Author: Jae-Yeon Hwang
Methodology
Before starting an ultrasound, the doctor should be familiar with the clinical manifestations of gastrointestinal diseases that are urgent. The most common are: vomiting, pain, hematochezia, and diarrhea with or without fever. Some diseases have typical clinical manifestations, but many have overlapping symptoms and clinical manifestations.
An important factor influencing the quality of diagnosis is the choice of the correct sensor. A high-frequency (7.5–12.0 MHz) linear array probe is usually preferred for detecting GI problems. It provides a fairly high benefit in demonstrating dissection and thickening of the intestinal wall. For small children, it is recommended to use a smaller convex probe (5-8 MHz). Its smaller, rounded shape is also useful for compressing anatomical structures when needed. The large convex probe (1-5 MHz) is used for general screening of the entire abdomen in large patients or those with a poor sound window due to obesity.
Bowel gas may obscure abdominal organs, especially in patients with bloating and/or defence. The staged compression technique is a simple, highly effective method for bowel imaging in such cases because it eliminates bowel gas, thereby reducing the distance between the transducer and the target organ. Additionally, it can be used to isolate abnormal segments of intestine by pushing away adjacent normal segments.
Thus, gradual compression has the added value of differentiating abnormal segments of the intestine (eg, in the presence of acute appendicitis or other intestinal inflammation) from normal parts. In addition, when direct pressure is applied to abnormal areas of the intestine (for example, in a patient with appendicitis or intussusception), a lack of compressibility and mobility is detected, while segments of healthy intestine compress and move under the pressure generated by the transducer. The gradient compression technique should begin with gentle pressure on the abdominal wall so as not to alarm the patient experiencing gastrointestinal tenderness (Video 1).
Normal anatomy
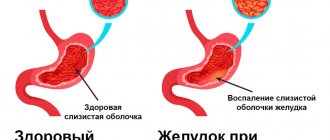
Many gastrointestinal diseases manifest as thickening of the intestinal wall, including inflammatory bowel disease, non-Hodgkin's lymphoma, intussusception, and Henoch-Schönlein purpura. Normal intestinal wall thickness in adults is approximately 2-4 mm, although this may vary depending on peristalsis and the degree of distension. In addition, normal intestinal wall thickness may change with age. In children, the maximum thickness is 1.5 mm in the small intestine and 2.0 mm in the colon. The study showed that the normal neonatal intestinal wall can be up to 2.6 mm thick. Thus, intestinal wall thickness ≥3 mm can be considered a sign of pathology in the small intestine. Colon wall thickness ≥4 mm also indicates a pathological condition. On color Doppler, normal intestinal walls rarely show vascularity, although flow may increase after feeding.
The intestinal wall has five layers: the mucosa, the muscularis mucosa, the submucosa, the muscularis layer, and the outer layer known as the serosa. On ultrasound, the innermost echogenic layer is seen as the superficial mucosa or mucosa-lumen. The next hypoechoic layer is the deep mucosa. The submucosa is visible as a hyperechoic layer and is most pronounced in the colon. The muscle layer is a hypoechoic ring that is secreted in the intestinal wall. The outermost serosa is very thin and not very visible on ultrasound (Figure 1). Tissue harmonic imaging may offer better visualization of bowel wall stratification on ultrasound. A disadvantage of harmonic tissue imaging is that beam penetration is limited (Figure 2).
Figure 1: Diagram of normal intestinal stratification using ultrasound.
Figure 2: Improved visualization of intestinal stratification using tissue harmonic imaging. A, B. Conventional grayscale image (A) and tissue harmonic image (B) show the terminal ileum (arrows).
The jejunum, especially the proximal part, is usually located in the left upper quadrant of the abdominal cavity and is usually compressed with a noticeable fold. The ileum has less pronounced folding than the jejunum, and internal fluid is often present normally. The terminal ileum has a thicker wall and a more prominent fold than other parts of the small intestine. In addition, the hypoechoic deep mucosa is emphasized on ultrasound. The terminal ileum and ileocecal valve are good landmarks for locating the appendix (Figure 3)
Figure 3: Sonogram of the small intestine.AD Sonograms show the jejunum (A), ileum (B), terminal ileum, and ileocecal valve (C, D).
