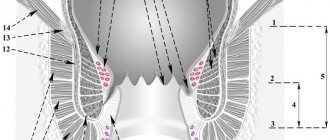
Scheme of the pathogenesis of acute anal fissure.
Anal fissure (AF) ranks third in frequency of occurrence in the structure of diseases of the colon (11-15%), and the incidence ranges from 20 to 23 cases per 1000 adults [1-4]. AT most often affects women (more than 60%) and about 1/3 of patients are of working age [5], which determines the social significance of the problem. In 85% of cases or more, AT is detected along the posterior circumference of the anal canal at 6 o'clock and in 8-9% - along the anterior wall at 12 o'clock, mainly in women [5], in 0.5% - on the lateral walls of the anal canal [6]. In 3-4% of cases, 2 ATs are found along the posterior and anterior walls of the anal canal [7].
Etiology and pathogenesis
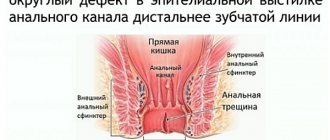
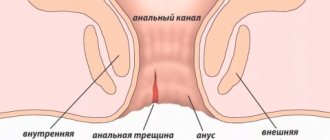
AT is a defect in the wall of the anal canal, reaching the pectineal line, sometimes higher. There are acute and chronic AT. An acute fissure has smooth edges, and its bottom is the muscle tissue of the anal sphincter [6]. Under unfavorable conditions and lack of treatment, over time, the bottom and edges of the crack become covered with granulation tissue. Next, granulation tissue grows and is replaced by connective tissue. In the area of the edges (usually the inner edge), excess tissue appears - the anal tubercle, which can hypertrophy into a fibrous anal “polyp” [1]. The resulting inflammatory changes involve nerve endings in the process and contribute to the formation of a non-healing ulcer. In this case, a spasm of the anal sphincter occurs and a vicious circle develops [6].
The causes of AT are numerous. In 1994, W. Schouten et al. [8, 9] revealed a relationship between pressure in the anal canal and blood supply to the anoderm. The study showed that the pressure of the internal anal sphincter is inversely proportional to the blood supply of the anodermal zone at 6 o'clock and that the blood supply of the anoderm is worse at 6 o'clock than in other areas [10]. A significant decrease in blood supply in this area was also revealed in patients with chronic AT compared to healthy people in the control group [8, 9].
These facts correlate well with the known mechanism of injury to the anoderm with the formation of an acute fissure and the occurrence of pain, which causes spasm of the internal anal sphincter and increased pressure in the anal canal, which, in turn, leads to a decrease in blood supply to the anoderm, tissue ischemia, forming a vicious circle [ 11] (see diagram).
It is known that a decrease in anal sphincter pressure after sphincterotomy is associated with improved blood supply to tissues in this area [8, 9, 12, 13].
The most common cause of acute AT is considered to be a defect in the mucous membrane of the anal canal due to the passage of hard stool. In the posterior wall of the anal canal, the deeper parts of the anal crypts (sinuses) are located, and the tendon endings of the anal sphincter muscles converge here, which causes the more frequent occurrence of posterior fissures [1, 6].
In women, the vulva, vagina and fibrous center of the perineum converge in the anterior part of the anal canal, and therefore anterior fissures are more common in women [5, 14]. Cracks on the side walls are quite rare. Often the fissure is combined with chronic hemorrhoids [5]. The walls of internal hemorrhoids are located in the area most susceptible to injury during defecation. In addition, poor circulation may be accompanied by the appearance of linear ulcers, similar to the ulcers that form with varicose veins. The presence of chronic inflammation in this area can also lead to loss of elasticity and fibrotic changes in this area, and subsequently to injury and ruptures with the formation of AT [5]. Neurogenic disorders can lead to the formation of AT due to prolonged spasm of the external and internal sphincters.
The mucous membrane, exposed to the action of intestinal flora, becomes scarified, then thickens and deepens, thus forming AT - a longitudinal defect of the mucous membrane with a clearly differentiated bottom and edges. At the bottom of the crack, the nerve endings are exposed, which leads to increased pain. The proximal edge of the fissure does not extend to the rectal mucosa and remains within the dentate line [6].
