Quite often, spicy and fatty foods, poor nutrition and an unhealthy lifestyle lead to dyspepsia, the symptoms of which are heartburn, unpleasant heaviness in the stomach, sour belching and bloating. Especially often, such symptoms can appear during pregnancy, when the constantly growing belly of the expectant mother contributes to the release of gastric juice directly into the esophagus. To get rid of these symptoms, you need a drug that is completely safe for the fetus, and one of these drugs is Gaviscon, the instructions for use of which should always be in a visible place.
Composition and release form
The medicine Gaviscon has 2 release forms: tablets and suspension for oral use.
Tablets with a chewable base, slightly flat with a round shape and a light shade. They have minor inclusions over the entire surface, with a pleasant smell of mint or lemon. Packaged in cardboard packaging with 2-4 blisters, each containing 8 pieces. Or in a polypropylene container of 16 tablets with attached instructions for use.
Gaviscon suspension for oral administration has a uniform consistency, light color and menthol flavor. Sold in a dark glass bottle with a volume of 100, 150 or 300 ml.
The active components of the drug are sodium bicarbonate and alginate, calcium carbonate. Their concentration varies depending on the dosage form. Excipients: mint flavor, magnesium stearate, macrogol, aspartame, mannitol, acesulfame potassium.
pharmachologic effect
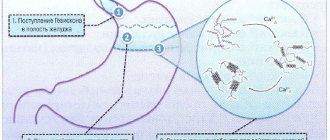
Gaviscon is a drug for symptomatic treatment. Thanks to the antacid group, it has the property of neutralizing hydrochloric acid in the stomach cavity.
After oral administration, the active ingredients immediately react with the acidic environment. As a result, a gel base with a neutral pH is formed. Due to it, a protective film is formed on the mucous surface of the stomach, preventing gastroesophageal reflux from developing within 4 hours.
In case of severe pathological process, the gel penetrates into the digestive tract, ahead of the entry of gastric contents, where it helps to reduce the irritant factor on the esophageal mucosa.
The therapeutic effect of the drug occurs 3-4 minutes after administration.
The drug does not have general bioavailability. Its pharmacological activity does not extend beyond the gastrointestinal tract. Therefore, the active ingredients rarely penetrate into the bloodstream. This occurs when the therapeutic dose of the drug is increased. Then it is excreted from the body through the kidneys with urine unchanged.
Gaviscon Double action mint suspension, 300 ml
Manufacturer
Reckitt Benckiser, UK
Briefly about the product
Compound
10 ml of suspension contains: active ingredients: sodium alginate 500 mg, sodium bicarbonate 213 mg, calcium carbonate 325 mg; excipients: carbomer (974P) 65 mg, methyl parahydroxybenzoate 40 mg, propyl parahydroxybenzoate 6 mg, sodium hydroxide 26.67 mg, sodium saccharinate 10 mg, mint flavor 6 mg, purified water up to 10 ml.
pharmachologic effect
This drug is a combination of alginate and antacids (calcium carbonate and sodium bicarbonate). Pharmacodynamics
When taken orally, the active ingredients of Gaviscon® Double Action quickly react with the acidic contents of the stomach.
In this case, an alginate gel is formed with a pH value close to neutral. Within three minutes, the gel forms a protective barrier on the surface of the stomach contents, preventing the occurrence of gastroesophageal reflux (return of stomach contents into the esophagus) for a period of up to four hours. In severe cases of reflux (regurgitation), the gel enters the esophagus, ahead of the rest of the gastric contents, where it reduces irritation of the esophageal mucosa. Calcium carbonate quickly neutralizes the hydrochloric acid of gastric juice, relieving the feeling of heartburn. This effect is enhanced by the presence of sodium bicarbonate in the drug, which also has a neutralizing effect. The total acid neutralizing activity of the drug in a minimum dose of 10 ml is approximately 10 mEq. Pharmacokinetics
The mechanism of action of the drug Gaviscon® Double Action is physical and does not depend on absorption into the systemic circulation.
Indications
Symptomatic treatment of diseases associated with digestive disorders, increased acidity of gastric juice and gastroesophageal reflux (heartburn, sour belching), a feeling of heaviness in the stomach after eating, including during pregnancy.
Use during pregnancy and breastfeeding
Pregnancy Clinical studies involving more than 500 pregnant women and the volume of data obtained during the post-registration period did not show congenital, feto- and neonatal toxicity of the active substances. Gaviscon® Double Action can be used during pregnancy if clinically necessary. Taking into account the presence of calcium carbonate in the composition, it is recommended to reduce the duration of use of the drug. Breastfeeding period The effect of the active ingredients of the drug on newborns, infants, and lactating women has not been demonstrated. Gaviscon® Double Action can be used during breastfeeding.
Contraindications
- Hypersensitivity to any of the components of the drug;
- moderate to severe renal failure;
- children's age up to 12 years.
With caution:
If you have the following diseases or conditions, you should consult your doctor before using the drug: hypercalcemia, nephrocalcinosis, urolithiasis with the formation of calcium oxalate stones, congestive heart failure, hypophosphatemia, mild renal failure).
Side effects
The incidence of adverse reactions was assessed based on the following criteria: very often (≥ 1/10), often (≥ 1/100, < 1/10), infrequently (≥ 1/1000, < 1/100), rarely (≥ 1 /10000, <1/1000), very rare (<1/10000) and unknown frequency (frequency cannot be calculated from available data).
Immune system disorders: unknown frequency - anaphylactic and anaphylactoid reactions, hypersensitivity reactions (urticaria).
Disorders of the respiratory system, chest and mediastinal organs: unknown frequency - respiratory effects (bronchospasm).