To distinguish the large intestine from the small intestine, one must note its location, lack of folds, haustral markings, thicker wall than the small intestine, and prominent submucosal and muscular layers. The ascending and descending colon are easily found in close proximity to both paracolic gutters (Fig. 4), whereas the transverse colon is usually located at the inferior portion of the lesser curvature of the stomach. The sigmoid colon is located in the left lower quadrant and crosses the left psoas muscle. This landmark can be useful for tracking the descending colon and rectum (Video 2).
Figure 4: Sonogram of the colon. A, B. Sonograms show the transverse colon (arrows) (A) and descending colon (arrows) (B). Note the haustral markings, prominent echogenic submucosa, and peripheral location of the colon.
Causes of disturbances in the formation of the gastrointestinal tract in the fetus, statistics
Anomalies in the structure of the gastrointestinal tract are associated with a violation of embryogenesis at the stage of 4-8 weeks of pregnancy, when the opening of the digestive tube is formed. Initially, it ends at both ends, but by the end of the 8th week, channels are formed, and the mucous epithelium closes the lumen of the intestinal tube. Among the most common pathologies are stenosis (narrowing or stretching of the walls) or atresia (fusion).
The duodenum suffers the most, which is due to the peculiarities of its embryogenesis. 1/2 of cases are accompanied by defects of other internal organs - heart, blood vessels, rectum, liver, stomach. Some cases are so severe that the baby will have to undergo many operations during his lifetime, and they will not guarantee his normal existence.
Abnormalities of the gastrointestinal tract are visible on ultrasound at 11 weeks. Ultrasound diagnostics is not a 100% guarantee that the baby will have serious abnormalities, so its results are the basis for a more detailed examination of the woman.
A pregnant woman undergoes karyotyping to detect chromosomal abnormalities. She also undergoes an amniotic fluid test, and based on the results of the examination (if they are bad and the diagnosis is confirmed), she is recommended to terminate the pregnancy
Intestinal malrotation
The normal fetal colon rotates 270° counterclockwise in the womb. Any defect during this process results in malposition of the duodenum and ileocecal valve, a short mesenteric root, and close proximity of the cecum and duodenum. If not properly diagnosed, it can lead to proximal intestinal obstruction.
Although examination of the upper gastrointestinal tract remains the modality of choice in such cases, ultrasound may be an alternative imaging modality. An abnormal connection between the superior mesenteric artery (SMA) and the superior mesenteric vein (SMV) is an ultrasound finding of suspected intestinal malrotation.
For assessment, the patient should be in the supine position and the probe should be applied to the upper third of the midline of the abdomen using a gradual compression technique. Typically, the SMA is located in the right anterior aspect of the SMA and is easily compressed by the pressure of the transducer (Figure 5). Color Doppler can help differentiate the two vessels. If the SMV is located ventrally or on the left side of the SMA, it is malpositioned (Figure 6).
Figure 5: Normal position of the superior mesenteric artery, superior mesenteric vein and third part of the duodenum. The illustration shows the normal relationship between the vessels and the duodenum. B, C. In the grayscale image, the SMA (arrow) is larger than the SMA (arrow) and is more compressible by pressure from the transducer. IVC - inferior vena cava.
Figure 6: Abnormal position of the superior mesenteric artery and superior mesenteric vein. A, B. Grayscale image (A) and color Doppler image (B) show the SMA (arrows) located on the left side of the SMA (arrows).
Recently, direct imaging of the retroperitoneal location of the third portion of the duodenum or the duodenum in the left upper quadrant of the abdomen has been reported to be an alternative diagnostic indicator of intestinal malformation (Figure 7). Drinking small amounts of water may facilitate visualization of the normal course of the third portion of the duodenum. However, this ultrasound imaging method is highly physician dependent (Video 3). If ultrasound findings are inconclusive or abnormal, an upper gastrointestinal examination should be performed to confirm the diagnosis, as sonographic diagnosis cannot completely rule out an intestinal malformation.