Diagnostics
The main symptoms of AT are pain and discharge of red blood during and after defecation [1, 5—7]. In acute AT, the pain is intense, but relatively short-lived, it occurs during and immediately after defecation and goes away on its own within 15-20 minutes [4, 11]. When examining such patients, a pronounced spasm of the internal anal sphincter is noted, bleeding is minimal. Upon examination, as a rule, a painful area in the anal canal is identified. With a chronic fissure, the pain may be more prolonged, and sometimes a symptom such as stool phobia appears. Patients begin to use laxatives and enemas, insomnia appears, and patients become irritable [11]. Complications of the fissure include severe pain caused by spasm of the anal sphincter, repeated bleeding, and in rare cases, acute or chronic paraproctitis (rectal fistula) due to infection of the perirectal tissue through a defect in the mucous membrane [7].
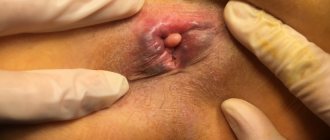
An external examination may reveal a longitudinal or triangular ulcer extending proximally into the anal canal [6].
A thorough examination with separation of the anal folds should precede a digital examination, which is not always feasible due to the severity of the pain syndrome. During a digital examination, you can determine the localization of the crack, the condition of its edges, the presence of a “sentinel tubercle”, pay attention to the degree of sphincter spasm, the presence of discharge (for differential diagnosis with an incomplete internal fistula) [5].
The use of instrumental research methods (anoscopy, sigmoidoscopy) is possible only after adequate anesthesia of the anal canal [7]. Significant methods for diagnosing AT include profilometry (sphincteromanometry) - measuring the pressure of the internal and external sphincters to assess the pathogenesis of the disease in a particular patient [5, 15, 16]. It is advisable to conduct research both before and after a course of treatment or surgery. Electromyography using a special sensor can be used to assess the contractility of the sphincter muscles [5, 17].
AT must be differentiated from an incomplete internal rectal fistula, in which the pain is usually less intense, and purulent or mucopurulent discharge from the rectum is noted. Upon digital examination, a funnel-shaped depression is identified at the bottom of the ulcer - the internal opening of the fistula [6].
An ulcerative defect can also be observed with cancer of the anal canal - in this case, the edges and bottom of the wound are very dense (stony density). The history shows the rapid formation of a significant ulcerative defect, the discrepancy between the pain syndrome and its size (unexpressed pain with a significant ulcerative defect), which makes it possible to suspect a malignant disease in the early stages [18]. There are often cases of diagnosing squamous cell carcinoma after excision of such an ulcerative defect. A case of myeloid sarcoma of the anal canal, clinically defined as AT, in a patient with previously diagnosed acute myloid leukemia is described [19].
AT should also be differentiated from manifestations of syphilis (gumma) [20, 21], tuberculosis [22, 23], HIV [24, 25] or AT in Crohn's disease [26-28]. In such cases, the diagnosis helps to establish a thorough history, since the clinical picture of AT can occur in different ways and it can be difficult to differentiate it from a specific lesion during digital examination and anoscopy [1, 7].
Treatment
Treatment methods for AT include conservative and surgical methods [2, 3, 6, 7]. Conservative methods include therapeutic methods that help reduce the tone of the anal sphincter (medicinal relaxation) [29-31]. The goal of treatment in this case is to break the vicious circle (see diagram), which improves blood supply to the tissue in the area of the crack and can lead to its healing. About 50% of patients with acute AT are cured with conservative treatment, including abundant consumption of fiber-rich foods and warm (sitz) baths [4, 32].
A special study [33] showed that local use of drugs containing nitroglycerin is more effective than placebo in the treatment of AT (healing was observed in 49% of patients using nitroglycerin, and 36% with placebo). However, later relapse occurs in approximately 50% of patients. It was also found that calcium channel blockers had comparable efficacy to topical nitroglycerin [34]. Topical application of Diltiazem cream was shown to have better results than oral tablets (65% versus 36%) [29].
However, the use of nitroglycerin and nifidipine is currently most widespread. A double-blind, randomized study evaluating the effectiveness of topical nifidipine 0.3% and isosorbide dinitrate 0.2% included patients from 20 to 60 years of age [30]. After 21 days of follow-up, complete healing was achieved in 77.1% of patients in the nifedipine group and in 51.4% of patients in the isosorbide dinitrate group. The mean visual analog scale pain intensity on day 21 after the start of treatment was 0.91 in the isosorbide dinitrate group and 0.45 ± 0.78 in the nifedipine group.
In a study by M. de Rosa et al. [33] (142 patients) compared the effectiveness of internal lateral sphincterotomy (LIS) and topical nifidipine combined with anal dilation. In the latter case, patients were asked to use a warm anal dilator with nifidipine ointment for 5 minutes, 2 times a day for 4 weeks. In 51 (68.9%) patients treated conservatively and in 60 (88.2%) patients after lateral sphincterotomy, the AT healed after 8 weeks. There was no statistical difference in the occurrence of side effects between the groups of patients after LIS and after topical application of nifidipine.
