Development of anemia in certain diseases of the gastrointestinal tract in children
Anemia is a fairly common secondary syndrome in gastroenterological diseases. The main types of anemia detected in gastroenterological patients are: iron deficiency anemia (IDA), anemia of chronic diseases (ACD), hemolytic anemia, B12 and folate deficiency anemia, hypo-, aplastic anemia.
IDA is the most common form of anemia in gastroenterology, and its development is caused by bleeding, impaired iron absorption, and dietary restrictions. The second most common after IDA in pediatric gastroenterology is ACD, which is characterized by sufficient iron reserves in the body, but a decrease in transferrin saturation with iron, and a reduced or normal level of serum iron. The reason for the development of ACD is the action of pro-inflammatory cytokines (interleukin 1-alpha and interleukin 1-beta, tumor necrosis factor alpha, interferon gamma, etc.).
Inflammatory bowel diseases
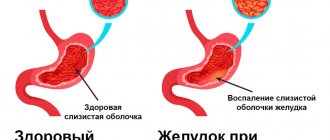
Inflammatory bowel diseases (IBD), primarily ulcerative colitis (UC) and Crohn's disease (CD), are often accompanied by the development of anemia. The frequency of anemia in IBD, according to the literature, is quite variable and ranges from 6% to 74% [17]. In most studies, this value is 35–50% [9, 12, 13, 19, 25, 27]. Anemia is detected with a slightly higher frequency in patients with ulcerative colitis. Thus, S.?Schreiber et al. [26] reported the presence of anemia in 25% of patients with CD and 37% of patients with UC.
Several mechanisms always play a role in the development of anemia in IBD. Conventionally, the following groups of causes of anemia in IBD can be distinguished [2, 4, 7, 13, 17, 18, 20]:
- Malabsorption. Malabsorption of various nutrients occurs in both UC and CD, but they reach their greatest severity in patients with CD when the jejunum and ileum are involved in the process or resection of the latter. From the perspective of the development of anemia, of particular importance is impaired absorption of iron (iron deficiency anemia), folic acid, vitamin B12 (B12-folate deficiency anemia), impaired absorption of proteins with the development of protein-energy deficiency, which can lead to hypotransferrinemia, impaired iron transport and adequate erythropoiesis.
- Chronic blood loss. Chronic blood loss is the most common condition in IBD, and its degree can vary, sometimes reaching significant proportions, especially in UC, resulting in the development of iron deficiency posthemorrhagic anemia.
- Anemia of chronic diseases (ACD). ACD develops as a result of a long-term increase in the level of pro-inflammatory cytokines, such as TNF-alpha, interleukins 1, 6, 10, interferon gamma, which leads to both impaired iron metabolism and inhibition of bone marrow hematopoiesis.
- Impact of drugs. Many drugs used in the treatment of IBD, such as sulfasalazine, mesalazine, azathioprine, mercaptopurine, have a pronounced inhibitory effect on erythropoiesis, leading to the development of hyporegenerative anemia.
- Hemolytic anemia. This type of anemia is described mainly in UC and is caused by autoimmune mechanisms.
- Myelodysplastic syndrome and aplasia. These disorders are recorded mainly in patients with IBD over 60 years of age.
- Hereditary hemolytic anemia. Some researchers have described a combination of IBD with hereditary hemolytic anemia caused by a deficiency of erythrocyte glucose-6-phosphate dehydrogenase activity. However, to date, no reliable genetic connection has been established between these diseases.
Most often, in patients with IBD, ACD and iron deficiency anemia are combined [1, 4, 17, 18], however, the role of other factors is also large and the severity of anemia may increase due to repeated episodes of bleeding, deficiency of vitamins and nutrients, and the influence of medications.
Anemia of chronic disease was first described by Maxwell Wintrobe and George Cartwright in 1949. The main criteria for its diagnosis are a decrease in serum iron levels and transferrin saturation with iron with intact iron reserves in the reticuloendothelial tissue (normal ferritin levels).