Ingestion of large amounts of calcium carbonate can cause alkalosis, hypercalcemia, acid rebound, milk-base syndrome, and constipation. These conditions usually occur with an overdose.
If any of the side effects indicated in the instructions get worse, or you notice other side effects not listed in the instructions, tell your doctor.
Interaction
Since calcium carbonate, which is part of the drug, exhibits antacid activity, at least 2 hours should pass between taking Gaviscon® Double Action and other drugs, especially when taken simultaneously with H2-histamine receptor blockers, antibiotics from the tetracycline group, digoxin, fluoroquinolone, iron salts, ketoconazole, antipsychotics, thyroid hormones, levothyroxine sodium, penicillamine, beta-blockers (atenolol, metoprolol, propranolol), glucocorticosteroids, chloroquine, bisphosphonates and estramustine.
How to take, course of administration and dosage
Inside.
Adults and children over 12 years of age: 10–20 ml after meals and before bed (up to 4 times a day).
The maximum daily dose is 80 ml.
For elderly patients, no dose change is required.
The drug should not be used for a long time; if symptoms persist after 7 days of taking the drug, you should consult a doctor to review therapy.
Overdose
Symptoms: Abdominal bloating may occur.
Treatment: symptomatic.
Special instructions
In 10 ml of suspension the sodium content is 127.25 mg (5.53 mmol). This should be taken into account when a salt-restricted diet is required, for example in congestive heart failure and mild renal failure. 10 ml of suspension contains 130 mg (3.25 mmol) of calcium. Therefore, caution should be exercised when treating patients with hypercalcemia, nephrocalcinosis and urolithiasis with the formation of calcium oxalate stones. Gaviscon® Double Action contains antacids, which can mask the symptoms of serious gastrointestinal diseases. The effectiveness of the drug may be reduced in patients with low acidity of gastric juice. Children with gastroenteritis or suspected renal failure are at increased risk of hypernatremia. The drug contains methyl parahydroxybenzoate and propyl parahydroxybenzoate, and therefore may cause allergic reactions (including delayed ones). Effect on the ability to drive vehicles and machinery The drug does not affect the ability to drive vehicles and machinery, as well as engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Release form
Oral suspension [mint]. 150 ml, 200 ml, 300 ml or 600 ml in dark glass bottles, wrapped in shrink film, with a polypropylene cap that provides first-opening control. Instructions for use are located under the label. 10 ml of suspension in multilayer bags (polyester, aluminum, polyethylene). 4, 6, 8, 10, 12, 16, 20 or 24 sachets along with instructions for use in a cardboard box.
Storage conditions
At a temperature not exceeding 30 °C. Keep out of the reach of children.
Best before date
2 years.
Active substance
Sodium alginate, Sodium bicarbonate, Calcium carbonate
Dosage form
suspension for oral administration
Barcode and weight
Barcode: 4640018991592, 5000158071773 Weight: 0.546 kg
Instructions for use
According to the instructions, the medication is taken only orally. It is important to observe the therapeutic dose per day and not exceed it.
The tablets are chewed and washed down with a sufficient amount of water. The suspension in powder is kneaded to a homogeneous viscous consistency. As a rule, the course of therapy lasts a week. If there is no pharmacological effect, you should consult your doctor.
Depending on the dosage form, the drug is taken according to the following scheme:
- Tablets - taken after meals and before going to bed, chewing thoroughly. The therapeutic dosage for adults and children over 12 years of age is 2-4 tablets up to 4 times a day. Persons with liver disease and the elderly do not require course correction.
- Suspension – can be used by patients over 6 years of age. Adults are prescribed 10-20 ml, no more than 80 ml per day after meals. For a child 6-12 years old, no more than 40 ml per day, up to 4 times 5-10 ml.
Gaviscon tablets and suspension should be taken with caution if the excretory system is disrupted. Treatment dose adjustment is required depending on the condition.
Contraindications and precautions for the use of Gaviscon
Although Gaviscon is a completely harmless drug, you should still take the drug very carefully, taking all possible precautions. After all, if you exceed the dosage of the drug, the patient may experience a symptom of bloating, which can only be removed with the help of special symptomatic therapy.
If anti-bloating remedies do not help, it is best to then consult a doctor.
Before using the drug, you must undergo a thorough examination in the hospital. After which the doctor will carefully check whether you are allergic to any components of the drug, and only then can you safely begin treatment. After all, if you start taking Gaviscon without consulting a doctor, you may experience an allergic reaction after taking it.
Gaviscon. Mint tablets
In addition, the doctor must check you for the presence of heart disease or kidney disease in order to prescribe the correct dosage of the drug. After all, 4 tablets of Gaviscon contain 246 mg of sodium, which means that in the presence of impaired renal function and heart failure, you need to slightly reduce the dosage so as not to violate the diet, which requires limiting salt intake.
special instructions
The drug contains the chemical element sodium, so it is important for people with pathologies of the heart, kidneys, and when following a therapeutic diet to limit salt to take this into account.
Dose adjustment and strict medical supervision are necessary for nephrocalcinosis, urolithiasis and high calcium levels in the body.
An auxiliary component of the drug is aspartame. It belongs to the group of nonspecific sweeteners, so the medicine can be used for diabetes. On the contrary, it is contraindicated in patients with phenylketonuria.
During clinical trials, Gaviscon did not reveal mutational or teratogenic properties during embryonic development. Pregnant women and nursing mothers can use the drug as prescribed by a specialist.
Since Gaviscon is an antacid, it does not have a direct effect on the nervous system and blood flow. The medicine does not impair memory, does not suppress thinking and concentration, and therefore does not prohibit personal driving.