Currently, AT treatment with botulinum toxin is actively used. In this case, the healing rate reaches 60–80% [24, 35–41]. However, relapse is possible in 42%, and repeated treatment has the same effectiveness. The most common side effect is temporary incontinence of gas (18%) and stool (up to 5%) [32]. In a number of studies [31, 40], botulinum toxin injection shows similar results to topical nitroglycerin and calcium channel blockers.
Surgical treatment of AT must be carried out in case of chronic disease that is not amenable to conservative therapy. The operation consists of excision along the plane of the crack itself within the healthy mucous membrane. The wound usually heals easily and quickly (on average within 5-6 days) with a thin scar. The main thing in this operation is to decide whether an additional sphincterotomy needs to be done, and if necessary, then in what way. Without this additional manipulation, in most cases the operation is not successful; a recurrence of the crack occurs due to renewed tonic contractions of the sphincter. Patients in whom spasm was not detected before surgery or was only slightly expressed can be permanently cured only by excision of a crack within the healthy mucous membrane. If spasm is pronounced, then lateral subcutaneous sphincterotomy is indicated [41-47].
If in the last century most coloproctologists used the technique of open posterior dosed sphincterotomy, now a different trend has emerged. Additional dissection of the anal sphincter through the wound is difficult to accurately dose, if only because the thickness of the dissected wall of the anal canal is different in patients of different sexes and constitutions. These observations prompted many coloproctologists around the world to switch to lateral subcutaneous sphincterotomy, proposed by A. Parks [46]. In this case, only the internal sphincter is dissected, since it has been proven that its pathological condition is the source of spasm and pain. A narrow (ocular) scalpel is inserted into the groove separating the external and internal sphincters, and the scalpel blade is turned toward the lumen of the rectum under the control of a finger inserted into the anus. Then, in one movement, the internal sphincter is cut from the side, away from the excised fissure, to the mucous membrane of the anal canal. After the operation, a thin tube-catheter and a gauze turunda soaked in water-soluble antiseptic ointment are inserted into the anus.
In the postoperative period, usually on an outpatient basis, only hygienic measures are necessary. For a week, you should take daily sitz baths and apply ointment to the anal canal wound. The patient needs to achieve daily soft stools not only in the immediate postoperative period, but also in the future [46].
Surgical treatment is usually offered to patients when conservative treatment is ineffective. In the literature, it is often recommended that surgical treatment be performed within 4 weeks from the start of treatment, but a differentiated approach is necessary. The presence of a sentinel tubercle or fibrous polyp near the fissure, dense edges with scar changes, is certainly an indication for excision of the fissure at any stage of the disease [44].
Lateral sphincterotomy is a gentle method, healing is observed in approximately 95% of patients [31]. Compared with topical nitroglycerin, calcium channel blockers, and botulinum toxin injection, lateral sphincterotomy is superior to conservative treatments but has more complications. The most commonly observed (17%) are anal sphincter insufficiency, gas or stool incontinence (usually temporary), which in some cases cannot be completely corrected. Complications of lateral subcutaneous sphincterotomy also include hematoma formation, bleeding and suppuration of the postoperative wound [31, 38, 48, 49].
A systematic review [50] with a meta-analysis of 148 studies of AT treatment found that the use of lateral sphincterotomy was more effective than non-surgical methods. Stool incontinence, previously considered a common complication of this treatment method, was observed in 3.4-4.4% of patients. When comparing surgical treatment methods, complications were observed significantly more often with manual divulsion of the anal sphincter than with lateral sphincterotomy. In patients requiring surgical treatment, open and closed sphincterotomies have been found to be equally effective.
In recent years, there has been increasing interest in combined techniques, mainly with excision of the fissure and the use of other technology (injection of botulinum toxin, use of a repositionable flap, use of platelet-derived growth factors, etc.). A review study with long-term follow-up [51] showed that conventional excision of the fissure resulted in healing in 88% of patients, recurrence was observed in 11.6% of patients, and failure was observed in 2.3%.
The study [35] conducted a combined treatment of chronic AT using botulinum toxin after excision of the fissure. The study included 102 patients. In 68 patients, complete recovery was observed at the control examination after 12 weeks, in 29 patients additional injections of botulinum toxin were required, in 95 patients there were no postoperative complications, in 7 patients there was temporary stool incontinence, the latter was not noted at the control examination at week 12. There were no other complications.