Advances in deciphering the basic mechanisms of ACD have led to increased attention to this type of anemia, including in pediatrics. It has been established that ACD accounts for about 10–20% of all anemias.
Today, the main link in the pathogenesis of ACD is considered to be the overproduction of proinflammatory cytokines. They lead to inhibition of erythropoietin synthesis and significantly reduce its effects aimed at enhancing the proliferation and maturation of erythroid precursors [1–4, 13].
Impaired iron metabolism in ACD is largely mediated by the expression of hepcidin. Hepcidin is an acute-phase inflammatory protein that is synthesized by the liver in response to bacterial lipopolysaccharides and interleukin 6. Hepcidin reduces the absorption of iron in the duodenum, blocks the release of iron from macrophages and reduces the ability of the red bone marrow to absorb it. Insufficient supply of iron to erythroid cells leads to disruption of their maturation and decreased heme synthesis [3, 14].
The participation of pro-inflammatory cytokines in the genesis of ACD in IBD is due to both their direct inhibitory effect (primarily interferon gamma) on the maturation of erythroid cells and increased apoptosis processes, and indirect effects: suppression of erythropoietin synthesis, decreased sensitivity of erythroid cells to it, activation of synthesis hepcidin and impaired iron utilization, as well as increased free radical reactions [5, 10, 14].
Over the past twenty years, 155 patients with UC and 61 patients with CD have been observed in the gastroenterology department of the Russian Children's Clinical Hospital (RCCH). Anemia was detected in 35% of children with UC and was of an iron deficiency nature, mainly due to chronic blood loss. In 69% of cases the anemia was mild, in 27% it was moderate, and in 4% it was severe. The severity of anemia largely depended on the extent of damage to the colon and the severity of the ulcerative process.
A decrease in hemoglobin levels (110 g/l ≤ Hb < 120 g/l) was determined in 9% of children with UC, and a decrease in serum iron levels with normal hemoglobin levels was detected in 12%. A total iron deficiency state, including developed IDA, was observed in 56% of children with UC.
Among children with CD, anemia was detected in 33%. Of these, in 93% of cases it was mild, in 7% it was of moderate severity. There were no children with severe anemia. Anemia in CD was predominantly iron deficiency, however, chronic blood loss in this group of patients was less pronounced and significant, while the activity of immune inflammation and the level of proinflammatory cytokines were significantly higher than in patients with UC (especially interleukin 1-beta and tumor necrosis factor alpha) . Some patients had damage to both the large and small intestines, which led to disruption of absorption processes.
A decrease in hemoglobin levels (110 g/l ≤ Hb < 120 g/l) was observed in 5% of children with CD, and a decrease in serum iron levels with normal hemoglobin values was observed in 15%. A total of iron deficiency, including developed IDA, was present in 53% of children with CD.
According to our data, during the period of exacerbation there was a significant increase in the level of interleukin 1-alpha in 85% of children with UC and in 71% of children with CD (505.1 ± 77.92 and 345.93 ± 72.37 pg/ml, respectively, with normal 107.2 ± 11.59). While during the period of remission there was a decrease in these indicators with a tendency towards normalization: an increase in the level of interleukin 1-alpha was observed in 63% of children with UC and in 50% of children with CD, which amounted to 305.1 ± 86.73 and 170.2 ± 48 .91 pg/ml, respectively.
A slightly different picture was observed for interleukin 1-beta. Its level was increased in 66% of patients with UC (58.2 ± 10.92 pg/ml, normal 32.0 ± 3.7 pg/ml) and in 85.7% of patients with CD (141.37 ± 60, 62 pg/ml). During the period of remission, the level of interleukin 1-beta remained elevated in 58% of patients with UC (45.66 ± 8.14), and in patients with CD its normal values were observed (10.21 ± 7.68).
The severity of anemia in patients with UC correlated with the level of interleukin 1-alpha (R = 0.53) and interleukin 1-beta (R = 0.59). This correlation was more pronounced in patients with CD: for interleukin 1-alpha R = 0.64, for interleukin 1-beta R = 0.81.