Figure 7: Normal retroperitoneal position of the duodenum. A, B. Sonogram (A) and illustration (B) show the normal retroperitoneal position of the third portion of the duodenum. Transverse colon; SMV - superior mesenteric vein; SMA - superior mesenteric artery; IVC - inferior vena cava.
Midgut volvulus is a fatal complication of intestinal defects leading to proximal intestinal obstruction and ischemia. Approximately 75% of cases occur within a month after birth (mostly during the first week) and 90% within 1 year.
The sonographic sign in the midgut is the whirlpool sign, which refers to the clockwise twisting of the SMV, mesentery, and duodenum around the SMA. Color Doppler can help determine the relationship between the SMV and the SMA (Video 4).
Supporting findings include a thickened echogenic bowel wall due to edema or hemorrhage, a fluid-filled duodenum with varying degrees of distension, and a hyperpulsating SMA on Doppler. If this finding is positive, upper gastrointestinal exploration should be performed immediately, followed by surgery (Figure 8).
Figure 8: A, B. Grayscale image (A) and color Doppler image (B) show rotation of the duodenum (arrow) and superior mesenteric vein (arrows) around the superior mesenteric artery (open arrows). C. Sonogram shows the duodenum, which has been dilated, filling it with fluid (arrow). D. Barium study shows the appearance of a duodenal whirlpool (arrow) and proximal dilatation.
A common cause of false-positive results in the diagnosis of intestinal defects is non-central scanning. If the probe is not applied properly in the midline of the abdomen, the SMV to SMA ratio may appear abnormal. Another false-positive diagnosis is when the whirlpool sign corresponds to a normal counterclockwise rotation of the SMV against the SMA. This counterclockwise rotation of the jejunal branch of the SMV is often found normally and does not always accompany rotation of the duodenum or small intestine.
Severe bloating, defence, excessive bowel gas and/or an inexperienced physician can lead to false negative diagnoses. In these situations, the use of a more gentle gradual compression technique, convex probe, limited sedation, and emergency exploration of the upper gastrointestinal tract may be helpful to clarify the diagnosis.
DO YOU CARE CORRECTLY FOR YOUR ULTRASOUND DEVICE?
Download your care guide now
Download PDF
What it is?
In order to understand what “hyperechoic intestine” is, it is necessary to understand the terminology used by ultrasound diagnosticians. Echogenicity is a property of the tissues of a living organism that allows them to reflect or absorb sound waves. Some tissues, such as those filled with fluid, have low echogenicity, so ultrasound passes through them and only a faint gray shadow remains on the image. Echogenicity increases with tissue density; for example, bones almost completely reflect ultrasound and appear as a dark spot on the image.
Normally, the echogenicity of the fetal intestine is moderate, but higher than that of nearby organs - the liver, kidneys or lungs. With increased intra-abdominal structure, the organ becomes comparable to bone tissue, and then doctors talk about hyperechoic intestines.
If this pathology is detected in the early stages of pregnancy, there is no reason to worry. Increased brightness of the organ on ultrasound is normal in the first trimester and disappears at 16-20 weeks of gestation. The reason for additional research is the detection of hyperechogenicity at a period of 24 weeks.
Hypertrophic pyloric stenosis
Hypertrophic pyloric stenosis (HPS) is the most common reason for surgery in young children. A typical symptom of HPS is non-bilious, intermittent vomiting in a previously healthy child. HPS is not a clear emergency unless complications such as severe dehydration and electrolyte imbalance occur. Symptoms usually appear during the fourth week after birth, but the timing can vary from the third to the twelfth week. Ultrasound is the imaging modality of choice for diagnosing HPS, with a sensitivity and specificity of approximately 100%.
The examination begins with the patient lying on his back. A high-frequency linear/convex sensor is placed in the epigastric region. When scanning downward, the operator should begin with the liver and gastroesophageal junction and continue to the hepatic cruciate ligament. On careful scanning, normal pylorus is visible between the liver and the head of the pancreas.
