Genetic engineering biological therapy (GEBT) for inflammatory bowel diseases (IBD) has become firmly established in the treatment regimens of patients with ulcerative colitis (UC) and Crohn's disease. In recent years, it has come to the realization that drug therapy regimens need to be assessed not only from the point of view of “expensive” or “cheap”, but primarily from the point of view of the ratio of costs, clinical effectiveness and safety. There is a shift in the paradigm of modern healthcare towards a value-based model, when when treating a specific patient with IBD, the focus shifts from cost to the result of treatment.
An integral part of the work of the medical organization, which includes the IBD Center, is the choice of GIBT taking into account costs and effectiveness, timely generation of annual applications according to the true needs of patients with IBD who are registered at the dispensary, targeted justification for the appointment of GIBT taking into account the individual “profile” of the patient. Understanding the safety aspects of new drugs (BD) in the treatment of IBD is important to achieve and maintain clinical results, minimize risks and manage side effects or complications if they occur.
Patients with IBD are known to have an increased risk of infectious complications, including influenza, pneumonia, herpes zoster, Clostridium difficile, tuberculosis, and others, compared with the population without IBD. According to multicenter studies, vedolizumab (Entyvio) is a drug with a minimal risk of activating infection [1–3].
This article describes our own clinical observation of the management of a patient with a long course of total UC, who had a history of pulmonary tuberculosis.
Description of Salofalk candles
These suppositories have an anti-inflammatory effect due to the active substance they contain called mesalazine. At the same time, the medicine Salofalk is available not only in the form of suppositories, but also in the form of tablets, granules and enema.
The dosage form is chosen by the attending physician depending on the extent and location of intestinal damage.
For example, for ulcerative nonspecific colitis, patients are prescribed Salofalk in tablets, for proctitis and proctosigmoiditis - in the form of suppositories and enemas. Sometimes it is recommended to combine suppositories with tablets.
Salofalk suppositories are prescribed for:
- Crohn's disease
- ulcerative colitis
- diverticulitis
They can also be used to prevent colon cancer in people who are prone to this disease.
However, there are a number of contraindications in which you will have to look for an analogue of Salofalk with another active substance.
Among them:
- high sensitivity to salicylic acid
- disorders of the kidneys and liver
- acute peptic ulcer of the stomach and duodenum
- age up to 2 years
As you can see, you cannot use the drug independently. In addition, it is sold in pharmacies only by prescription.
Instructions for use SALOFALK® tablets
General properties of mesalazine
Suction
Mesalazine is absorbed most efficiently in the proximal intestine and least efficiently in the distal intestine.
Distribution
Plasma protein binding of mesalazine and N-Ac-5-ASA is 43% and 78%, respectively. Approximately 1% of an oral dose of mesalazine is excreted in breast milk, mainly as N-Ac-5-ASA.
Metabolism
Mesalazine undergoes first-pass metabolism to form inactive N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA), both in the intestinal mucosa and in the liver. The pattern of acetylation is independent of the patient's acetylation phenotype. Part of mesalazine is also acetylated due to the action of bacterial microflora of the large intestine.
Removal
Mesalazine and its metabolite N-Ac-5-ASA are excreted in excrement (most of it), kidneys (the amount varies from 20% to 50% depending on the route of administration, dosage form and mechanism of release of the active substance) and bile (minor part) . It is excreted by the kidneys mainly in the form of N-Ac-5-ASA.
Specific features of the drug Salofalk® in the form of tablets 250 mg
Distribution
A combined pharmacoscintigraphic/pharmacokinetic study demonstrated that when taken with food (breakfast test), enteric film-coated tablets dissolve in approximately 3-4 hours in the ileum. The average time for evacuation of gastric contents was approximately 3 hours. After approximately 7 hours, the tablets reached the colon. In a subsequent study in volunteers, the evacuation time from the duodenum to the ileum was approximately 3 hours, with the highest concentration of 5-ASA in the intestinal lumen observed 7-8 hours after co-administration of the tablets with a test breakfast. Approximately 75% of the mesalazine dose reaches the large intestine unmetabolized.
Suction
The release of mesalazine from Salofalk® 250 mg enteric film-coated tablets begins after a lag phase of approximately 3-4 hours. The highest plasma concentration is achieved after approximately 5 hours (in the ileum) and with the administration of mesalazine at a dose of 500 mg 3 times/day (3×2 tablets. Salofalk® 250 mg) under the condition of steady-state equilibrium is 2.1±1.7 µg/ml for mesalazine and 2.8±1.7 µg/ml for its metabolite, N-A4-5-ACK.