Interleukin 1-beta is known to be the most sensitive marker of inflammation. It has a significant effect on erythropoiesis in the red bone marrow. Our data suggest a more significant contribution of ACD to the development of anemia in patients with CD compared to patients with UC.
Malabsorption syndrome
Anemia is the most common concomitant of diseases occurring with malabsorption syndrome, and, first of all, celiac disease. According to the literature, anemia in celiac disease is observed in 40% of children [24]. The reason for its development, first of all, is a severe violation of intestinal absorption, including iron, folic acid, vitamin B12, amino acids and other nutrients necessary for full erythropoiesis. However, iron absorption is most affected, which determines the predominantly iron deficiency nature of anemia in celiac disease. On the other hand, latent celiac disease in IDA of unknown origin is detected in 2.8% of cases [22].
In case of exudative enteropathy, caused by malformations of the intestinal lymphatic vessels, anemia is also predominantly of an iron deficiency nature, but its cause is primarily the loss of serum iron and plasma carrier proteins (transferrin) with the lymph.
Among 218 children with celiac disease observed at the Russian Children's Clinical Hospital (RCCH), IDA was detected in 68% of cases. Of these, 82% of patients had mild anemia, 12% had moderate anemia, and 6% had severe anemia. There were no signs of obvious or hidden bleeding.
The severity of anemia correlated to a certain extent with the degree of malnutrition and disease activity. Thus, IDA occurred in all children with grade III malnutrition. At the same time, it was of a severe and moderate nature, while with less severity of malnutrition, mild anemia was more often observed.
A decrease in hemoglobin levels (110 g/l ≤ Hb < 120 g/l) was determined in 7% of children with celiac disease, and a decrease in serum iron levels with normal hemoglobin levels was detected in 18%. Thus, a total iron deficiency state, including developed IDA, was observed in 93% of children with celiac disease.
In addition, 1.5% of children with celiac disease had megaloblastic hyperchromic anemia, which appears to be due to a predominant malabsorption of folic acid and vitamin B12.
Differential diagnosis of anemia in diseases of the gastrointestinal tract (GIT)
The diagnosis of anemia itself in gastrointestinal diseases is made on the basis of the clinical picture, laboratory signs of anemia and, as a rule, does not cause difficulties. The main problem may be the differentiation of IDA and ACD, which is sometimes impossible due to the combination of these types of anemia. IDA is characterized by hypochromic (CP < 0.85) anemia of varying severity, a decrease in the average concentration of hemoglobin in erythrocytes, an increase in the number of reticulocytes, microcytosis and poikilocytosis of erythrocytes (in a peripheral blood smear); decrease in the number of sideroblasts in bone marrow aspirate; decrease in iron content in blood serum (<12.5 µmol/l); an increase in the total iron-binding capacity of serum (TIBC) of more than 85 µmol/l (an indicator of “starvation”); an increase in the level of transferrin in the blood serum, with a decrease in its saturation with iron (less than 15%); decreased serum ferritin levels (<15 µg/L).
ACD is usually characterized by a moderate decrease in hemoglobin (95–80 g/l), normocytosis, normal or moderate hypochromia of erythrocytes, and a decrease in the number of reticulocytes. A decrease in serum iron levels and transferrin saturation is observed in both anemias, but the reason for this is different. If in IDA these changes are associated with absolute iron deficiency, then in ACD the iron reserves are sufficient, but they cannot be utilized from the reticuloendothelial system (reticuloendothelial block).
An indicator of the state of the iron depot is the level of serum ferritin, which in ACD has normal or increased values, while in IDA it is always reduced (less than 15 ng/mg). However, when iron deficiency and chronic disease are combined in patients with IBD, the significance of this indicator is low. For example, in CD, patients with anemia in most cases show a decrease in serum ferritin levels by a factor of two or more, compared with patients without anemia [17].
To date, the most reliable criterion for distinguishing these two forms of anemia is determining the level of soluble transferrin receptors in the blood. In IDA, their level increases significantly due to iron deficiency erythropoiesis, while in ACD it decreases due to the suppression of their synthesis by proinflammatory cytokines.