Back in the 60s of the last century, attempts were made to minimally invasive treatment of AT. In 1964, J. Watts et al. [52] reported a treatment method used in 99 patients with AT, divulsion of the anal canal with two and then four fingers, applying significant force to the lateral walls of the anal canal. Dilatation was carried out for at least 4 minutes. A satisfactory effect was reported in 95% of patients; recurrence of the fissure occurred in 16% of patients.
To standardize the anal divulsion technique, pneumatic balloon dilatation (PBD) was developed. N. Sohn et al. [53] first described PBD using a 40 mm rectosigmoid balloon.
In a randomized controlled trial [54], PBD was used instead of manual dilatation compared with lateral internal sphincterotomy (LIS) for the treatment of chronic A.T. Overall healing rates at 6 weeks for PBD and LIS were 83 and 92%, respectively. As assessed by preoperative and postoperative manometry, both methods reduced anal pressure by approximately 30%. In the PBD group, temporary anal incontinence was observed in some cases; however, at 24-month follow-up, no incontinence was noted in the PBD group, while 16% of patients in the LIS group had it. Thus, PBD has a significantly higher risk of relapse with fewer complications.
According to Russian studies [17], a decrease in the incidence of postoperative complications by more than 20% was revealed compared with patients who underwent lateral subcutaneous sphincterotomy, as well as a decrease in the severity of pain, which significantly reduced the risk of urinary retention and eliminated the need for narcotic analgesics. There was a reduction in the duration of hospital treatment to 2.1±0.3 days compared to 6.4±1.1 days in patients after lateral subcutaneous sphincterotomy. In the long-term period, in patients who underwent pneumodivulsion, stable elimination of spasm of the internal sphincter was observed, while no changes in functional contractility or disruption of the integrity of the sphincter muscle structures were detected. There was also no relapse or development of anal sphincter insufficiency when using balloon pneumodivulsion, whereas after lateral subcutaneous sphincterotomy, sphincter insufficiency occurred in 12% of patients, and relapse in 8%.
Various studies have evaluated the technique of a repositioned mucocutaneous flap from the anal margin of the anoderm. Two independent studies [55, 56] revealed a 98% chance of healing of chronic AT with improved anoplasty, regardless of the value of the initial anal tone.
The study [57] showed success in 10 (100%) patients who underwent excision of anal fissure and Y-en-V anoplasty with botulinum toxin injection for anterior chronic AT with hypertonicity of the internal anal sphincter, with no relapse within up to 12 month after surgery.
Although these studies have shown promising results, larger prospective studies with longer follow-up are needed before further recommendations for the use of this method can be made [44].
Currently, laser technologies are actively developing in medicine [58], including in the treatment of AT. In the 80s and 90s, due to the reduction in cost and increased availability of high-tech medical devices in our country, lasers began to be actively used in medicine in general and in coloproctology in particular [59-62]. However, since the 2000s, interest in this technology began to fade.
The authors of the study [63] compared the effectiveness of laser therapy and the surgical method (lateral sphincterotomy) in 300 patients with acute AT in whom conservative therapy was ineffective. In the main group ( n
=150) 10-20 laser therapy sessions of 5-10 minutes each were performed, and during treatment the patients did not receive medication. There were no significant differences during the early postoperative period in the main and control groups, however, in the late period, patients in the main group had fewer complications and relapses of the disease were less frequent. The disadvantage of this technique is the significantly longer duration of treatment.
The study, which was carried out among 25 patients with chronic AT aged 20-75 years, used a carbon dioxide laser. To remove fibrous and granulation tissue, the bottom and edges of the crack were treated with a laser in a continuous mode, and the area around the crack was treated in a fractional mode to stimulate reparative processes. There was a significant regression in the frequency and severity of pain, bleeding and constipation [64]. None of the patients had a relapse of AT after 1 year of follow-up and no symptoms of incontinence were diagnosed. The results of the study show that the use of laser surgery in the treatment of anal fissure is very promising. The authors of the article argue that laser surgery is a simple, safe and effective procedure for the treatment of AT, which can be performed under local anesthesia in an outpatient clinic, with minimal pain. Due to the small sample of patients, this method requires further research and study.