When the sound window is poor due to abundant gas in the antrum, duodenal bulb, or transverse colon, the patient should be placed in the right lateral decubitus position, which allows visualization of the pylorus with fluid moving into the antropyloric canal and displacement of the pylorus anteriorly while the gas moves to the bottom of the stomach. In addition, after filling the stomach with glucose solution or water, the passage of fluid through the pylorus can be assessed.
Pyloric muscle thickness >3 mm and canal length >17 mm provide high diagnostic accuracy. The pylorus is a dynamic structure, and the thickness and length of the pyloric muscle may change during real-time examination. Therefore, muscle thickness >3 mm throughout the study and failure to distend the pylorus by gastric peristalsis and minimal gastric emptying, mucosal prolapse into the gastric antrum, and trapped fluid in mucosal clefts (double track sign) are unequivocal findings associated with HPS (Fig. 9).
Figure 9: Hypertrophic pyloric stenosis in a 2-week-old patient. A, B. Transverse (A) and longitudinal (B) images show concentric hypertrophy of the muscle layer of the pyloric canal (arrows). Note the double track (arrows) and bloating despite fasting for 2 hours. C. Longitudinal image shows the nipple antrum sign, which refers to the prolapse of echogenic mucosa into the antrum (open arrows).
When the muscle thickness is between 2 mm and 3 mm, it is considered a questionable finding. In this case, a diagnosis of pylorospasm or the development of HPS is considered. If pyloric muscle thickness returns to normal and the pyloric canal opens on long-term examination, pyloric spasm can be considered.
The main cause of a false negative result is an overdistended stomach because the antropyloric channel is directed back into the depths of the abdomen (Fig. 10).
Figure 10: Change in direction of the pylorus after feeding. Before feeding with milk, the pylorus (arrows) is directed to the right side and forward. B. After feeding, the axis of the pyloric canal (arrows) is directed to the left and back
Intestinal obstruction
Intussusception is the most common cause of small bowel obstruction in children, with 60% of cases occurring during the first year of life and 90% before the age of 2 years. Both the sensitivity and specificity of ultrasound reach 100%. Although most cases of intussusception in infants are idiopathic, they may also be associated with hypertrophied lymphoid tissue in the terminal ileum. Approximately 25% of these pediatric patients have a focal mass or diffuse bowel wall abnormality, such as abnormal lead points.
Ileocecal intussusception usually occurs in the subhepatic region in the right upper quadrant of the abdomen, although it has also been found in the upper abdominal midline and left upper quadrant. If ultrasound does not detect intussusception, the entire abdomen should be examined to look for other causes of abdominal pain.
Simple ileocolic intussusception consists of three segments of intestine. The entering and returning ends of the intestinal tract are the terminal ileum, and the outer E. coli is the colon. Ultrasound revealed that the most thickened segment is the recurrent limb of the intussusception (due to impaired blood supply). The mesentery and lymph nodes are often visible between the two segments of the intestinal artery (Fig. 11).
Figure 11: Ileocolic intussusception. The inverting part (red) and the permanent part (purple) of the intussusception (corresponding to the colon). Note the mesentery (yellow) enclosed between the two limbs of the intussusception. B. At the leading edge of the intussusception, the central part (arrow) and peripheral parts (arrow) form a ring sign. The central intussusception is separated by echogenic lines of serosa and mesentery (open arrow).
It has been reported that ultrasound can detect approximately two-thirds of abnormal leads. Meckel's diverticulum, duplication cyst, polyp, lymphoma, and intramural hematoma of the HPS are common pathologic lead points (Figure 12). Care should be taken not to miss an abnormal lead point in unusual clinical settings, for example, a patient who does not fall into the usual age group (eg, <1 month or >5 years) has an abnormal type of intussusception (bell, small bowel intussusception). ), has long-term symptoms or experiences a relapse.
Figure 12: 7-year-old boy with small bowel intussusception. A. The sonogram shows small bowel intussusception with one pedunculated polyp (arrow) and two sessile polyps (arrowheads) as pathological leading points. B. The surgical specimen shows the same results as seen on the sonogram. Note the pedunculated (arrow) and sessile polyps (arrows).