Establishing the genesis of anemia in gastrointestinal diseases is of fundamental importance, since it allows one to determine adequate ways of its correction. Numerous studies show that correction of anemia in gastrointestinal diseases helps improve regenerative processes due to normal tissue oxygenation, reduces the severity of the disease and improves its outcome, reduces the frequency of hospitalizations, and improves the patient’s quality of life. Of particular importance in childhood is the improvement of cognitive (cognitive) functions, normalization of the central nervous system and psycho-emotional sphere of the child [1, 20, 25].
Principles of correction of anemia in children with gastroenterological pathology
Achieving success in treating anemia in children with gastrointestinal pathology is impossible without treating the underlying disease that caused it. This is of particular importance in IBD, because... The severity of anemia directly correlates with the degree of disease activity, thus, successful treatment of the underlying disease is also treatment of anemia [6, 17].
Treatment of the underlying disease includes:
- elimination, if possible, of the etiological factor;
- decreased activity of the pathological process;
- normalization of gastrointestinal motility, processes of digestion, absorption, etc.
In addition to reducing the activity of the underlying disease, in most cases correction of the anemia itself is necessary. In cases of severe anemia, especially due to blood loss, we can talk about transfusions of donor blood. But this method allows only to temporarily improve the situation, moreover, it is fraught with the development of some complications (risk of infection, sensitization, iron overload, tissue hemosiderosis, etc.). Therefore, blood transfusions are rarely used and cannot be the basic method of correcting anemia.
Correction of anemia in gastrointestinal diseases, due to its predominantly iron deficiency nature, should include the administration of iron supplements. The effectiveness of prescribing iron supplements is ambiguous and largely depends on the genesis of anemia, the choice of drug and the method of its administration.
The use of oral iron supplements is not always effective. Firstly, the maximum adsorption capacity of the intestine to absorb iron does not exceed 2 mg per day, but on average it is 1 mg, which is clearly not enough for the effective correction of anemia. Secondly, in many gastrointestinal diseases, even these amounts of iron do not come from the intestinal cavity due to impaired absorption processes. And finally, oral iron supplements cause or aggravate inflammatory processes in the intestinal mucosa, promote the development of free radical reactions up to oxidative stress, and have a carcinogenic effect in relation to the colon. To the greatest extent, these complications are observed when using divalent iron salts (ferrous sulfate, etc.) and in more than 20% of cases they are the basis for discontinuation of therapy. Polymaltose hydroxide compounds of ferric iron (Maltofer and others) do not cause such complications and from a safety point of view can be used, but their effectiveness is limited. A number of studies have shown that the administration of oral iron supplements for IBD leads to a slight increase in hemoglobin levels, sometimes even to normalization, but there is no accumulation of iron, i.e. ferritin levels remain low, and only partial and temporary replenishment of iron deficiency occurs .
Enteral iron supplements are indicated for patients with gastroenterological pathology for the correction of iron deficiency and the treatment of mild to moderate iron deficiency anemia in the absence of bleeding in the upper gastrointestinal tract and severe intestinal absorption disorders. However, the prescription of oral medications to children with gastroenterological pathology requires compliance with certain conditions: iron preparations must be safe and not cause toxic and oxidative reactions, including due to uncontrolled absorption; do not irritate the mucous membranes of the gastrointestinal tract; have high bioavailability and are well absorbed. It is optimal to use hydroxide polymaltose compounds of ferric iron (Maltofer), which meet all the requirements.
Intravenous iron preparations have a great advantage - iron sucrose (Venofer), which are devoid of the above-mentioned side effects, do not depend on absorption and are able to quickly and effectively replenish iron deficiency and its reserves (increased transferrin levels).
However, the use of iron supplements will be effective only if iron deficiency anemia is predominant. In many cases, especially with IBD, the basis of anemia is ACD, in which there is no true iron deficiency: its reserves in the reticuloendothelial tissue are sufficient, and sometimes excessive, while its release from the depot and the ability to be utilized by erythroid precursors of the red bone marrow is impaired. Prescribing iron supplements in this case not only does not lead to a significant and lasting correction of anemia, but is also fraught with iron overload with the development of hemosiderosis.