Removal
During long-term treatment with Salofalk® 250 mg enteric film-coated tablets at a daily dose of 500 mg mesalazine 3 times a day (assuming steady state), the total rate of renal excretion of mesalazine and N-A4-5-ACK was approximately 55% (value obtained within 24 hours after the last dose). The proportion of unmetabolized mesalazine was approximately 5%. T1/2 is 0.7-2.4 hours (on average 1.4±0.6 hours) when using mesalazine at a dose of 500 mg 3 times a day.
Specific features of the drug Salofalk® in the form of tablets 500 mg
Distribution
A combined pharmacoscintigraphic/pharmacokinetic study demonstrated that when taken with food (breakfast test), enteric film-coated tablets dissolve in approximately 3-4 hours in the ileum. After approximately 4-5 hours, the tablets reach the colon. The total transit time in the large intestine is about 17 hours.
Suction
The release of mesalazine from Salofalk® 500 mg enteric film-coated tablets begins after a lag phase of approximately 3-4 hours. The highest plasma concentration is achieved after approximately 5 hours (in the ileum) and with the administration of mesalazine at a dose of 500 mg 3 times/day, subject to steady state equilibrium, is 3±1.6 µg/ml for mesalazine and 3.4±1.6 µg/ml for its metabolite, N-Ac-5-ASA.
Removal
The total rate of renal excretion of mesalazine and N-Ac-5-ASA within 24 hours with repeated doses (1 tablet 500 mg 3 times a day for 2 days; and 1 tablet on the third control day) was approximately 60% ( value obtained within 24 hours after the last dose). The proportion of unmetabolized mesalazine was approximately 10%.
Causes and symptoms of ulcerative colitis
Ulcerative colitis is a chronic disease that affects the lining of the colon. At the same time, it swells, becomes inflamed, and ulcers form on it.
If we talk about the causes of ulcerative colitis, scientists put forward a number of theories. Some are inclined to believe that ulcerative colitis is an infectious disease, but the causative agent has not yet been identified.
Other doctors believe that this disease is autoimmune, that is, the human immune system produces antibodies that destroy the mucous membrane of the colon. And, of course, we must not forget about the genetic factor.
Predisposing factors:
- following a diet low in proteins but rich in carbohydrates
- dysbacteriosis
- stress
- passive lifestyle
According to statistics, people who have had their appendix removed suffer from ulcerative colitis much less often. Nobody knows what this is connected with.
Ulcerative colitis occurs differently in each patient, but common symptoms include: malaise, fever, weakness, cramping abdominal pain, frequent bowel movements (up to 5 times a day). There may be blood in the stool.
As you can see, you shouldn’t hesitate to see a doctor using folk remedies. This is fraught with serious complications that are much more difficult to cure.
ULCERATIVE COLITIS: PHARMACEUTICAL ASPECTS
T. L. Mikhailova, O. V. Golovenko State Scientific Center of Coloproctology, Ministry of Health of the Russian Federation Moscow
Ulcerative colitis (UC) is a chronic recurrent disease of the colon of unknown etiology, characterized by hemorrhagic purulent inflammation of the colon with the development of local and systemic complications.
Accurate data on the prevalence of UC is difficult to obtain, since mild cases of UC, especially in the initial period of the disease, often remain unreported. Patients in these cases are usually observed in non-specialized outpatient facilities and are difficult to account for.
Epidemiology . UC is most common in urbanized countries, particularly in Europe and North America. In these regions, the incidence of UC (primary incidence) ranges from 4 to 20 cases per 100,000 people, with an average of 8-10 cases per year. The prevalence of UC (number of patients) is 40 - 117 patients per 100,000 inhabitants. The largest number of cases occurs between the ages of 20 and 40 years. The second peak of incidence is observed in the older age group - after 55 years. The highest mortality rates are observed during the first year of the disease due to cases of extremely severe fulminant course of the disease and 10 years after its onset due to the development of colorectal cancer in a number of patients.
The role of environmental factors, in particular smoking, remains unclear. Numerous epidemiological studies have shown that UC is more common in non-smokers. This even made it possible to propose nicotine as a therapeutic agent. People who have had an appendectomy have a lower risk of developing UC, as do people who do excessive exercise. Compared to healthy individuals, the diet of patients suffering from UC contains less dietary fiber and more carbohydrates. In the anamnesis of UC patients, cases of childhood infectious diseases are more often observed than in the general population.
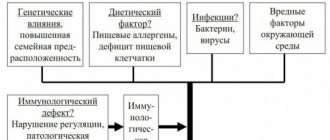
Etiology . The exact etiology of UC is currently unknown. Three main concepts are discussed:
— the disease is caused by the direct influence of some exogenous environmental factors that have not yet been established. Infection is considered the main cause;
— UC is an autoimmune disease. If there is a genetic predisposition of the body, the impact of one or more “trigger” factors triggers a cascade of mechanisms directed against its own antigens. A similar pattern characterizes other autoimmune diseases;
— UC is a disease caused by an imbalance of the immune system of the gastrointestinal tract. Against this background, exposure to a variety of unfavorable factors leads to an excessive inflammatory response, which occurs due to hereditary or acquired disorders in the regulatory mechanisms of the immune system.