Temporary intussusception of the small bowel is sometimes discovered incidentally. Most cases of transient intussusception are associated with small bowel hyperperistalsis. They are usually smaller than ileocecal intussusception (diameter <1 cm) and are located in the periumbilicus, left upper quadrant, or left lower quadrant of the abdomen. Repeated or prolonged examination is recommended to determine whether the lesion has resolved. If the lesion is persistent and symptomatic, with prolonged segmental involvement >3.5 cm, the patient should be carefully evaluated to determine the pathologic point of abduction.
Diagnosis and further actions
If an ultrasound showed a hyperechoic intestine in the baby, then the pregnant woman is sent for additional diagnostics:
- repeat ultrasound;
- biochemical screening - analysis of venous blood for chromosomal pathologies;
- analysis for TORCH infections;
- cordocentesis - taking blood from the umbilical cord to determine genetic abnormalities;
- amniocentesis - analysis of amniotic fluid.
Intrauterine infections are treated with medication. Treatment is complicated by the fact that many antibiotics are contraindicated while pregnant. The expectant mother is prescribed a course of immunomodulators and immunoglobulins, which do not have a detrimental effect on the development of the fetus.
If Down syndrome or other genetic abnormalities are confirmed, parents are strongly advised to have an abortion. Children with intestinal pathologies come under the close attention of doctors immediately after birth. For Meckel's diverticulum or Hirschsprung's disease, surgery is performed in utero or immediately after the birth of the baby.
Acute appendicitis
Acute appendicitis is the most common condition that requires abdominal surgery in children. Unlike adults, approximately one third of patients experience nonspecific symptoms such as fever, diarrhea, and vague abdominal pain, which can lead to misdiagnosis.
The disease often occurs in young children (<6 years) due to an inability to communicate their symptoms. Consequently, perforation rates are higher in these younger patients: 83% among neonates and 51%-100% among children under 5 years of age. The sensitivity and specificity of ultrasound for diagnosing appendicitis are 80–95% and 90–100%, respectively.
A high-frequency linear matrix sensor is required when using the graduated compression method. Slow compression at the site of maximum tenderness is important to optimize patient tolerance. Additionally, sudden removal of the sensor should be avoided to prevent rebound pain. A convex transducer (1-5 MHz) may be an alternative if the sound window is weak or the appendix is believed to be located in the retrocecal region.
The ileocecal valve is a good landmark for identifying the appendix. The base of the appendix is almost always located in the medial part of the cecum, just caudal to the ileocecal valve. The location of the tip of the appendix, however, varies. Once the ileocecal valve has been located, the base of the appendix can be located by scanning 1-2 cm from the ileocecal valve or ileum. Thickened deep mucosa, a prominent fold, and the presence of peristaltic movements of the terminal ileum are clues to the differentiation of the appendix.
The normal appendix has a diameter of ≤6 mm and is compressed using a graduated compression technique. The echogenic line, representing the luminal surface, is located in the central part of the appendix. The normal wall thickness of the appendix is <3 mm. Various amounts of luminal air or fluid may be seen.
The most consistent sonographic findings associated with appendicitis are loss of compressibility and a maximum outer diameter of >6 mm (Figure 13). Supporting findings include a short-axis target-like appearance, thickened echogenic submucosa, a fluid-filled lumen, the presence of appendicoliths, increased periappendiceal echogenicity, enlarged mesenteric lymph nodes, and small amounts of pericecal or periappendicular fluid. Hyperemia may be seen on color Doppler (Fig. 14).
Figure 13: Acute appendicitis. A, B. Uncompressed (A) and compressed (B) sonogram shows an uncompressible dilated appendix greater than 6 mm in diameter (arrows). Note thickening of the echogenic submucosa of the appendix and increased echogenicity of the periappendiceal fat (arrowhead).
Figure 14: Ancillary signs of acute appendicitis. Short axis view shows a target-like appendix (arrow) with prominent periappendicular fat (arrowhead) and a small amount of periappendiceal fluid (open arrow). B. Long axial color Doppler view shows thickening of the echogenic submucosa and hyperemia of the appendix growth.