The most effective and pathogenetically justified for the correction of anemia of chronic diseases is the administration of erythropoietin. The introduction of erythropoietin compensates for its insufficient endogenous production and can overcome the low sensitivity of red bone marrow cells to erythropoietin. In addition, erythropoietin has a blocking effect on the synthesis of proinflammatory cytokines and the transmission of the cytokine signal into the nucleus of target cells, promotes the release of iron from the reticuloendothelial system, its absorption by red bone marrow cells and heme biosynthesis. Moreover, treatment with erythropoietin, by suppressing proinflammatory cytokines, reduces the activity of the underlying disease [11, 15, 16, 23].
Prescribing erythropoietin to patients inevitably leads to high iron consumption by erythroid precursors and rapid depletion of its reserves in the body. The result of this is iron deficiency erythropoiesis. Therefore, in parallel with erythropoietin, it is necessary to prescribe intravenous iron supplements, which is especially indicated for patients with mixed genesis of anemia, i.e. AHZ and ZhDA. The use of erythropoietin in combination with iron supplements leads to a faster achievement of normal hemoglobin levels [11].
Literature
- Anemia in inflammatory bowel diseases. In the book. Anemia - a hidden epidemic / Transl. from English M.: MegaPro, 2004, p. 57–59.
- Glikman R.?M. Inflammatory bowel diseases: ulcerative colitis and Crohn's disease / Internal diseases (ed. T.? R.? Harrison et al.) Translated. from English M.: Medicine. 1996. T. 7. pp. 113–136.
- Kozlovskaya L.?V., Rameev V.?V., Sarkisova I.?A. Pathogenesis and clinical significance of anemia of chronic diseases // Anemia. 2005. No. 4. P. 4–10.
- Malkoch A.?V., Belmer S.?V., Anastasevich N.?A. and others. Anemia in pediatric gastroenterology // Anemia. 2006. No. 1–2. pp. 59–63.
- Andrews N.?C. Anemia of inflammation: the cytokine-hepcidin link // J.?Clin. Invest., 2004; 113:1251–1253.
- Best W.?R. et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study // Gastroenterology, 1976; 70: 439–444.
- Beeken W.?L. Absorptive defects in young people with regional enteritis // Pediatrics. 1973; 52:69–74.
- Blanchard J.?F., Bernstein C.?N., Wajda A., Rawsthorne P. Small-area variations and sociodemographic correlates for the incidence of Crohn's disease and ulcerative colitis // Am. J.?Epidemiol. 2001. 154: 328–335.
- Burbige E.?J., Huang S.?H., Bayless T.?M. Clinical manifestation of Crohn's disease in children and adolescents // Peditrics. 1975; 55:866–690.
- Denz H., Huber P., Landmann R. et al. Association between the activation of macrophages, changes of iron metabolism and the degree of anemia in patients with malignant disorders // Eur. J.?Haematol., 1992; 48: 244–248.
- Dohil R., Hassall E., Wadsworth L.?D. et al. Recombinant human erythropoietin for the treatment of anemia of chronic disease in children Crohn's disease // J Pediatr. 1998; 132: 155–159.
- Dyer N.?H., Child J.?A., Mollin D.?L. et al. Anaemia in Crohn's disease // QJ Med. 1972; 41:419–436.
- Friedman S., Bloomberg R.?S. et al. Inflammatory bowel diseases. In: Braunwald E., Fauci A.?S., Kasper D.?S. et al., eds. Harrison's Principles of Internal Medicine. 15 th ed. New York, NY: McGraw-Hill; 2001: 1679–1692.
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation // Blood, 2003; 102:783–788.
- Gasche S., Dejaco C., Reinisch et al. Sequential treatment of anemia in ulcerative colitis with intravenous iron and erythropoietin // Digestion. 1999; 132: 155–159.
