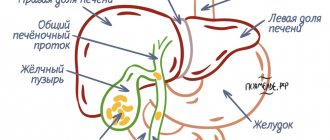
A gastric polyp is a benign formation on the gastric mucosa. It is rare and usually does not manifest itself clinically. Most often it is discovered by chance during examination for other reasons.
The size of stomach polyps ranges from 0.5 to 8-10 cm. They can develop in any area of the stomach, but most often they are localized in the pylorus - near the exit from the stomach, where the duodenum begins.
Most polyps are benign, although some increase the risk of developing stomach cancer. Treatment of such polyps involves their removal; in other cases, the doctor is limited to observation only.
Polyps in the stomach
What causes the development of polyps in the stomach?
The content of the article
A stomach polyp develops when the mucous membrane of this organ is damaged. What increases the likelihood of its occurrence the most is:
- Chronic gastritis
. Chronic inflammation of the stomach leads to hyperplasia of polyps and adenomas. Hyperplastic polyps are less susceptible to malignant transformation, but if the lesion is larger than one centimeter, the risk increases. Adenomas are the most susceptible to malignant neoplasms among polyps. Because of this, it is recommended to remove the adenoma. Fortunately, adenoma is the rarest form of gastric polyp. - Familial polyposis
. This disease has already been mentioned in the review of colorectal polyps. In this rare congenital pathology, polyps also arise from the fundus of the stomach. These polyps are also characterized by malignancy. Familial polyposis also causes the development of adenoma, so treatment is also surgical. - Regular use of certain medications for stomach upset
. The occurrence of polyps can be promoted by proton pump inhibitors taken to reduce the acidity of gastric juice. In this case, the polyps are small and usually do not cause dangerous complications, but if they increase in size, the risk of malignancy increases, so if such polyps are present, the gastroenterologist will either change the medicine that caused the polyp, or suggest removing the polyp, or both.
Gastric polyps are a common diagnostic finding during endoscopic examination of the upper gastrointestinal tract. On average, during esophagogastroduodenoscopy (EGD), they are detected in 2% of patients [1, 2]. At the same time, the tactics for their management are not sufficiently standardized and often raise questions from clinicians.
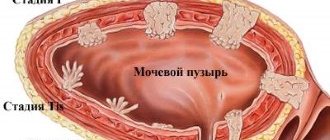
A polyp is any formation on the mucous membrane that protrudes into the lumen of the organ. This term reflects only certain macroscopic changes detected by endoscopy and does not denote an independent nosological unit. The nature of the polyp can only be established after a morphological examination, although some endoscopic signs tentatively indicate a presumptive diagnosis. To date, there is no generally accepted classification of gastric polyps. Depending on the number of formations, single and multiple polyps are distinguished. If more than 20 polyps are detected, they speak of gastric polyposis.
The endoscopic classification proposed by S. Yamada in 1966 distinguishes four types of polyps [3]:
- Type I – flat, raised, with unclear boundaries;
- Type II – protruding, semicircular, with fairly clear boundaries;
- Type III – clearly protruding, rounded, with a retracted base;
- Type IV – pedunculated.
In addition to the shape, it is also necessary to evaluate the nature of the surface of the polyp, which can be smooth, lobulated or villous (papillomatous). From a clinical point of view, the morphological classification of gastric polyps is extremely important, because the histological structure largely determines further management tactics. In accordance with the World Health Organization (WHO) classification of gastrointestinal tumors, gastric polyps are divided into epithelial and non-epithelial.
In each of these categories, tumors and tumor-like changes are distinguished [4].
- Epithelial polyps:
- adenomatous (adenomas);
- fundic glandular;
- hyperplastic;
- hamartomas:
- juvenile polyp;
- Peutz-Jeghers syndrome;
- Cowden's syndrome;
- juvenile polyposis;
- polyposis syndromes (not hamartomas):
- familial adenomatous polyposis;
- Cronkhite–Canada syndrome.
- Nonepithelial intramural polyps:
- inflammatory fibroids;
- xanthoma/xanthelasma;
- heterotopia of pancreatic tissue;
- lipoma
Of the epithelial polyps, only adenomatous polyps (adenomas) are classified as tumors, while the remaining polyps of this category, according to their histological structure, are not true neoplasias and represent tumor-like changes. Among non-epithelial polyps, gastrointestinal stromal tumors, leiomyomas, lipomas, fibromas and fibromyomas, neurogenic and vascular neoplasias are classified as tumors, while fibroid polyps, xanthomas and heterotopia of pancreatic tissue are classified as tumor-like changes.
During endoscopic examination, the form of polyps can be gastric adenocarcinoma, MALT lymphoma, gastrointestinal stromal tumors, carcinoid and gastric metastases of tumors of other localizations.
