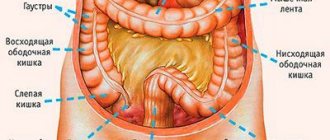
Intestinal neoplasms are often characterized by a long asymptomatic course and manifest themselves only as they grow significantly.
Signs of this disease depend on the type of tumor and location of origin. Thus, tubular adenoma of the rectum can manifest itself as pain and bleeding. When diagnosing such pathologies, it is important to distinguish a benign tumor from oncology.
Introduction
The possibility of endoscopic treatment of early colon cancer has been discussed for a long time. It is a generally accepted fact that tumor removal within healthy tissue is sufficient for cancer limited to the mucous membrane. Opinions on the endoscopic treatment of early invasive colorectal cancer (CRC) range from complete denial of this possibility [2] to assessment of endoscopic removal as radical in all cases where the tumor is removed within healthy tissue [8]. Both of these approaches currently have only historical significance. This review is devoted to modern views on endoscopic treatment of early invasive colon cancer.
How to prevent colorectal cancer?
Rectal cancer can be avoided. The disease develops in the presence of long-existing polyps. Their appearance is asymptomatic, so in order to detect these benign formations in time, people after 45 years of age need to undergo a colonoscopy.
If during the examination the doctor finds polyps in the patient, he will be able to remove them painlessly. After such removal, the patient should undergo a colonoscopy annually to prevent the development of new polyps.
If no formations in the rectum are detected, colonoscopy can be repeated after 10 years. In the interval between these examinations, it is enough to do a blood culture test for occult blood from time to time.
Terminology
Early colon cancer is adnocarcinoma limited to the mucosa or submucosa [7].
According to the revised Vienna classification [4], the concept of early cancer can include points 4-2, 4-3, 4-4 and 5 (Fig. 1).
Vienna classification:
- No intraepithelial neoplasia.
- Doubtful intraepithelial neoplasia.
- Low grade intraepithelial neoplasia.
- Adenoma/dysplasia.
- High-grade neoplasia without invasion (intraepithelial or intramucosal). Adenoma/dysplasia.
- Non-invasive carcinoma.
- Suspicion of invasive carcinoma.
- Intramucosal carcinoma (invasion of the lamina propria).
The macroscopic classification of early colon cancer corresponds to the Parisian classification of epithelial neoplasia shown in Fig. 2.
In both surgical and endoscopic treatment of colon cancer, the tumor must be removed within healthy tissue. However, unlike surgical treatment, during endoscopic mucosal resection, regional lymph nodes cannot be removed. Thus, local tumor removal is indicated only for patients with minimal risk of metastasis to regional lymph nodes.
Treatment
The main treatment method is removal of the tubular adenoma. Sometimes it is also necessary to remove part of the affected intestine.
Possible surgical options:
- Removal of individual intestinal polyps (polypectomy). The indication for such an operation is a polyp size of more than 1 cm. The advantage of such an operation is rapid rehabilitation.
- Laparoscopic removal of polyps is a minimally invasive technique that allows you to quickly and safely remove rectal adenoma.
- Removal of part of the colon and rectum. Such treatment is required in rare cases with severe disease.
Before choosing a surgical technique, a thorough endoscopic and histological examination is performed. If malignant degeneration of the polyp is confirmed, more extensive surgery may be required to completely remove neoplastic cells.
This video will show you how to remove a tubular adenoma of the rectum:
Risk factors
Lymphatic vessels do not extend to the mucous membrane of the colon, but are present only in the submucosal and muscular layers [6]. Thus, non-invasive (i.e., not spreading to the muscular plate of the mucosa) carcinomas cannot metastasize to regional lymph nodes and therefore endoscopic removal is always radical for them (provided that the tumor is locally removed within healthy tissue).
A number of factors are considered as possible indicators of the presence or absence of regional lymph node metastases in early invasive CRC. These are qualitative criteria, such as: the degree of tumor differentiation, the presence or absence of vascular invasion, screenings and the macroscopic type of early CRC. The only quantitative risk factor for lymph node involvement is the depth of submucosal invasion.
Tumor differentiation
It is generally accepted that low tumor differentiation is an independent risk factor for metastasis to lymph nodes and hematogenous dissemination [9, 11, 16, 17]. However, according to some data, it occurs in no more than 10% of cases of early colon cancer and most often in combination with other risk factors [11]. The greatest role is played by tumor differentiation at the site of the deepest semimucosal invasion.
Vascular invasion
Vascular invasion refers to invasion of lymphatic or venous vessels (Fig. 3).
There are two opposing approaches to this issue. Thus, Nedzer believes that venous invasion itself cannot be considered as an isolated factor determining the performance of laparotomy in early CRC [11]. He also believes that isolated lymphatic invasion is rare. There is also an opinion about the difficulty of differential diagnosis between lymphatic and venous invasion or artifacts [18].
According to other data, there is no need to differentiate between venous or lymphatic invasion, and vascular invasion itself is a risk factor for the presence of metastases in the lymph nodes [17]. A similar opinion is shared by Egashira et al. [5] and Yamamoto S., et al. [20].
In our work, we also consider vascular (irrespectively lymphatic or venous) invasion as an independent risk factor for metastasis to the lymph nodes.
Dropouts
Screenings are isolated tumor cells or groups of cells located in otherwise healthy tissues (Fig. 4).
Dropouts can be considered as the initial form of invasion, preceding vascular invasion. It is an independent risk factor for lymph node involvement not only in early but also in advanced CRC [12, 14].
Macroscopic tumor type
A number of researchers believe that flat forms of early CRC are associated with a greater risk of metastasis to lymph nodes than polypoid tumors [10]. However, according to other data, such a relationship is absent [1, 14].