- Gasche S., Dejaco C., Waldhoer T. et al. Intravenous iron and erythropoietin for anemia associated with Crohn disease: a randomized, controlled trial // Ann Intern Med. 1997; 126:782–787.
- Gasche C., Kulnigg S. Intravenosus Iron in Inflammatory Bowel Disease // Seminars in Hematology. 2006, Vol. 43, No. 4, Suppl 6, S18–S22.
- Gasche S., Reinisch W., Lochs H. et al. Anemia in Crohn's disease: importance of inadequate erythropoietin production and iron deficiency // Dig Dis Sci. 1994; 39: 1930–1934.
- Harries A.?D., Fitzsimons E., Dew M.?J. et al. Association between iron deficiency anemia and mid-arm circumference in Crohn's disease // Hum Nutr Clin Nutr. 1984; 38:47–53.
- Horina J.?H., Petritsch W.?Schmid C.?R. et al. Treatment anemia in inflammatory bowel diseases with recombinant human erythropoietin: results in three patients // Gastroenterology.1997; 126:1828–1831.
- Hugot J.?P., Zouali H., Lesage S. et al. Etiology of the inflammatory bowel diseases // Int J Colorectal Dis. 1999; 14:2–9.
- Karnam U.?S., Felder L.?R., Raskin J.?B. Prevalence of occult celiac disease in patients with iron-deficiency anemia: a prospective study // South Med J. 2004; 97 (1): 30–34.
- Kulnigg S., Gasche C. Systematic review: managing anemia in Crohn's disease // Aliment Pharmacol Ther. 2006, 24, p. 1507–1523.
- Rashid M., Cranney A., Zarkadas M. et al. Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children // Pediatrics. 2005; 116(6):754–759.
- Revel-Vilk S., Tamary H., Broide E. et al. Serum transferrin receptor in children and adolescents with inflammatory bowel disease // Eur J Pediatr. 2000; 159:585–589.
- Schreiber S., Howaldt S., Schnoor M. et al. Recombinant erythropoietin for the treatment of anemia in inflammatory bowel diseases // N.?Engl. J.?Med. 1996; 334:619–623.
- Walker A.?M., Szneke P., Bianchi L.?A. et al. 5-Aminosalicylates, sulfasalazine, steroid use, and complications in patients with ulcerative colitis // Am J Gastroenterol. 1997; 92:816–820
A. V. Malkoch*, Candidate of Medical Sciences S. V. Belmer*, Doctor of Medical Sciences, Professor N. A. Anastasevich* E. V. Semenova* L. M. Karpina** N. E. Shchigoleva** I. A. Matina** A. P. Ponomareva**
*RGMU, **Russian Children's Clinical Hospital, Moscow
Contact information for authors for correspondence
Vitamin B12 deficiency
Vitamin B12 (cyanocobalamin) is necessary for the maturation of red blood cells - erythrocytes. It is not synthesized in the human body and must be constantly supplied with food.
Vitamin B12 is absorbed in the lower small intestine. But in order to go into a digestible form, it must first contact a protein called intrinsic factor of Castle, which is produced by the cells of the mucous membrane of the fundus and body of the stomach. After a gastrectomy, Castle factor is no longer produced and the absorption of vitamin B12 is impaired. Pernicious anemia develops.
Possible manifestations of vitamin B12 deficiency: weight loss, loss of taste, nausea, diarrhea or constipation, burning, enlarged tongue, bright red tongue, “lacquered tongue”, slight yellowing of the skin, vitiligo (discolored patches on the skin), early graying of hair.
In addition, vitamin deficiency can lead to the death of nerve cells and neurological disorders: numbness, tingling in the arms and legs, disruption of the coordination of different muscle groups.
Symptoms of vitamin B12 deficiency may appear as early as one year after surgery. They do not appear immediately, because the liver stores a fairly large supply of the vitamin.
How to detect B12 deficiency anemia? A blood test reveals an increased MCV (average erythrocyte volume), normal MCH (average hemoglobin content in one erythrocyte), anisocytosis (change in the size of erythrocytes), poikilocytosis (change in the shape of erythrocytes), a decrease in the number of reticulocytes (young forms of erythrocytes), megalocytes (large oval-shaped cells), leukopenia (decreased number of white blood cells). A blood chemistry test may detect decreased levels of vitamin B12, but this is not a very reliable test.