Adenomatous polyps (adenomas)
Adenomatous polyps are formations consisting of papillary and tubular structures lined with dysplastic epithelium. The great clinical significance of this type of polyps is determined by the high risk of malignancy, which allows them to be classified as precancerous diseases of the gastric mucosa [4]. In Western countries, the proportion of adenomas among other gastric polyps ranges from 0.5 to 3.75%. In regions with a high prevalence of gastric adenocarcinoma, this figure is much higher and amounts to 9–20%. The male to female ratio is 2:1 [1].
Macroscopically, adenomatous polyps are characterized as single (in approximately 80% of cases) formations on a stalk or broad base with a velvety lobular surface. Their size is usually less than 2 cm. However, much larger adenomas also occur. The surface of the polyps may be ulcerated. Adenomas are most often localized in the antrum. Less often they are detected on the mucous membrane of the body of the stomach. In most cases, this type of polyps develops against the background of atrophic gastritis associated with Helicobacter pylori infection [1].
As a rule, adenomatous polyps do not have clinical symptoms. In some patients, ulceration of their surface causes gastric bleeding, which is rarely significant. In some cases, chronic hidden blood loss leads to the development of iron deficiency anemia. Large polyps can cause obstruction of the pyloric canal of the stomach.
The WHO classification identifies three main types of adenomas:
- papillary (villous);
- tubular;
- papillo-tubular.
Papillary adenoma consists of narrow or wide finger-like outgrowths of various shapes, the basis of which is the lamina propria of the mucous membrane. Tubular adenoma is formed by branched glands enclosed in the tissue of the lamina propria of the mucous membrane. Papillo-tubular adenoma is characterized by an intermediate morphological structure. The surface of adenomatous polyps is covered with epithelium, which always has signs of low or high degree dysplasia [5]. Based on morphological and histochemical characteristics, two main phenotypes of adenomatous polyps can be distinguished. The most common are intestinal-type adenomas, the epithelium of which is represented by enterocytes, goblet cells and Paneth cells. Gastric type adenomas are recorded much less frequently and may consist of superficial pitted epithelium of the body of the stomach (foveolar adenoma) or cells of the pyloric gastric glands (pyloric adenoma). Pyloric adenomas often develop in older women and are localized primarily in the body of the stomach. According to some data, in 26% of cases, the tissue of pyloric adenomas contains foci of adenocarcinoma [1, 6, 7].
Adenomatous polyps occur sporadically or may be associated with familial adenomatous polyposis. Papillary adenomas are usually sporadic, while tubular adenomas are more common in familial polyposis. Most sporadic adenomas are formed against the background of chronic atrophic Helicobacter gastritis with foci of intestinal metaplasia. The risk of developing them increases with age. These polyps are one of the stages of carcinogenesis leading to the development of stomach cancer. Formations larger than 2 cm in size are especially dangerous in terms of the high risk of neoplastic progression. Histological examination of the tissues of such adenomatous polyps reveals adenocarcinoma foci in 50% of cases [2, 4, 8].
In 30% of patients, at the time of detection of adenomatous polyps, histological signs of a malignant neoplasm are recorded in other areas of the mucous membrane. Thus, adenomatous polyps are a marker of a high risk of developing adenocarcinoma in the surrounding gastric mucosa [2, 4, 8]. All adenomatous polyps, regardless of size and histological structure, must be removed using endoscopic polypectomy or endoscopic resection of the mucous membrane. All patients after removal of adenomas require regular monitoring to identify new or previously undetected polyps and early gastric cancer. Recurrence of adenomas is observed in no more than 5% of cases. In 1.3% of patients with adenomatous polyps, gastric cancer is subsequently detected [9].
The first control endoscopic examination should be performed one year after polypectomy. If the results are normal, the examination interval can be increased to 3–5 years. If a histological examination of polyp tissue shows the presence of high-grade dysplasia, then observation should be individualized, and the first gastroscopy should be performed no later than 6 months [4, 9].
Fundic glandular polyps
Fundic glandular polyps are among the most common gastric polyps. The proportion of these formations among all gastric polyps, according to various sources, ranges from 13 to 77% [4]. Fundal glandular polyps are well-circumscribed, round, smooth, shiny, pale pink formations of small size (less than 1 cm) on a broad base. As a rule, they are multiple and localized on the mucous membrane of the fundus and upper body of the stomach. Cases of giant polyps reaching 3–8 cm have been described. The surrounding mucous membrane is usually unchanged [2, 10, 11].
Histologically, fundic glandular polyps are significantly expanded in the form of cysts of the main glands of the body of the stomach, the lumen of which is lined with flattened mukocytes, parietal and chief cells [1]. Fundic glandular polyps may occur sporadically or be associated with proton pump inhibitors (PPIs) and hereditary adenomatous polyposis.
The etiology and pathogenesis of fundic polyps have not been sufficiently studied. Previously it was believed that they belonged to hamartomas. However, reports of the occurrence of these formations during long-term PPI therapy led to the assumption that mechanisms associated with the suppression of hydrochloric acid secretion are involved in their pathogenesis [12]. Sporadic polyps are often solitary or few in number (10). They are detected predominantly in H. pylori-negative individuals without atrophic gastritis. According to some data, helicobacteriosis has a protective effect against the development of these polyps. Histological examination of the tissue of sporadic fundal polyps reveals areas of epithelial dysplasia in less than 1% of cases [1].
The association of fundic glandular polyps with PPI use remains a matter of debate. According to studies, this type of polyps occurred in 36% of patients who took antisecretory drugs for more than a year, and only in 12% of people who did not receive acid suppressive therapy (4-fold increase in risk). The average period of PPI use required for the formation of polyps is 32.5 months, and regression of these formations was noted 3 months after discontinuation of acid suppressive therapy.
In patients receiving PPIs, fundic glandular polyps tend to be more numerous compared with sporadic cases [13, 14]. The reasons for the formation of polyps while taking PPIs are not clear.
Most patients receiving antisecretory therapy never develop them. One theory suggests an important role for hypergastrinemia. Gastrin causes hyperplasia and protrusion of parietal cells into the lumen of the main glands, which in turn leads to their expansion and the formation of cysts. Subsequently, with an even greater increase in size of these cysts, fundic glandular polyps appear [15]. At the same time, the etiological relationship between PPIs and this type of polyps needs to be confirmed in prospective studies, because a number of studies did not reveal such an association [4].
Fundic glandular polyps are found in 53–88% of patients with familial adenomatous polyposis. In such a situation, polyps are always multiple and can cover the entire surface of the body of the stomach. Histological examination, in contrast to sporadic fundal polyps, reveals dysplasia in 25–41% of cases. In particular, in a study by LK Bianchi et al. Low-grade dysplasia was detected in 38% of patients, and high-grade dysplasia in 3% of examined patients [16, 17]. Sporadic fundic glandular polyps and fundic polyps associated with PPI use have a very low risk of malignancy. The possibility of spontaneous regression is shown. In the presence of a typical endoscopic picture and small size polyps (0.5 cm), the diagnosis is established based on histological examination of one biopsy. If polyps measuring 0.5–1.0 cm are detected, a biopsy should be taken from each such formation. Polyps larger than 1 cm should be removed. The issue of PPI withdrawal should also be decided individually. For small polyps, therapy can be continued, while for polyps larger than 1 cm, antisecretory drugs should be discontinued [2].
In patients with familial adenomatous polyposis, dysplasia of the epithelium of fundal polyps, according to some data, is detected in 40% of cases. The risk of its development increases with a large size of polyps (>1 cm), the presence of polyps in the duodenum and antral gastritis [2]. It is impossible to accurately differentiate sporadic fundic glandular polyps and fundic polyps in familial adenomatous polyposis during EGD. To identify colon polyposis, patients under 40 years of age with multiple fundal polyps are advised to undergo sigmoid or colonoscopy. A study of the colon also needs to be carried out if foci of dysplasia are detected in the biopsy samples of these polyps. In turn, the complex of dispensary measures for patients with adenomatous polyposis must necessarily include EGDS.
Hyperplastic polyps
In terms of frequency of occurrence, hyperplastic polyps are in second place after fundic glandular polyps. According to various sources, their share among all gastric polyps ranges from 18 to 70%. They can develop at any age, but are most often detected after 60–65 years. Hyperplastic polyps are localized mainly in the antrum and body of the stomach, but they can form on the mucous cardia and fundus. In most cases, these are single formations measuring 0.5–1.5 cm. Giant polyps up to 13 cm in size have been described. Small polyps have a smooth surface and a wide base. Larger formations have a stalk and a lobed, often eroded surface. In most cases, this type of polyps develops against the background of atrophic Helicobacter gastritis with foci of intestinal metaplasia. Multiple hyperplastic polyps may be detected in patients with Ménétrier's disease.
Hyperplastic polyps in most cases do not have any clinical symptoms.
In some patients, ulceration of their surface causes gastric bleeding and iron deficiency anemia. Cases of obstruction of the pyloric canal and ampulla of Vater's papilla with the development of secondary pancreatitis have been described [1, 2]. Histologically, hyperplastic polyps are characterized by significant elongation and branching of the foveal epithelium-lined gastric pits, which subsequently form cysts or corkscrew-like structures. Another characteristic feature is the infiltration of the lamina propria of the mucous membrane with plasma cells, lymphocytes, eosinophils, mast cells, macrophages and neutrophils. Between the pits there may be bundles of smooth muscle fibers.
In the epithelium of hyperplastic polyps, areas of dysplasia are quite rare (2–3%). Cases of detection of carcinoma foci have also been described. Neoplastic changes are difficult to differentiate from regenerative atypia of the epithelium and stroma, which often occurs in the tissue of these polyps [1]. The reasons for the development of hyperplastic polyps are currently unclear. As a rule, they are formed during excessive regeneration in response to damage to the mucous membrane and are often associated with chronic Helicobacter gastritis and pernicious anemia. These formations are often located next to erosions, ulcers and gastroenteroanastomoses [1].
Hyperplastic polyps can either increase in size and number or regress spontaneously or after eradication of H. pylori. Malignant transformation of the epithelium of polyps is rare (0.6–2.1%) and is more often observed when polyps are larger than 2 cm. At the same time, this type of polyp is associated with a high risk of developing neoplasia in the surrounding mucosa. Large hyperplastic polyps require complete removal followed by endoscopic observation after several months. The need for polypectomy for small lesions is not recognized by all authors. In this case, observational tactics with repeat endoscopy after a year may be justified. A thorough histological examination of the gastric mucosa for the presence of foci of dysplasia and adenocarcinoma is advisable. Biopsies are taken from five standard sites and any suspicious areas [4]. Eradication of H. pylori and elimination of chronic active inflammation of the mucous membrane, against which hyperplastic polyps develop, can contribute to the regression of these formations in 70% of cases [2, 18].
Hamartomas
Gastric hamartomas are rare clinical findings and are represented by juvenile polyps and polyps in Peutz-Jeghers and Cowden syndromes.
Juvenile polyp
Juvenile polyps are single (no more than two) hamartomas that can be detected in childhood. Unlike juvenile polyposis, sporadic juvenile polyps do not carry the risk of developing neoplasia [19].
Small juvenile polyps have a round shape and a wide base. Large polyps acquire a lobular structure and a stalk. They are localized mainly in the antrum of the stomach. During histological examination, juvenile polyps are difficult to differentiate from hyperplastic ones. They consist of cystically dilated glandular and foveal structures, lined with normal gastric epithelium and surrounded by edematous stroma with elements of inflammation. There are no smooth muscle fibers in the lamina propria of the mucous membrane, which distinguishes them from Peutz–Jeghers polyps [4].
Juvenile polyposis is a rare autosomal dominant disorder with variable penetrance. It is characterized by the presence of hamartomatous polyps of the gastrointestinal tract and an increased risk of developing gastric (15–20% lifetime) and colon cancer. It is detected in childhood or adolescence [20]. In juvenile polyposis, there are multiple round-shaped gastric polyps, often with an eroded surface. Clinical manifestations of this type of polyp may include pyloric obstruction, anemia and hypoproteinemia [1]. Morphological examination reveals tortuous, elongated and cystically dilated glands. Adjacent mucosa with signs of edema and inflammation [4]. The high risk of developing stomach and colon cancer dictates the need for endoscopy and colonoscopy at intervals of 1–3 years [2, 4].
Peutz–Jeghers syndrome
Peutz-Jeghers syndrome is a rare autosomal dominant inherited disease, which is characterized by the presence of hamartomatous polyps of the stomach, small and large intestine, pigment spots on the skin and mucous membranes, as well as an increased risk of developing malignant tumors of the gastrointestinal tract, breast, endometrium, ovaries and lungs . The lifetime risk of developing stomach cancer in Peutz-Jeghers syndrome is about 30%. However, cancer rarely develops in the tissues of hamartomatous polyps [21].
In patients with this hereditary disease, polyps are localized in the antrum and body of the stomach. They can be located on a wide base or have a leg; the surface of the polyps is velvety or papillary. The size of the formations usually does not exceed 1 cm. The surrounding mucous membrane is not changed. Histological examination reveals hyperplastic glands lined with foveal epithelium, separated by bundles of smooth muscle, the source of which is the muscular plate of the mucous membrane. The last morphological feature makes it possible to distinguish this type of polyps from polyps in juvenile polyposis.
Patients with Peutz-Jeghers syndrome and the presence of gastric polyps are advised to undergo EGD once every 3 years, which should be part of a comprehensive monitoring program. At the age of 50, this period should be reduced to 1–2 years. Polypectomy does not appear to reduce the risk of developing gastric cancer and therefore may be recommended to eliminate complications [21].
Cowden's syndrome
Cowden syndrome is a rare inherited autosomal dominant disease characterized by the presence of multiple hamartomas and neoplasias of the skin, gastrointestinal mucosa, thyroid and mammary glands, genitourinary system and brain. The disease is most often diagnosed in childhood or adolescence [22]. Hamartomatic polyps of the stomach or duodenum in Cowden syndrome are detected in 66–100% of patients. This type of polyp is usually benign in nature. Malignant transformation is rare. The prognosis is determined by the high risk of developing cancer of extragastric localization. Histological examination reveals elongated, cystically dilated glands with papillary folds, between which smooth muscle components are identified. Once the diagnosis has been clarified, further observation of gastric polyps in patients with Cowden syndrome is not required [19].
Polyposis syndrome
Familial adenomatous polyposis
Familial adenomatous polyposis is an autosomal dominant disease associated with a mutation in the APC gene and characterized by the presence of multiple colon polyps. Almost all patients with familial adenomatous polyposis develop colorectal cancer during their lifetime. In 15–20% of cases, the disease develops “de novo” with an uncomplicated hereditary history [23].