In our practice, we do not consider the macroscopic type as an independent risk factor for metastasis to the lymph nodes, recognizing, however, that tumors with a type 0-IIc component often have a greater depth of invasion into the submucosal layer than other macroscopic types. However, the greater likelihood of metastasis in this case will be associated with a greater depth of invasion, and not with the type of tumor itself.
Depth of invasion
The depth of invasion is the distance from the muscular plate of the mucosa (or its presumed location) to the deepest border of the tumor (Fig. 5).
Nagasako K., reported the absence of metastases to the lymph nodes with invasion into the submucosal layer with a depth of up to 500 µm and a width of up to 2000 µm (invasion of the sm-s type). However, according to other data, even such a minimal degree of invasion can be combined with the presence of metastases to the lymph nodes. Thus, Tanaka S., et al., identified metastases to the lymph nodes in 4% of patients with this type of invasion [15].
Diagnosis of rectal cancer
To detect colorectal cancer in a timely manner, modern medicine uses various types of diagnostics:
- Digital rectal examination.
- Occult blood test (hemoculture test).
- Colonoscopy.
- Ultrasound (ultrasound examination).
- Tumor markers.
- MRI (magnetic resonance imaging).
- Sigmoidoscopy.
- PET (positron emission tomography).
- Rectoscopy.
- CT (computed tomography).
The simplest method is a rectal examination using fingers. The specialist can feel the formations in the intestine with his index finger, assess their nature and take a biopsy.
If rectal cancer is suspected, diagnosis continues. The stool is examined for the presence of occult blood. If it is detected, the patient is referred for a colonoscopy.
This method is the only and most informative way to verify the presence of a tumor in the rectum. An endoscope is inserted into the anus to examine the inner surface of the intestine, previously cleansed with an enema or laxatives. During the procedure, the doctor not only takes tissue samples, but may also remove any polyps found.
When performing a colonoscopy, a specialist can examine the sigmoid colon (sigmoidoscopy) or rectum (rectoscopy).
During an ultrasound, the organ is examined using an ultrasound probe. This examination allows you to find out how far the tumor has penetrated the intestinal wall, whether there are neoplasms in neighboring tissues, or metastases in the lymph nodes.
Tumor markers make it possible to determine the degree of activity of tumor cells.
MRI most accurately shows the picture of the presence of tumor processes in the pelvis. This study is mandatory when confirming the need for chemoradiotherapy before surgery.
A PET scan identifies cancer cells, which are made visible by special agents administered intravenously.
CT scans use contrast agents to help visualize malignant neoplasms in the rectum, as well as detect organs affected by metastases.
Literature
- Ajioka Y., et al. Early Colorectal Cancer with Special Reference to the Superficial Nonpolypoid Type from a Histopathologic Point of View Online publication: 5 June 2000.
- Colacchio TA, et al. Endoscopic polypectomy: inadequate treatment for invasive colorectal carcinoma. Ann Surg 1981;194:704–707.
- Cooper HS, et al. Endoscopically removed malignant colorectal polyps: clinicopathologic correlations. Gastroenterology 995; 108:1657–1665.
- Dixon MF Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002;51:130-131.
- Egashira Y., et al. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol. 2004 May;17(5):503-11.
- Fenoglio CM, et al. Distribution of human colonic lymphatics in normal hyperplastic, and adenomatous tissue. Gastroenterology 1973; 64: 51-66.
- Japanese Research Society for Cancer of the Colon and Rectum (1983) General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Jpn. J. Surg. 13:574.
- Lipper S., et al. The significance of microscopic invasive cancer in endoscopically removed polyps of the large bowel. A clinicopathologic study of 51 cases. Cancer 1983;52:1691–1699.
- Masaki T. Muto T. Predictive value of histology at the invasive margin in the prognosis of early invasive colorectal carcinoma.J Gastroenterol. 2000;35(3):195-200.
- Minamoto T., et al. Early invasive colorectal carcinomas metastatic to the lymph node with attention to their nonpolypoid development. Am. J. Gastroenterol. 1994; 88:1035
- Nedzer P., et al. Risk factor assessment of endoscopically removed malignant colorectal polyps. Gut 1998;43:669-674.
- Okuyama T., et al., Budding as a risk factor for lymph node metastasis in pT1 or pT2 well-differentiated colorectal adenocarcinoma. Dis Colon Rectum 2002;45:628–634.
- Sakuragi M. et al. Predictive factors for lymph node metastasis in T1 stage colorectal carcinomas. Dis Colon Rectum. 2003 Dec;46(12):1626-32.
- Shimomura T., et al. New Indication for Endoscopic Treatment of Colorectal Carcinoma With Submucosal Invasion. J Gastroenterol Hepatol 19(1):48–55, 2004.
- Tanaka S. Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis.J Gastroenterol. 1995 Dec;30(6):710-7.
- Tanaka S., et al. Conditions of curability after endoscopic resection for colorectal carcinoma with submucosally massive invasion. Oncol Rep. 2000 Jul-Aug;7(4):783-8.
- Ueno H., et al. Risk Factors for an Adverse Outcome in Early Invasive Colorectal Carcinoma. Gastroent. 2004;127:385–394.
- Volk E. E., et al. Management and outcome of patients with invasive carcinoma arising in colorectal polyps. Gastroenterology 1995;109:1801–1807.
- Williams B. The Rationale for Current Practice in the Management of Malignant Colonic Polyps. Endoscopy - 1993. - 25.
- Yamamoto S., et al. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004 Jul-Aug;51(58):998-1000.
| back |