Classification
Pathology can be characterized by:
- according to the speed of onset of symptoms: typical (chronic) and fulminant (rapidly developing);
- along the course: recurrent and continuous;
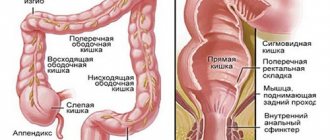
- by localization: left-sided with involvement of the descending colon and sigmoid, proctitis (damage to the rectum), total colitis with ileoreflux, colitis with extraintestinal manifestations and complications;
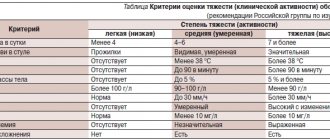
- according to the severity of clinical manifestations: mild, moderate, severe;
- by activity: minimal, moderate, pronounced.
Pathomorphology indicates a superficial diffuse pathological process.
The future of MAT
Recently, bispicephic or polyclonal antibodies have been created that act on more than one target and significantly increase the effectiveness of therapy. Thus, the drug Abatacept (Orencia) for the treatment of rheumatoid arthritis binds simultaneously to CD80 and CD86.
Fc-fusion proteins (fusion proteins, Fc-fusion proteins) are chimeric proteins obtained as a result of translation of a hybrid gene (“cross-linked” from two genes that originally encoded different proteins). The greatest commercial and clinical success has been achieved by protein preparations that include the Fc fragment of an antibody molecule (usually IgG).
Fig. 3 Scheme of the structure of MAT and Fc-fusion proteins
The Fc fragment causes antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity of the hybrid protein. The end result of hybridization is the prolongation of the therapeutic effect of the molecule connected to the Fc region and increased clinical efficacy. Three similar substances are registered for use in Russia: Aflibercept, Etanercept, Romiplostim.
Where are MAT preparations used?
MAT-based drugs are used for the treatment of chronic diseases with a long, progressive course. More than half of all MAbs have been created for cancer immunotherapy. Another broad area of application is autoimmune diseases, especially severe forms such as rheumatoid arthritis, systemic lupus erythematosus, psoriasis, and multiple sclerosis. In addition, MAT are used in allergology for the treatment of resistant, severe forms of bronchial asthma, in transplantology for the prevention of transplant rejection, in the treatment of certain viral, chronic inflammatory and orphan diseases (paroxysmal nocturnal hemoglobinuria, cryopyrin-associated periodic syndromes).
Difference from Crohn's disease
The pathology clinic resembles another common disease in which the erosive-ulcerative process affects the gastric part of the digestive system and the walls of the distal intestine - Crohn's disease.
A distinctive differential feature between the two pathologies is the localization of ulcers and erosions. Ulcerative colitis affects only the mucous membrane of the large colon and rectum, and Crohn's syndrome is localized in any part of the gastrointestinal tract. In addition, ulcerative colitis is associated with immune changes, and Crohn's disease is chronic inflammation.
What the pathologies have in common is their clinical manifestations. In addition, studies in a group of monozygotic twins showed a small probability of the appearance of a certain trait in both twins with UC (about 10%) in contrast to Crohn's disease (up to 50%), which directly proves the dominant role of external influences in the occurrence of UC before genetic problems.
To the surprise of scientists, smoking behaved especially unconventionally: it simply blocks UC, but stimulates Crohn's disease.
Methods for producing monoclonal antibodies
Obtaining monoclonal antibodies is a complex multi-step process that goes through the following stages:
- Animal immunization. Typically mice or rats are used. This is necessary in order to increase the number of lymphoblasts - cells that produce the necessary antibodies and transfer these cells to an active state. Once isolated from the body, these cells cannot exist for long in laboratory conditions; they will die even on nutrient media containing growth factors. To prevent this, they are crossed with malignant myeloma cells.
- Preparation of myeloma cells. In parallel with immunization of animals, tumor myeloma cells are prepared. They, firstly, have the ability to synthesize monoclonal antibodies, and secondly, they have unlimited life potential (they are immortal and capable of endless reproduction). To prevent myeloma cells from dying outside the body, they are cultured in special media using growth factors.
- Hybridization (fusion) of lymphoblasts and myeloma cells to form a hybridoma. To do this, cells are treated with various antibodies to change the structure of their membranes and provoke the formation of cytoplasmic contacts. In this case, different types of cells are formed that have a double set of chromosomes (dicaryons). These can be dikaryons formed only by lymphocytes, or only by myeloma cells. But for the production of monoclonal antibodies, it is dikaryons formed by a lymphocyte and a myeloma cell—hybrid cells—that are needed.