Thus, AT is a common disease, which determines its social and economic significance, since many patients are of working age. Recently, there has been a trend towards a decrease in the use of invasive methods and an increase in the frequency of use of combination treatment - the prescription of several minimally invasive or medicinal techniques to obtain an optimal result (reduction of surgical trauma, length of hospitalization and the number of complications). The most effective methods of treatment include the use of botulinum toxin, including in combination with excision of the AT in the presence of fibrous changes, sentinel tubercle or fibrous anal polyp, the use of a CO2 laser, as well as the use of nifedipine ointment for the treatment of fissures with severe sphincter spasm and in the absence of pronounced fibrotic or inflammatory changes.
The authors declare no conflict of interest.
What is the best method to operate on an anal fissure?
In total, there are three main methods of surgical treatment of rupture of the mucous membrane of the anal canal:
1. Scalpel
: the crack is excised and the edges of the wound are sutured. As of today, the method is practically not used anywhere due to its high invasiveness and low efficiency.
2. Laser
: using a laser beam of a given power, the edges of the crack are skinned, and the gap itself is coagulated. This method is quite painful and practically useless for treating old cracks.
3. By radio wave
: the gap is removed non-contactly using the Surgitron device, which generates a focused “beam” of radio waves that allows you to cut tissue and coagulate wounds. The method is painless and has a minimal risk of subsequent relapse.
Thus, if the patient has a choice, it is best to give preference to the radio wave method of therapy.
Rectal fistulas
According to statistics, approximately 95% of patients with rectal fistulas associate the onset of the disease with a history of purulent inflammation (acute paraproctitis). It is not advisable to postpone radical surgical treatment for a long time, since exacerbation of paraproctitis can recur and the inflammatory process can lead to deformation of the surrounding tissues, including the development of anal muscle insufficiency. For most rectal fistulas, excision of the fistula is performed (with or without suturing the wound). Surgical treatment of simple fistulas leads to a permanent cure in approximately 95% of cases and is not accompanied by any serious complications. Complex fistulas (so-called deep trans- and extrasphincteric) can also be cured without functional impairment.
Epithelial coccygeal tract
In proctological practice, every 10 patients (in men, on average, three times more often) are found to have fistulas in the coccyx area. This is a congenital anomaly - the epithelial coccygeal duct. Patients are often unaware of their illness, or their complaints are limited to a small constant discharge above the anus, wetness of the skin between the buttocks, and itching. In other cases, often after an injury, suppuration suddenly occurs in the coccyx area, after opening which a non-healing fistula remains. Prolonged course of the epithelial coccygeal tract without proper treatment or self-medication leads to the formation of secondary fistulas; the entire sacrococcygeal region is affected by the inflammatory process. The prognosis for radical treatment of the epithelial coccygeal tract at any stage of the disease is favorable, complete recovery occurs.
Perianal genital warts
Pointed perianal condylomas are gray-pink papillary formations between which unaffected skin is visible, sometimes in the form of papillae that merge and form whole conglomerates that can cover the anus. Sometimes there are giant condylomas up to 20 cm or more in diameter. It has now been established that condylomas are caused by human papillomavirus (HPV) types 6 and 11. The disease is usually sexually transmitted. They must be distinguished from flat condylomas, characteristic of syphilis and lesions in patients with AIDS. Surgical treatment is most often undertaken in the presence of large nodes and damage to the anal canal. Excision is performed with a scalpel, or an electric knife, or a laser. Sometimes, in the presence of large condylomas, surgical removal is not performed at once, since the resulting extensive wounds can lead to deformation in the area of the anus.
Patient consultations in our department are free and are held daily on weekdays. You can pre-agreed the time of your visit by phone. Read more on the website: https://www.surgery-future.ru/proktologiya
Can an anal fissure heal on its own?
This is possible subject to the following conditions:
- switching to a dietary diet that excludes foods that tend to irritate the rectum and anal fissure in particular (spicy, salty, smoked foods, marinades);
- normalization of stool - achieving regularity and softening of stool;
- decreased physical activity, especially weight lifting.
All of the above conditions must be met within the next 5-7 days, otherwise independent healing is regarded as unlikely.
Diagnosis of the disease
Only a doctor can make an accurate diagnosis; a person without a medical education cannot do this on his own, since a full examination is needed. For that? To make an accurate diagnosis, the doctor will examine the patient and also prescribe tests:
- A general blood test, the main purpose of which is to identify anemia, which directly indicates the presence of bleeding from a fissure
- General analysis of stool, the main purpose of which is to exclude inflammatory processes in the intestines, identify pathogenic flora, exclude bleeding, as well as the presence or absence of worms
Along with the above tests, the doctor may send the person for the following procedures: