Composition and release form
The drug is available in the form of tablets for oral administration. They are oval in shape, convex on both sides and have a soluble yellow-brown shell. The inside structure of the tablet is rough, the color ranges from white to yellow. The medicine is packaged in contour cells of 14 pieces and a cardboard box. One package contains 1-4 cells and includes instructions for use.
The active compound of the drug is pantoprazole sodium in an amount of 20 mg. Auxiliary components: becomes calcium, mannitol, sorbitol, anhydrous sodium carbonate solution.
pharmachologic effect
Nolpaza is a proton pump inhibitor and has a pronounced hypoacid effect, namely, it suppresses the secretion of hydrochloric acid in the stomach.
After use, the drug is quickly absorbed and distributed throughout the body through the bloodstream. The bioavailability of the drug is high; it does not depend in any way on the time of food intake. The maximum concentration of the drug is achieved 2 hours after oral administration.
The breakdown of medicinal components is observed in the liver. The half-life of the drug is 1 hour.
Correction of the therapeutic dose is necessary for persons with severe liver pathologies.
Indications for Nolpaza
A hypoacid agent is prescribed for the treatment of the following conditions:
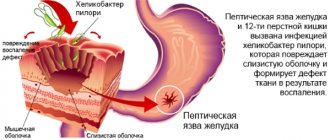
- Infection of the digestive organs with Helicobacter pylori.
- Open form of ulcerative process in the cavity of the stomach and duodenum.
- Uncomplicated gastroesophageal pathology.
- Therapy and prevention of reflex esophagitis of any severity.
In addition, the use of Nolpaza is recommended for persons undergoing long-term treatment with anti-inflammatory drugs to prevent the development of ulcers.
Instructions for use NOLPAZA®
It is necessary to regularly monitor the activity of liver enzymes throughout the entire period of use of pantoprazole (especially during long-term therapy). If the activity of liver enzymes increases, treatment with pantoprazole should be discontinued.
The use of Nolpaza® in a dose of 20 mg as a prophylactic agent for the treatment of erosive and ulcerative lesions of the stomach and duodenum caused by NSAID therapy should be limited only to the group of patients continuing NSAID therapy and at risk of developing gastrointestinal complications. This risk is assessed by the presence of individual risk factors, for example, advanced age (over 65 years), gastric or duodenal ulcers and a history of gastrointestinal bleeding.
When conducting combination therapy for Helicobacter pylori eradication, it is necessary to observe and take into account the characteristics of the relevant drugs. In the presence of alarming symptoms (eg, significant unintentional weight loss, recurrent vomiting, dysphagia, hematemesis, anemia or melena) or in the presence or suspicion of a gastric ulcer, it is necessary to exclude the presence of a malignant neoplasm, because The therapy carried out, masking the symptoms, can delay the correct diagnosis. Re-evaluation should be carried out if symptoms persist despite adequate therapy with pantoprazole.
In patients with Zollinger-Ellison syndrome and other pathological conditions associated with increased gastric secretion that require long-term treatment, pantoprazole may reduce the absorption of vitamin B12 cyanocobalamin against the background of suppressed hydrochloric acid secretion. This should be taken into account in patients with reduced body weight or the presence of risk factors for reduced absorption of vitamin B12 during long-term therapy or in the presence of clinical symptoms.
Long-term therapy, especially lasting more than 1 year, requires regular monitoring of the patient.
When using pantoprazole, like other proton pump inhibitors, it is possible to increase the number of bacteria normally present in the upper gastrointestinal tract. Treatment with Nolpaza® may lead to a slight increase in the risk of developing bacterial gastrointestinal infections caused by bacteria such as Salmonella spp. and Campylobacter spp.
Severe hypomagnesemia has been observed in patients taking proton pump inhibitors, incl. pantoprazole, more than 3 months, and in most cases - within a year. Severe manifestations of hypomagnesemia may include fatigue, tetany, delirium, seizures, dizziness, and ventricular arrhythmia. In most patients, their condition improved after magnesium replacement therapy or cessation of treatment with proton pump inhibitors. If long-term therapy with proton pump inhibitors is planned, as well as during the simultaneous use of digoxin or drugs that cause hypomagnesemia (for example, diuretics), magnesium levels should be monitored before starting therapy and periodically during treatment.
Proton pump inhibitors, when used long-term (more than 1 year) and when used in high doses, may slightly increase the risk of fractures of the femur, wrist and spine, especially in elderly patients or patients with established risk factors. Research suggests that proton pump inhibitors may increase the overall risk of fractures by 10-40%. However, this increase may be due to other risk factors. Patients at risk of developing osteoporosis need to get adequate amounts of vitamin D and calcium; Such patients should be treated in accordance with current clinical guidelines.
Nolpaza® contains sorbitol, so the drug is not recommended for use in patients with rare hereditary conditions associated with fructose intolerance.
Use in pediatrics
Nolpaza® is not recommended for use in children under 12 years of age
due to limited safety data.
Impact on the ability to drive vehicles and machinery
When using the drug, adverse reactions such as dizziness and visual disturbances may occur. In such cases, the patient should not drive vehicles or potentially dangerous machinery.
Contraindications
Absolute contraindications to the use of the drug are:
- Individual intolerance to the main and auxiliary components.
- Dyspepsia of a non-secondary nature.
- The presence of serious diseases of the liver and excretory system.
With extreme caution, a hypoacid drug is prescribed to patients at risk for the development of gastrointestinal bleeding and ulcers. Regular monitoring of patients with uncomplicated liver pathologies and vitamin B12 deficiency is also necessary.
Nolpaza®
Liver dysfunction In patients with severe liver dysfunction, liver enzyme activity should be regularly monitored during treatment with pantoprazole, especially with long-term use. If the activity of liver enzymes increases, treatment should be stopped (see section “Method of administration and dosage”).
Combination therapy When combining therapy, you should follow the instructions for use of the appropriate medications.
Malignant neoplasms of the stomach Before starting treatment with Nolpaza®, the possibility of a malignant neoplasm should be excluded, since the drug can mask symptoms and delay the correct diagnosis. If there are risk factors (unintentional weight loss, anemia, gastrointestinal bleeding (stool discolored black - melena), swallowing disorder, persistent vomiting or vomiting with blood), as well as if a gastric ulcer is suspected or confirmed, a malignant neoplasm should be excluded. If symptoms persist despite appropriate treatment, further evaluation should be considered.
Concomitant use with HIV protease inhibitors Pantoprazole is not recommended for use with HIV protease inhibitors, the absorption of which depends on the pH of the stomach (for example, atazanavir), due to a significant decrease in their bioavailability (see section “Interaction with other drugs”).
Effect on vitamin B12 absorption In patients with Zollinger-Ellison syndrome and other pathological hypersecretory conditions who require long-term treatment, pantoprazole, like other drugs that block gastric acid secretion, may reduce the absorption of vitamin B12 (cyanocobalamin) due to hypo- and achlorhydria . This should be taken into account when treating patients with vitamin B12 deficiency in the body, or during long-term treatment of patients with risk factors for developing vitamin B12 deficiency, as well as in the presence of corresponding clinical symptoms.
Long-term therapy Long-term therapy, especially those lasting more than a year, requires regular monitoring of patients.
Gastrointestinal infections caused by bacteria When taking drugs that reduce the acidity of gastric juice, the risk of gastrointestinal infections caused by bacteria of the genus Salmonella spp., Campylobacter spp. slightly increases. or C. difficile.
Hypomagnesemia There have been reports of severe hypomagnesemia in patients receiving PPIs for at least 3 months, and in most cases for a year. Serious manifestations of hypomagnesemia, such as fatigue, tetany, delirium, convulsions, dizziness and ventricular arrhythmia, may occur, but hypomagnesemia may develop unnoticed and not be recognized in a timely manner. In most patients with hypomagnesemia, it decreases after magnesium replacement therapy and PPI discontinuation. In patients planning long-term treatment or in patients receiving PPIs concomitantly with digoxin or with other drugs that can cause hypomagnesemia (for example, diuretics), serum magnesium levels should be determined before starting PPI treatment and periodically during treatment.
Bone fractures PPIs, especially when used in high doses and over a long period of time (> 1 year), may slightly increase the risk of fractures of the femur, wrist and spine, mainly in patients who are elderly or have other recognized risk factors. Observational studies indicate that PPIs may increase the overall risk of fracture by 10% to 40%. Some of these fractures may be due to other risk factors. Patients at risk of developing osteoporosis should receive treatment in accordance with current clinical guidelines and adequate amounts of vitamin D and calcium.
Subacute cutaneous lupus erythematosus When treated with PPIs, the development of PCLE is very rarely observed. If skin lesions occur, especially in sun-exposed areas, or in the presence of concomitant arthralgia, the patient should seek immediate medical attention and a healthcare professional should evaluate the need to discontinue treatment with Nolpaza. The occurrence of PCLE after previous PPI treatment may increase the risk of developing PCLE when treated with other PPIs.
Impact on the results of laboratory tests When conducting laboratory tests, it is necessary to take into account that increased levels of CgA in the blood serum can distort the results of diagnostic tests to identify neuroendocrine tumors. In this regard, the use of the drug Nolpaza should be discontinued at least 5 days before testing the CgA content (see section “Pharmacological properties. Pharmacodynamics”). If CgA and gastrin levels have not returned to normal values after the first determination, the test should be repeated 14 days after stopping the PPI.
Special information on excipients
Sorbitol 1 tablet of Nolpaza® 20 mg contains 18 mg of sorbitol.
1 tablet of Nolpaza® 40 mg contains 36 mg of sorbitol.
Patients with rare hereditary fructose intolerance should not take this drug. It is necessary to take into account the additive effect of concomitantly used drugs containing sorbitol (or fructose) and dietary intake of sorbitol (or fructose).
The sorbitol content of oral medicinal products may affect the bioavailability of other oral medicinal products taken at the same time.
Sodium 1 film-coated tablet, 20 mg and 40 mg of Nolpaza® contains less than mmol of sodium (23 mg), i.e., practically no sodium.
Side effects
In most cases, taking the medicine does not cause negative reactions. But sometimes the following side effects are possible:
- Gastrointestinal tract dysfunction - nausea followed by vomiting, flatulence, epigastric discomfort, diarrhea or constipation, changes in the level of liver enzymes. Symptoms of jaundice are rarely observed.
- Dizziness, decreased visual acuity, headache and unstable psycho-emotional state.
- Tissue swelling, nephritis may develop.
- Allergic reaction - accompanied by itching, skin rashes, hives, photophobia. Angioedema rarely occurs.
- In some cases, muscle pain and high fever are noted.
Instructions for use of Nolpaza
The proton pump inhibitor should be taken orally with plenty of water. It is recommended not to chew the tablets, but simply swallow them. Taking the medicine does not depend on food, but it is better to drink it before meals in the morning.
The therapeutic dose and duration of therapy depends on the diagnosis and condition of the patient. Taking these criteria into account, the drug is prescribed according to different regimens:
- Gastroesophageal disease - it is recommended to use 1 tablet per day. Duration of treatment is at least 2 weeks and maximum a month. In some cases, taking into account clinical data, the doctor may prescribe the medicine for 2 months.
- Reflux esophagitis in a state of exacerbation - you should drink 20 mg for therapy and prevention.
- Infestation with Hilicobacter pylori - prescribe 2 tablets twice a day - morning and evening, in combination with antimicrobial agents.
- High secretion of hydrochloric acid - 40 mg per day is indicated, the maximum permissible therapeutic dosage is 80 mg.
Patients with liver pathologies are prescribed the drug in a dose of no more than 20 mg.
Nolpaza
Nolpaza ®
(lat.
Nolpaza ®
) is an antiulcer drug that reduces gastric acidity, a high-quality Slovenian generic of pantoprazole, a proton pump inhibitor.
Composition and release form of Nolpaza
Active substance of Nolpaza: pantoprazole (lat. pantoprazole
). Nolpaza is available in the form of enteric-coated tablets in two versions containing pantoprazole sodium sesquihydrate equivalent to 20 and 40 mg of pantoprazole.
Indications for use of Nolpaza
- heartburn
- belching sour
- pain when swallowing
- gastroesophageal reflux disease
- reflux esophagitis
- erosive and ulcerative lesions of the stomach and duodenum associated with the use of non-steroidal anti-inflammatory drugs
- treatment and prevention of gastric and duodenal ulcers
- eradication of Helicobacter pylori
(only as part of complex therapy, monotherapy with nolpase is ineffective) - Zollinger-Ellison syndrome
- other diseases and conditions associated with high stomach acidity.
Method of use of Nolpaza and dosage
The Nolpaza tablet is swallowed whole, without chewing and breaking, with a small amount of liquid before meals. When taken once a day, Nolpaza is taken before breakfast. When taken twice - before breakfast and before dinner.
- To relieve the symptoms of gastroesophageal reflux disease
(GERD): heartburn, acid regurgitation, pain when swallowing and in the treatment of GERD, including erosive and ulcerative reflux esophagitis daily dose: 1 tablet of Nolpaza 20 mg for mild GERD or 1-2 tablets of Nolpaza for 40 mg - for moderate to severe cases. Relief of symptoms usually occurs within 2–4 weeks. The duration of treatment with Nolpaza is from 4 to 8 weeks. For preventive purposes, during long-term maintenance therapy, take one Nolpaza 20 mg tablet per day. This dose, if necessary, can be increased to 40 or 80 mg. If symptoms of GERD appear, Nolpaza can be taken “on demand”. - When treating erosive and ulcerative lesions of the stomach and duodenum resulting from taking non-steroidal anti-inflammatory drugs
(NSAIDs), take 1-2 tablets of nolpaza 40 mg per day. The duration of treatment with Nolpaza is from 4 to 8 weeks. To prevent erosive lesions during long-term use of NSAIDs - 1 tablet of Nolpaza 20 mg per day. - For the treatment and prevention of stomach and duodenal ulcers,
take 1–2 Nolpaza 40 mg tablets per day. For exacerbation of duodenal ulcers, the duration of therapy is 2 weeks, for gastric ulcers - 4–8 weeks. The treatment period may be extended. - When eradicating
Helicobacter pylori, Nolpaza is taken 2 times a day, one 40 mg tablet, always in combination with antibiotics. The duration of eradication is from 7 to 14 days. - Treatment of Zollinger-Ellison syndrome
and other pathologies associated with increased secretion of hydrochloric acid in the stomach begins with taking a Nolpaza 40 mg tablet in the morning and a second 40 mg tablet in the evening. In the future, the daily dose of nolpaza can be adjusted depending on the level of gastric secretion. It is possible to temporarily increase the daily dose to four Nolpaza 40 mg tablets. The duration of therapy is determined by the doctor. - In case of severe liver diseases,
no more than one Nolpaza 40 mg tablet is allowed per day. Monitoring the activity of liver enzymes is desirable, especially with long-term treatment with Nolpaza. If the activity of liver enzymes increases, Nolpaza should be discontinued. - older
patients and with
kidney disease,
no more than one Nolpaza 40 mg tablet per day is allowed. - In older
Helicobacter pylori
eradication therapy should not exceed 7 days.