If inflammation progresses and purulent appendicitis develops, heterogeneous echogenicity and hyperemic changes in the periappendiceal tissue may be present. Gangrenous changes are suggested by the presence of increasing dilatation of the appendix and loss of echogenic submucosal layer with absence of vascularity on Doppler examination (Fig. 15). In addition, perforation is suspected in the presence of inflammatory cellulitis, seen as a mixed echogenic mass, poorly defined bowel loops in the right lower quadrant, focal bowel wall thickening, intraperitoneal fluid, localized fluid collection, or abscess formation. It may be difficult to visualize the inflamed appendix when perforation has occurred (Figure 16).
Figure 15: Gangrenous appendicitis. A, B. Grayscale image (A) and color Doppler image (B) show low echogenicity of the submucosal layer and absence of vascular outflow of the appendiceal wall. Note the appendicoliths (arrows).
Figure 16: Perforated appendix in a 10-month-old patient with fever of unknown origin. A, B. Grayscale images show the appendage (arrows) dilated by collecting periappendiceal fluid (arrows) with low echogenicity. Note the appendicolith in the appendix (open arrow) and increased echogenicity of the periappendiceal fat. C. CT scan image shows inflammatory cellulitis and abscess formation (arrowheads) in the right lower quadrant of the abdomen.
Mesenteric lymphadenitis
Mesenteric lymphadenitis is a benign, self-limiting disease. It occurs as a result of a primary inflammatory process or secondary inflammatory changes caused by abdominal disease. Mesenteric lymphadenitis is a clinical disease that is diagnosed after excluding other causes of abdominal pain. Since mesenteric lymph node enlargement is the only obvious finding, ultrasound is important to rule out other possible causes of abdominal pain.
Various normal ranges of LN sizes have been reported, typically falling between 4 and 20 mm in the short axis. LNs >5 mm in short axis are common in normal, healthy children. Enlarged mesenteric lymph nodes are common in pediatric patients, however, especially in those <10 years of age. Three or more LNs >5 mm in the short axis can be considered abnormal.
Enlarged mesenteric lymph nodes are often oval, with or without increased echogenicity of perinatal fat tissue. The preserved hilar fat serves as an echogenic area in the center of the LN. On color Doppler scanning, central vascular pedicles are visible with or without increased vascularity (Fig. 17). It is important that radiologists evaluate the presence of inflammation in the appendix and gastrointestinal tract. Malignant LNs should be suspected when they are round rather than oval in shape, there is loss of fat pad, and/or the LN has an eccentrically thickening cortex.
Figure 17: Mesenteric lymphadenitis in a 7-year-old patient with abdominal pain. A, B. Grayscale (A) and color Doppler images (B) show an enlarged mesenteric lymph node with hyperemic changes. The lymph node is oval in shape and contains preserved adipose tissue and a central vascular pedicle.
Causes of congenital fetal pathology
What factors can adversely affect the fetus?
The causes of various pathologies are:
- burdened heredity;
- Mom's age is over 35 years, dad's age is over 40;
- unfavorable environmental conditions;
- working with toxic substances;
- bad habits - alcohol abuse, smoking, drug use;
- viral and bacterial diseases, especially in the first trimester - influenza, rubella, toxoplasmosis, hepatitis B, sexually transmitted diseases;
- taking certain medications;
- excessive psycho-emotional stress.
The more factors are present, the greater the likelihood of fetal pathologies. That is why you need to start planning for the birth of a child in advance, at least six months before conception. This time is enough to undergo a comprehensive examination, identify and cure diseases, and prepare for the conception of a healthy baby. To highlight the impact of bad habits on the fetus, we present some numbers.
With maternal alcoholism, toxicosis is observed in 26% of cases, antenatal death and asphyxia in 12%, miscarriages in 22%, premature birth in 34%, birth injuries in 8%, and intrauterine growth retardation in 19%.
Hemorrhagic vasculitis
GV is a systemic vasculitis that can affect the gastrointestinal tract. As a common cause of abdominal pain in children, it manifests as abdominal pain and thickening of the intestinal wall in 50-60% of patients. It often involves the duodenum and small intestine, although it can involve the entire gastrointestinal tract, mimicking acute appendicitis. Abdominal pain can prevent skin flare-ups. Therefore, if a patient has severe bowel wall thickening in the duodenum and proximal small intestine, GV should be considered, even if skin lesions have not yet appeared.