- Selection of hybrid cells. For this purpose, special solutions are used that allow only lymphoblastic and hybridoma dikaryons to survive. The former soon die, because they do not have the ability to divide without limits, but hybridoma cells remain viable.
- Recloning of hybridoma clones.
- Identification and selection of hybridomas producing monoclonal antibodies. Typically, an enzyme immunoassay is used for this.
- Massive build-up of antibodies.
- Purification of the resulting antibodies. The degree of purification will be determined by the area of application of the drug. If this is diagnostic, 70-95% purity is sufficient. If the drug is to be used for immunotherapy, a higher degree of purity is required. Affinity and ion exchange chromatography are used for purification.
- Removing remaining impurities and disinfecting the resulting drug from viruses and bacteria.
Currently, there is a trend towards refusing to use animal antibodies for therapeutic purposes. Firstly, they are foreign agents to the body and can provoke allergic reactions, including anaphylaxis, which directly threatens the lives of patients. Secondly, the human immune system, recognizing such antibodies as foreign, will try to inactivate them, which will reduce the effectiveness of antitumor treatment. It is not possible to obtain human monoclonal antibodies using the method described above due to the following problems:
- Immunizing humans with various antigens is unethical.
- Even if you obtain immunized human lymphocytes, there will be problems at the stage of their fusion with mouse myeloma cells—the resulting hybridomas will be unstable.
- Human myeloma cell lines that could be effectively used within biotechnologies to produce antibodies have not yet been obtained.
In this regard, it was necessary to look for new technologies for producing antibodies. The solution to the problem was hybrid, humanized and single-chain antibodies, the production of which involved the use of hybridoma technology, briefly described above, and recombinant DNA technology.
- A hybrid or chimeric antibody is an antibody in which its constant domain is replaced with human immunoglobulin. They are obtained using recombinant DNA technology, when the fragment of mouse DNA responsible for the synthesis of the constant domain is removed and replaced with a fragment of human DNA. Thus, the antibody will have a human protein as a constant domain, which has immunogenic and effector properties, which will allow the body to perceive it as “one of its own,” and the variable domain, which specifically interacts with the antigen, will remain mouse. All together, this will preserve the specificity and reduce the allergenicity and immunogenicity of the drug used.
- The humanized antibody contains even less mouse protein due to only the antigen-binding hypervariable regions of the variable domain. This further reduces the likelihood of complications from the immune system.
- A single-chain antibody is the smallest fragment of an antibody that is still able to bind well to the antigen and exert its effect. It does not contain a constant domain at all.
Mechanism of action of monoclonal antibodies
Monoclonal antibodies are widely used in the treatment of diseases in which an immune component is involved in the pathogenesis. They are used to treat psoriasis, autoimmune diseases, rheumatoid arthritis, and multiple sclerosis. These technologies have also shown great promise in oncology as part of targeted therapy. Moreover, their effect is based on various mechanisms, which are discussed below.
Changing Cell Signals
An example of changes in cellular signals is growth factor receptors. Some malignant cells have on their surface a large number of receptors for growth factors that activate a cascade of reactions aimed at enhancing cell reproduction. The more such receptors, the more active this process occurs. If the receptor is blocked with a monoclonal antibody, it will not be able to contact the ligand (growth factor), and accordingly the cascade of these reactions will not be launched. The cell will not reproduce as actively and will eventually die.
Complement-dependent cytotoxicity
This mechanism is implemented as follows. The antibody binds to the antigen located on the surface of the malignant cell, which leads to the activation of the multi-stage complement system (immune response mechanism). The final stage of these reactions is the formation of a special protein C 9, which perforates the cell membrane of the cancer cell, which ultimately leads to its death.
Strengthening the cytotoxic effects of immune cells
Monoclonal antibodies can stimulate immune cells such as macrophages. They will recognize malignant tumor cells and “devour” them, thereby destroying them.
Development of adaptive immunity
One of the reasons why the formation and development of a malignant tumor in the body becomes possible is that the human immune system does not recognize such cells as foreign. Monoclonal antibodies allow the immune system to “get to know” the cancer and make it available for binding and subsequent destruction. Thus, the body gets the opportunity to fight the tumor on its own.