Polyps of the stomach and duodenum are registered in 30–100 and 50–90% of patients with familial andenomatous polyposis, respectively. In most cases, gastric polyps are represented by fundic glandular polyps, and only in 5% of cases are adenomas of the antrum detected. Differential diagnosis requires mandatory taking of tissue biopsies from at least five polyps. In the presence of adenomas and fundal polyps larger than 1 cm, polypectomy is indicated. Duodenal polyps have malignant potential and are the leading cause of mortality after prophylactic colectomy [4].
Endoscopic surveillance should be carried out once every 1–2 years. For patients with large multiple duodenal polyps containing foci of dysplasia, the possibility of surgical treatment should be considered [4, 24].
Cronkite–Canada syndrome
Cronkite–Canada syndrome is a very rare non-hereditary disease of unknown etiology that manifests itself at the age of 60–70 years.
About 400 patients with this pathology have been described in the world. This syndrome is characterized by the presence of gastrointestinal polyps and a dermatological triad consisting of onychodystophy, alopecia, and skin hyperpigmentation. Polyposis of the digestive system often causes diarrhea, malabsorption, hypoproteinemia and weight loss. The five-year survival rate is 55% [24].
Gastric polyps in Cronkite–Canada syndrome have a wide base and a reddish color. Histologically, pitted hyperplasia and cystically dilated glands are revealed, surrounded by inflamed and edematous stroma. Morphologically, these formations are indistinguishable from juvenile and hyperplastic polyps. Some differential features may include marked inflammation of the surrounding mucosa, which remains normal in other hamartomatous polyposis syndromes [1].
Nonepithelial intramural polyps
Inflammatory fibrous polyps
Inflammatory fibroid polyps are rare formations in the submucosal membrane and account for no more than 1–3% of all gastric polyps. This type of polyps can be detected in any part of the gastrointestinal tract, but 80% occur in the antrum of the stomach. The reasons for the development remain unknown. Most often detected in women aged 50–60 years. Inflammatory fibroid polyps are often associated with atrophic gastritis and hypochlorhydria, but do not have malignant potential [2, 4].
Endoscopic examination reveals single, well-defined formations measuring 1–5 cm, with a wide base or stalk. The mucous membrane covering the polyp is not changed, but large formations have a central depression or ulceration [4]. Histological examination reveals the presence of submucosal vessels surrounded by circularly located fibroblasts and an inflammatory infiltrate, in which eosinophils predominate. Morphology may give false negative results, because formations are located in the submucosal layer [4].
There are no clinical manifestations in most cases, but as with any large polyps, pyloric obstruction, bleeding and anemia may occur. For large polyps, polypectomy is recommended, but complete removal of the formation can be difficult due to its growth into the stomach wall [2].
Xanthoma/xanthelasma
Xanthoma of the gastric mucosa is a benign formation that has no independent clinical significance. The formation of xanthomas is not associated with hyperlipidemia and reflects the presence of reparative processes. These formations are detected during EGD in 1–7% of patients [2, 25].
Macroscopically, xanthomas are characterized as small (3–10 mm), nodules or plate-shaped flat formations of light yellow color with clear boundaries. They are localized mainly in the antrum along the lesser curvature, around the pylorus or near areas of mucosal regeneration (ulcers, erosions, anastomoses). Often associated with chronic Helicobacter gastritis and may be multiple. Xanthomas consist of clusters containing cholesterol and neutral fat macrophages localized in the lamina propria of the mucous membrane.
Xanthoma does not require endoscopic observation, but histological examination is required to confirm the diagnosis and exclude signet ring cell carcinoma of the stomach [2].
Heterotopia of the pancreas
Pancreatic heterotopia accounts for less than 1% of all gastric polyps. As a rule, it is a single round submucosal formation with a diameter of about 10 mm, localized in the prepyloric region. In the center there is often a depression marking the opening of the pancreatic duct. The surrounding mucous membrane is not changed. Histologically, normal pancreatic tissue with acinar cells and ducts is revealed. Due to the submucosal location of the heterotopia, adequate collection of biopsy material may be difficult. Endoscopic ultrasonography, which can be supplemented by fine needle aspiration, helps in diagnosis [2].
Heterotopia of the pancreas has a benign course and in most cases is not accompanied by clinical manifestations.
In extremely rare situations, pain in the upper abdomen, pyloric obstruction, pancreatitis and adenocarcinoma of heterotopic tissue are observed. In these cases, surgical or endoscopic resection may be required [2, 26]. In uncomplicated cases, treatment or endoscopic monitoring is not required.
Lipoma
Gastric lipoma is detected relatively rarely. It accounts for about 1–2% of all benign tumors of this organ. A typical location is the antrum. In most cases, the formation is located in the submucosal layer.
Endoscopically, a gastric lipoma looks like a smooth submucosal formation protruding into the lumen of the organ. The mucous membrane over the tumor is usually not changed, but ulceration and a depression in the center may be observed. Histological examination reveals accumulations of differentiated adipocytes under the mucous membrane without signs of atypia.
In most cases, lipoma has no clinical manifestations, but epigastric pain, dyspepsia, bleeding, anemia and pyloric obstruction may occur [27].
Algorithm for managing patients with gastric polyps
The general algorithm for managing patients with gastric polyps is presented in the figure. Most gastric polyps have no symptoms and are detected by chance during an endoscopy.
In such a situation, it is critical for the endoscopist to obtain the maximum amount of diagnostic information to determine further tactics. This will avoid repeated endoscopic examination, which is burdensome for the patient.
The EGDS protocol must describe in detail the location, number, shape, surface and size of polyps, as well as the condition of the surrounding gastric mucosa. If technically possible, endophotographs should be attached to the protocol. Data from a full endoscopic report often allow us to make an assumption about the type of polyps even before receiving the results of a morphological study.
If gastric polyps are detected, a biopsy is required, followed by histological examination of the samples. If hyperplastic or adenomatous polyps are suspected, it is necessary to obtain biopsy samples of the surrounding mucous membrane according to the standard scheme (2 biopsies from the antrum, 1 from the angle of the stomach, 2 from the body). If fundic glandular polyps are suspected, a biopsy of the unaffected mucosa is usually not required [2, 28].
Large polyps larger than 1 cm in size, polyps with foci of dysplasia, as well as polyps with clinical symptoms require polypectomy or endoscopic resection of the mucous membrane. These manipulations should be performed in well-equipped clinical centers, because requires medication and carries a significant risk of serious complications (bleeding and perforation). Before endoscopic treatment, it is necessary to assess the patient’s general condition, concomitant diseases and the risk of bleeding [2, 28].
After removal of an adenomatous or hyperplastic polyp, a repeat endoscopy should be performed a year later. The presence of high-grade dysplasia in the polyp tissue dictates the need for shorter intervals, which in the first 2–3 years can be 6 months. Patients with simple fundic glandular polyps and inflammatory fibroid polyps do not require further observation. In polyposis syndromes, monitoring should be carried out in accordance with existing clinical guidelines [2, 28].
Since adenomas and often other types of polyps are often associated with Helicobacter pylori infection (Helicobacter chronic atrophic gastritis), based on the recommendations of the Kyoto Protocol (2015), eradication therapy is recommended for such patients regardless of the surgical treatment option (preferably before surgical treatment) [29, 30]. As the most effective eradication options, taking into account the relevance of the growing resistance of Helicobacter pylori to antibiotic therapy, primarily to clarithromycin, we recommend the regimens proposed by the Alliance of Clinical Chemotherapists and Microbiologists, developed for outpatient practice based on the latest consensus on the problem of Helicobacter pylori infection (Kyoto Consensus, Maastricht-IV–V, V Moscow Agreement, etc.): PPI in double dose 2 times a day + bismuth salts1 0.12 g 4 times a day + metronidazole orally 0.5 g 3 times a day + tetracycline orally 0.5 g 4 times a day. As an option - PPI in a double dose2 2 times a day + clarithromycin orally 0.5 g 2 times a day or josamycin orally 1.0 g 2 times a day + amoxicillin orally 1.0 g 2 times a day + bismuth salts 0.12 g 4 times a day. As an alternative therapy: PPI in double dose 2 times a day + clarithromycin orally 0.5 g 2 times a day or josamycin orally 1.0 g 2 times a day + amoxicillin orally 1.0 g 2 times a day, this regimen can be used in conditions of low levels of population resistance to macrolides in H. pylori (≤15–20%). All schemes are assigned for a period of 1014 days. Taking into account the fact that the effectiveness of H. pylori eradication, in addition to the antibiotic resistance of the microorganism, depends on a number of genetic characteristics of the patient (polymorphisms of the CYP2C19, MDR1, IL-1β genes), it is advisable to carry out mandatory control of eradication no earlier than after 4 weeks [31, 32].
Who is at risk from polyps?
Polyps are most often found in middle-aged and elderly people. Their appearance is expected when infected with Helicobacter pylori, since this bacterium increases the risk of developing chronic gastritis, and gastritis is considered one of the causes of polyps.
Multiple polyps are five times more likely to cause malignancy than single polyps. There is a close relationship between the size of the polyp and the risk of malignant neoplasm - a formation with a diameter of 1.5 cm is repeated in malignant disease in 6.8% of cases, and with a diameter of 3 cm - in 73.7%. Polyps with flat stalks are three times more likely to become cancerous than polyps with long stalks.
Classification
For the convenience of clarifying the details of the diagnosis and systematizing knowledge about polyposis, it is classified taking into account several signs: clinical, radiological, pathological and etiological.
In this regard, we should talk about formations:
- Hyperplastic, adenomatous and inflammatory. The former are characterized by excessive growth of the gastric mucosa, which outwardly becomes similar to a pavement lined with cobblestones. The second is the result of the development of atypical cells. Still others are one of the variants of long-term inflammation, and, as a rule, arise in its place. Of all the species presented, they are the safest in terms of degeneration.
- Single or multiple. In the first case, their number should not exceed three units. In the second - no more than 15 pieces. If there are so many of them that it is not possible to count, then we are talking about a diffuse process, which is extremely dangerous with the possibility of degeneration into a cancerous tumor.
- Sessile or with a leg. Here we are talking about the method of attaching the polyp to the gastric mucosa. If in the second case it is clearly visible, then in the first, the base of the growth may be wide and short, or absent altogether.
- Villous, tubular or mixed. This classification is based on the results of a microscopic examination of the tissues of the formation. The villi are located on its surface, and the tubes are an association that arose when studying the cells of the growth.
How do polyps appear in the stomach?
Stomach polyps are most often asymptomatic, although they may cause abdominal pain or indigestion. As the polyp grows, ulcers may appear on the surface of the stomach with pain, nausea, bloody stools or vomiting, and signs of anemia.
Pain due to polyps in the stomach
In rare cases, the polyp affects the gap between the stomach and the small intestine or the lumen of the duodenum, which leads to severe pain and obstruction.
The diagnosis of gastric polyp is made by endoscopic examination. During the examination, a biopsy may be done - a sample of polyp tissue is taken for morphological examination.
Clinical manifestations of duodenal polyps
Polyps of the duodenum are most often asymptomatic, due to their small size and slow growth. With large polyps, the functions of neighboring organs may be disrupted and clinical manifestations may appear. If the number and size of polyps increases, their size reduces the lumen of the intestine. The outlet section (pylorus) of the stomach may be infringed. Clinical manifestations may occur that are observed in various diseases of the digestive tract. The severity of the manifestation of a polyp of the duodenum with an increase in its size is the appearance of complications - bleeding and the development of a decrease in hemoglobin and the development of iron deficiency anemia.
Clinical manifestations:
- pain in the epigastric region or throughout the abdomen after eating,
- belching, nausea, sometimes vomiting blood,
- heartburn,
- constipation, diarrhea,
- weakness, dizziness (if anemia).
Polyps are characterized by pain, obstruction, and bleeding.
Treatment of polyps in the stomach
As mentioned, treatment for polyps depends on their type, size and number.
Small polyps that are not adenomas do not always require treatment, although neither one nor the other should be allowed - observation is necessary. So you will need to periodically undergo endoscopic examination by a gastroenterologist. If the polyp begins to grow or is detected clinically, it must be removed.
Antibacterial therapy is necessary for chronic gastritis and Helicobacter pylori infection. This will prevent the formation of polyps and may even cause hyperplastic polyps to disappear.
Antibacterial therapy for chronic gastritis
Large polyps and adenomas must be removed surgically. Polypectomy in this case is also performed during endoscopy. The introduction of this technique has greatly simplified the treatment of gastric polyps.
Disease prevention
The risk of new polyps will help reduce:
- Timely treatment of gastritis, gastric ulcer. Regular examination by a gastroenterologist for existing chronic diseases of the gastrointestinal tract;
- Elimination of Helicobacter pylori infection: treatment must be under the supervision of a specialist;
- Revision of drug prescriptions in favor of forms of drugs that are safer for the stomach;
- Refusal of self-medication, uncontrolled taking of pills;
- Reducing the amount of alcohol consumed, giving up strong alcoholic drinks, smoking;
- A balanced diet with a predominance of boiled and steamed food, fresh vegetables and fruits. Reducing the amount of hot spices and too hot food.
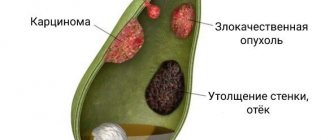
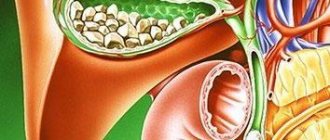
Polyps in the gallbladder
If you have an abdominal ultrasound report with suspected gallstone disease, and in the doctor’s report you read “polyps” instead of “stones,” do not be surprised - polyps in the gallbladder are not uncommon. According to statistics, gallbladder polyps occur in 0.3-9.5% of the population, although this condition is rarely detected clinically. Potentially dangerous formations are even rarer - 95% of gallbladder polyps are benign.
Polyps in the gallbladder
The cause of polyps in the gallbladder is unknown. We also don't know anything about risk factors. Scientists have not yet established a link between gallbladder polyps and age, gender, genetics or other factors that contribute to the development of gallbladder disease in general: obesity, diabetes, high cholesterol.
Clinical signs are thought to be rare. It also happens that the patient complains of nausea or vomiting, pain in the right navel, although polyps are most often detected by ultrasound for other reasons.
The size of the neoplasm indicates the ability of the polyp to assimilate. Tumors smaller than 1.3 cm in diameter are usually harmless and do not require treatment. However, this does not mean that we have the right to relax - the size of the polyp must be constantly monitored. Control is carried out by standard or endoscopic ultrasound examination.
If the growth of the polyp in the gallbladder exceeds 1.3 cm, we may already be dealing with a malignant tumor or the polyp may soon develop into such a disease. Polyps with a diameter of more than 1.9 cm are most likely already malignant.
Diagnostic methods
- Fibrogastroduodenoscopy
(FGDS) is the main method for diagnosing the disease; it allows not only to detect neoplasms, determine their shape and quantity, but also to perform a tissue biopsy for subsequent research. During the procedure, the doctor examines not only the stomach cavity, but also the esophagus and upper intestine. Additionally, FGDS allows you to take material to detect Helicobacter pylori infection in order to determine the degree of infection of the mucous bacterium H. pylori. - Histological examination
allows us to determine the type of polyp and its tendency to degenerate into a malignant tumor. It is carried out both after a preliminary biopsy and to study an already removed tumor. - Radiography with a contrast agent
(barium sulfate) is an important method in determining the shape and exact size of polyps, the degree of lumen overlap in various parts of the stomach. - Ultrasound examination of the abdominal organs is an auxiliary method that allows you to determine the size of polyps and identify concomitant pathologies. At the Yauza Clinical Hospital, endoscopic ultrasound is performed using a Hitachi Preirus device, which significantly increases the accuracy of diagnosis.
- Fecal occult blood analysis
makes it possible to detect even slight bleeding from damaged tissues.
At the Yauza Clinical Hospital, the ERK-17000 video processor is used to diagnose polyps of the stomach and intestines, which significantly improves the quality and accuracy of the study.
Tactics for treating polyps in the gallbladder
The treatment tactics for the gallbladder are also determined by the size of the polyp. Monitoring is sufficient for small polyps; treatment of large polyps involves a simple operation - laparoscopic cholecystectomy, excision of the gallbladder. The operation is performed under general anesthesia, the risk of complications is minimal, and the result is very good.
Laparoscopic cholecystectomy
Resection of the gallbladder is recommended even when the polyp is accompanied by cholelithiasis. In this case, the size of the polyp is no longer given special attention.
Endoscopic removal of polyps
Recently, more gentle methods for removing gastric polyps have become increasingly used. In the initial stage of development, endoscopic intervention can be successfully managed. With gastroscopy, both single and multiple polyps can be removed.
Through the channel of the gastroscope, a special loop is inserted into the stomach cavity, which is used to compress the leg of the polyp at the very base, then diathermocoagulation (cauterization with electric current) is carried out and the polyp dies and is removed. The surgical procedure is called electroexcision.
After the intervention, patients undergo a control gastroscopy 3 months after the operation. If necessary, additional electrocoagulation is prescribed - cauterization of residual tissue. Complete healing occurs after 2 months. For patients who have undergone electroexcision and electrocoagulation, regular examinations are recommended to detect possible re-formation of the polyp.
Nutrition rules after surgery
Refusal to eat is necessary only on the first day after the intervention. Then the patient should adhere to a certain diet; the diet should not include foods that cause chemical, thermal and mechanical irritation. This will allow the digestive system to work faster.
Products that can be included in the diet after gastric surgery:
- Soups should be prepared either without meat at all or in a weak meat broth.
- Boiled porridges, for which cereals are suitable: buckwheat, rice, oatmeal.
- Among flour products, sugar-free crackers, unsweetened biscuits, and dried bread are allowed.
- Sometimes you can include pasta and dietary ham from rabbit or poultry in your diet.
- Eggs are boiled or cooked like an omelet, but steamed.
- You can eat non-acidic cottage cheese, sour cream (low-fat), yoghurt and kefir.
- As for meat products, you can include chicken breast, veal, rabbit and turkey in your diet.
- Bananas and apples are allowed; zucchini, beets (once a week), carrots, pumpkin and cauliflower inflorescences are also not prohibited.
During the recovery period, it is prohibited to include in the diet:
- Of the cereals, only semolina is subject to restrictions.
- As for eggs, they should not be fried.
- Meat dishes in the diet should include lamb, pork and beef, as well as fatty poultry (duck and goose).
- As for flour products, you should avoid any fresh baked goods, cakes and pastries, baked goods and fried pies.
- Some vegetables and fruits are also prohibited for consumption. Among them are peas and white cabbage, radishes and radishes. You should not eat figs, plums and grapes.
- First courses should not be spicy or salty; therefore, you should not consume kharcho soup, solyanka and other soups made from fatty meat.
- Margarine cannot be added to dishes, and butter is limited. As for milk, you can drink it, if there is no individual intolerance, the fat content of the product should not be high.
- All sausages, canned food and marinades are prohibited. You should lighten your diet as much as possible by eliminating hot sauces and any food additives.
If an endoscopic procedure was performed, then, as a rule, rehabilitation does not take much time. Complete restoration of the organ mucosa will occur after a period of 10 to 40 days.