A common sonographic finding is thickening of the intestinal wall involving the duodenum and proximal jejunum due to intrauterine hemorrhage. GV, however, can involve any segment of the intestine (Fig. 18). A focal intramural hematoma may be considered an echogenic lesion of the intestinal mucosa or submucosa. Thickening of the intestinal wall due to intramural hematoma can have a maximum thickness of 9 mm. In addition, hyperemia of the intestinal wall can be seen on color Doppler examination. An intramural hematoma may be the leading point of intussusception (Fig. 19).
Figure 18: GV involving the gastrointestinal tract of a 6-year-old patient. A, B. Gray scale images of the duodenum (A) and jejunum (B) show concentric thickening of the bowel wall with slightly increased echogenicity of the bowel wall (arrows).
Figure 19: Small bowel intussusception due to gastrointestinal tract in a 6-year-old patient with abdominal pain. Color Doppler image shows small bowel intussusception with poor vascularity. B. CT scan shows small bowel intussusception (arrow). C. Photograph of the surgical specimen shows a long segmental small bowel intussusception with ischemia and severe thickening of the bowel wall.
Omental infarction
Omental infarction is a rare disease, although 15% of all cases occur in children. This disease is now being detected more frequently due to the increasing prevalence of obesity and the increasing use of computed tomography and ultrasound. Omental infarction is usually accompanied by pain in the right side of the abdomen, which is associated with gastrointestinal symptoms such as anorexia, nausea and vomiting. Thus, it mimics acute appendicitis.
The typical sonographic finding is an ovoid echogenic mass located between the abdominal wall and the intestine, often in the right upper quadrant (Fig. 20). This may appear as peripheral hyperemic changes on color Doppler. Although ultrasounds have good diagnostic accuracy for this diagnosis, the accuracy is variable because it depends on the physician. Ultrasounds play an important role in excluding appendicitis and other diseases of the gastrointestinal tract. Early detection of omental infarction may prevent unnecessary surgery or the need for a CT scan.
Figure 20: Omental infarction in a 9-year-old patient with acute abdominal pain. The sonogram shows an area of heterogeneously increased echogenicity adjacent to the abdominal wall (arrows). B. CT scan shows a focal area of adipose tissue coiled with a hypertonic peripheral halo (arrowheads).
Bottom line
Ultrasound is the modality of choice for the initial evaluation of acute abdominal pain in pediatric patients due to their small body size and the presence of less fatty tissue in the abdominal wall and abdominal cavity. Thus, it has an optimal sound window, unlike in adult patients. Proper selection of probes, optimal positioning, and use of progressive compression techniques improve visualization of bowel disease.
Ultrasounds can diagnose several conditions that cause abdominal pain and can differentiate between various medical and surgical problems in children. Careful assessment of bowel wall thickening, coupled with additional findings such as fluid accumulation, increased echogenicity of mesenteric fat, enlarged lymph nodes, hyperemic bowel changes, and abnormal bowel motility, enhances the diagnostic capabilities of ultrasound. Several disorders that mimic acute appendicitis (mesenteric lymphadenitis, HV, and omental infarction) should be considered when performing ultrasound in pediatric patients with right abdominal pain and a normal appendix.
Our catalog presents a large selection of devices with affordable high-frequency sensors for studies of the gastrointestinal tract. Contact our manager and he will advise you!
How often should you have an ultrasound during pregnancy?
The standard number of procedures is 2-3, but in some cases the patient has to visit the diagnostic room several times a month. These are women who are at risk due to the high likelihood of developing abnormalities in the fetus. People in this category include:
- over 35 years old;
- having children with congenital pathologies;
- with a family history;
- exposed to radiation, in contact with toxic substances;
- having a history of premature birth, cases of stillbirth or fetal death in the womb, miscarriages.
Do not be alarmed if the doctor prescribes an additional ultrasound - careful monitoring of the condition of the fetus allows you to avoid congenital pathologies and notice deviations from the norm in the early stages.