Pathogenesis . Numerous mechanisms of tissue and cellular damage are involved in the development of inflammation in UC. Bacterial and tissue antigens cause stimulation of T and B lymphocytes. During exacerbation of UC, a deficiency of immunoglobulins is detected, which facilitates the penetration of microbes and compensatory stimulation of B cells with the formation of immunoglobulins M and G. Deficiency of T suppressors leads to an increase in the autoimmune reaction. Enhanced synthesis of immunoglobulins M and G is accompanied by the formation of immune complexes and activation of the complement system, which has a cytotoxic effect, stimulates the chemotaxis of neutrophils and phagocytes with the subsequent release of inflammatory mediators, which cause the destruction of epithelial cells. Among the inflammatory mediators, the cytokines IL-1b, IF-g, IL-2, IL-4, IL-15 should be mentioned first of all, which affect the growth, movement, differentiation and effector functions of numerous cell types involved in the pathological process in UC. In addition to pathological immune reactions, active oxygen and proteases have a damaging effect on tissues; There is a change in apoptosis, that is, the mechanism of cell death.
An important role in the pathogenesis of UC is played by disruption of the barrier function of the intestinal mucosa and its ability to recover. It is believed that through mucosal defects, a variety of dietary and bacterial agents can penetrate into the deeper tissues of the intestine, which then trigger a cascade of inflammatory and immune reactions.
The patient’s personality traits and psychogenic influences are of great importance in the pathogenesis of UC and the provocation of relapse of the disease. An individual reaction to stress with an abnormal neurohumoral response may be a trigger for the development of the disease. The neuropsychic status of a patient with UC shows features that are expressed in emotional instability.
Clinic . The modern clinical classification of UC takes into account the prevalence of the process, the severity of clinical manifestations, and the nature of recurrence.
According to the length of the process, they are distinguished:
— distal colitis (in the form of proctitis or proctosigmoiditis);
— left-sided colitis (damage to the colon up to the right bend);
- total colitis (damage to the entire colon with involvement in the pathological process in some cases of the terminal segment of the ileum).
Based on the severity of clinical manifestations, the disease is classified into mild, moderate and severe.
Depending on the nature of the recurrence, there may be:
- acute form of ulcerative colitis (first attack);
— fulminant form (usually fatal);
- chronic relapsing form (with repeated exacerbations, often seasonal);
- continuous form of the disease (protracted exacerbation for more than 6 months, subject to adequate treatment).
There is a correlation between the extent of the lesion and the severity of symptoms, which, in turn, determines the volume and nature of treatment.
The diagnosis of UC is formulated taking into account the nature of the course (recurrence) of the disease, the prevalence of the process (distal, left-sided, total colitis), the severity of the disease (mild, moderate, severe), the phase of the disease (exacerbation, remission) with an indication of local and systemic complications. For example: nonspecific ulcerative colitis, total damage, chronic relapsing course, moderate severity.
By the time the diagnosis is made, approximately 20% of patients have total colitis, 30-40% have left-sided lesions, and 40-50% have proctitis or proctosigmoiditis.
The clinical picture of UC is characterized by local symptoms (intestinal bleeding, diarrhea, constipation, abdominal pain, tenesmus) and general manifestations of toxemia (fever, weight loss, nausea, vomiting, weakness, etc.). The intensity of symptoms in UC correlates with the extent of the pathological process in the intestine and the severity of inflammatory changes.
Severe total damage to the colon is characterized by profuse diarrhea mixed with a significant amount of blood in the stool, sometimes blood clots, cramping pain in the abdomen before defecation, anemia, symptoms of intoxication (fever, weight loss, severe general weakness). With this variant of UC, life-threatening complications can develop - toxic megacolon, colon perforation and massive intestinal bleeding. A particularly unfavorable course of UC is observed in patients with the fulminant form of UC. Mortality in severe UC during the first 5 years reaches 40% and half of these cases occur during the first attack.
With an exacerbation of moderate severity, frequent stools up to 5-6 times a day with a constant admixture of blood, cramping abdominal pain, low-grade fever, and fatigue are noted. A number of patients experience extraintestinal symptoms - arthritis, erythema nodosum, uveitis, etc. Moderate attacks of UC in most cases respond successfully to conservative treatment with modern anti-inflammatory drugs, primarily corticosteroids.
Severe and moderate exacerbations of UC are characteristic of total and in some cases left-sided lesions of the colon. Mild attacks of UC with total damage are manifested by a slight increase in stool frequency and a slight admixture of blood in the stool.