How to provide the body with vitamin B12? Classically, after gastric removal, patients are advised to receive vitamin B12 by intramuscular injection. However, research shows that taking B12 supplements orally (by swallowing) also helps because it introduces a different absorption pathway, independent of intrinsic factor. Subcutaneous injections may also be used. Vitamin B12 is rich in foods such as milk, red meat, fish, crustaceans, and beef liver.
Hemoglobin for gastritis
In recent years, new approaches to the treatment of iron deficiency anemia have emerged. Scientists have found that hemoglobin levels often decrease in patients with gastritis. It's simple: the inflamed gastric mucosa is not able to absorb iron well from food and medications.
Therefore, doctors now recommend that if you have anemia, you must be examined for the presence of Helicobacter. And if this bacterium is found on you, you must undergo a course of treatment.
The doctor will propose a treatment regimen that will need to be followed for 10–14 days. In most cases, getting rid of gastritis leads to normalization of iron absorption. And hemoglobin returns to normal.
1.General information
The oral mucosa is characterized by a number of distinctive features.
It is located in close proximity to vital organs, endocrine glands, lymph nodes; serves as a covering for the “entry gate” for the digestive tract and respiratory tract; it is intensively innervated, supplied with blood, moisturized, and renewed very quickly. Therefore, this mucous layer, extremely sensitive to any physiological, hormonal, biochemical changes in the body, can serve as a reliable early indicator of a pathological process, which sometimes begins in a completely different zone and system. Moreover, it is specific changes in the oral mucosa, discovered, for example, during a dental examination or treatment, that often turn out to be the first and at that time the only sign of a particular disease, sometimes formidable and severe, but progressing asymptomatically until a certain stage.
A must read! Help with treatment and hospitalization!
Symptoms of anemia
The most common symptoms of anemia are fatigue, weakness, shortness of breath, pallor, dizziness, dry skin, hair loss, rapid heartbeat, headache, tinnitus, loss of appetite, and increased symptoms of angina if a person has coronary artery disease.
It also happens that the symptoms of anemia are not clearly expressed and are not felt by the patient. That's why he doesn't see a doctor. Unfortunately, this is dangerous, because... with a long latent (imperceptible) course of anemia, heart failure may develop, or an existing one may worsen. That. Teremka Health doctors advise everyone, even healthy people, to periodically take a general blood test for preventive purposes.
What is hemoglobin
Hemoglobin (from ancient Greek αἷμα - “blood” and Latin globus - “ball”) is a complex iron-containing protein that is responsible for the delivery of oxygen from the lungs to the tissues of the body and for the transport of carbon dioxide in the opposite direction. In vertebrates, hemoglobin is found in red blood cells; in invertebrates, this protein is dissolved in the blood plasma and may be present in other tissues.
An increased level of hemoglobin is usually observed after physical exertion, while at high altitude (in pilots after flights, in residents of high mountains), as well as with the development of erythrocytosis (a disease in which too many red blood cells are produced), with thickening of the blood, congenital heart defects, intestinal obstruction, cardiopulmonary failure. Unbound hemoglobin (that is, not found in red blood cells) is very toxic and causes acute kidney failure.
Low hemoglobin levels, or anemia, occur due to a lack of vitamin B12 or iron, due to large blood loss, or due to blood diseases in which red blood cells are destroyed.
The level of hemoglobin in the blood of people is strictly individual and depends on age, gender and characteristics of the body, however, it is believed that the average hemoglobin level in men is 130-160 g/l, in women - 120-150 g/l. In children, the level of hemoglobin in the blood changes rapidly: one to three days after birth, the indicator is unusually high, 145-225 g/l, and after three to six months it drops to a minimum level of 95-135 g/l. During childhood and adolescence, the level of hemoglobin in the blood gradually rises and reaches normal levels by adulthood.