Materials for healthcare professionals regarding the use of Nolpaza
Articles and manual for doctors
- Diagnosis and treatment of gastroesophageal reflux disease // Russian Gastroenterological Association. A manual for doctors. - Moscow. — 2010
- Gubergrits N.B. And heartburn again... // Health of Ukraine. 2012.
- Paliy I.G., Ksenchin O.A. Clinical substantiation of the feasibility of an individual approach to the selection of pharmacological agents in patients with gastrointestinal pathology // Journal. Medicine. 2015. No. 1(151). pp. 58–66.
- Lapina T.L., Tertychny A.S., Nasretdinova E.R. and others. Results of long-term observation of patients with chronic gastritis after eradication of H. Pylori infection // RZHGGK. – 2015. – No. 4. pp. 101-108.
Nolpaza (pantoprazole) is the drug of choice if the patient must take a proton pump inhibitor and other medications at the same time.
59.3% of elderly patients with GERD have arterial hypertension, and 41.1% have coronary heart disease. Thus, a significant number of elderly patients with GERD require simultaneous use of several medications. If it is necessary to use a PPI to treat GERD in such a patient, then, taking into account the drug interaction profile, preference should be given to pantoprazole, for example, nolpase (Bordin D.S.). On the website GastroScan.ru in the “Literature” section there is a subsection “Pantoprazole”, containing articles for healthcare professionals regarding the treatment of the gastrointestinal tract with drugs containing the active ingredient pantoprazole, including Nolpaza.
Video
| Ilchishina T.A. GERD in everyday practice: patient misconceptions and doctor errors | |
| Maev I.V. Chest pain as a manifestation of GERD |
On the website GastroScan.ru in the “Video” section there is a subsection “Reports and Lectures”, containing video recordings of reports, lectures, webinars in various areas of gastroenterology for healthcare professionals.
For a detailed description of the pharmacological properties of Nolpaza, see the article “pantoprazole”.
We also recommend that you read the sections of the article “Proton pump inhibitors”
: “
Comparison of proton pump inhibitors
”,
“Selection of proton pump inhibitors”, “Mechanism of action of a proton pump inhibitor”
and others.
Other medicines containing the active ingredient pantoprazole
Registered in Russia:
Zipantol, Controloc, Crosacid, Sanpraz, Pantaz, Pantoprazole Canon, Pantoprazole sodium sesquihydrate, Panum, Peptazol, Pigenum-sanovel, Puloref, Ulthera.
Present on the pharmaceutical markets of countries - former republics of the USSR and not registered in Russia:
Zogast (BILIM Ilac San. ve Tic., AS, Turkey), Zolopent (Kusum Pharm LLC, Ukraine), Panotsid (Flamingo Pharmaceutical, India), Pantasan (Sun Pharmaceutical Industries Ltd, India), Pantap (Nobel Almaty Pharmaceutical JSC Factory, Kazakhstan), Proxium (JSC Lubnipharm, Ukraine), Protonex (Abdi Ibrachim, Turkey), Ulthera (Emcure Pharmaceuticals Ltd., India).
Branded pantoprazole approved in the USA (and not registered in Russia): Protonix (Pfizer Inc, USA).
general information
According to the pharmacological index, Nolpaza belongs to the group “Proton pump inhibitors”.
According to ATC, it belongs to the group “Proton pump inhibitors” and has the code A02BC02. Nolpaza is a prescription medicine
.
There are contraindications, side effects and application features; consultation with a specialist is necessary.
Instructions for medical use of the drug Nolpaza (enteric-coated tablets, 20 and 40 mg), (pdf, ).
The manufacturer of Nolpaza is KRKA (KRKA, dd), Slovenia.
Nolpaza has contraindications, side effects and application features; consultation with a specialist is necessary.
Back to section