In the clinical picture of patients with proctitis and proctosigmoiditis, very often it is not diarrhea that manifests itself, but constipation and false urge to defecate with the release of fresh blood, mucus and pus, and tenesmus. If the transit of intestinal contents through the inflamed distal sections of the colon is accelerated, then stasis is observed in the proximal segments. Constipation in distal colitis is associated with this pathophysiological mechanism. Patients may not notice blood in the stool for a long time; the general condition deteriorates unnoticeably, the ability to work is preserved. This latent period from the onset of UC to diagnosis can be very long - sometimes up to several years.
Complications of UC . With UC, a large number of different complications are observed. They can be divided into two categories - local and systemic.
Local complications include colonic perforation, acute toxic dilatation of the colon (or toxic megacolon), massive intestinal bleeding, and colon cancer.
Acute toxic dilatation of the colon is one of the most dangerous complications of UC. It develops as a result of a severe ulcerative-necrotic process and associated toxicosis. Toxic dilatation is characterized by expansion of a segment or the entire affected bowel during a severe attack of UC. Patients with toxic dilatation of the colon in the initial stages require intensive conservative therapy. If it is ineffective, surgery is performed.
Perforation of the colon is the most common cause of death in the fulminant form of UC, especially with the development of acute toxic dilatation. Due to an extensive ulcerative-necrotic process, the wall of the colon becomes thinner, loses its barrier functions and becomes permeable to a variety of toxic products located in the intestinal lumen. In addition to stretching the intestinal wall, microcirculation disturbances and proliferation of bacterial flora, especially Escherichia coli with pathogenic properties, play a decisive role in the occurrence of perforation. In the chronic stage of the disease, this complication is rare and occurs mainly in the form of a pericolytic abscess. Treatment for perforation is surgical only.
Massive intestinal bleeding is relatively rare and, as a complication, is a less complex problem than acute toxic dilatation of the colon and perforation. In most patients with bleeding, adequate anti-inflammatory and hemostatic therapy allows surgery to be avoided. With ongoing massive intestinal bleeding in patients with UC, surgical intervention is indicated.
The risk of developing colon cancer in UC increases sharply with a disease duration of more than 10 years, if colitis began before the age of 18 years and especially 10 years.
Systemic complications in UC are otherwise called extraintestinal manifestations. Patients may experience damage to the liver, oral mucosa, skin, and joints. The exact genesis of extraintestinal manifestations is not fully understood. Their formation involves foreign agents, including toxic agents entering the body from the intestinal lumen, and immune mechanisms. Erythema nodosum occurs not only as a reaction to taking sulfasalazine (associated with sulfapyridine), but is observed in 2 - 4% of patients with UC or CD and is not related to taking the drug. Pyoderma gangrenosum is a fairly rare complication and is observed in 1 - 2% of patients. Episcleritis occurs in 5 - 8% of patients with exacerbation of UC; acute arthropathy - in 10 - 15%. Arthropathy manifests itself as asymmetric damage to large joints. Ankylosing spondylitis is detected in 1 - 2% of patients. Liver lesions, according to our data, are observed in 33.3% of patients with UC and CD, manifesting in the majority either a transient increase in the level of transaminases in the blood or hepatomegaly. The most characteristic serious hepatobiliary disease in UC is primary sclerosing cholangitis, which is a chronic stenotic inflammation of the intra- and extrahepatic bile ducts. It occurs in approximately 3% of patients with UC.
Treatment . Therapeutic tactics for UC are determined by the localization of the pathological process in the colon, its extent, the severity of the attack, and the presence of local and/or systemic complications. Conservative therapy for UC is aimed at quickly stopping the attack, preventing relapse of the disease and progression of the process. Distal forms of UC - proctitis or proctosigmoiditis - are characterized by a milder course, therefore they are most often treated on an outpatient basis. Patients with left-sided and total lesions are usually treated in a hospital, since the course of the disease in them is characterized by greater severity of clinical symptoms and greater organic changes.
The food of patients should be high in calories and include foods rich in proteins, vitamins, limiting animal fats and excluding coarse plant fiber. Recommended are low-fat fish, meat (beef, chicken, turkey, rabbit), boiled or steamed, pureed cereals, potatoes, eggs, dried bread, walnuts. Raw vegetables and fruits are excluded from the diet, as they contribute to the development of diarrhea. Patients often have lactase deficiency, so dairy products are added only if they are well tolerated. These recommendations correspond to diets 4, 4B, 4B of the Institute of Nutrition of the Russian Academy of Medical Sciences.
All medications used in treatment regimens for UC can be divided into two large groups. The first combines basic anti-inflammatory drugs and includes aminosalicylates, that is, drugs containing 5-aminosalicylic acid (5-ASA, mesalazine), corticosteroids and immunosuppressants. All other drugs play either an auxiliary role in the treatment of UC or are at the stage of clinical study.
The first drug containing 5-ASA was sulfasalazine (salazosulfapyridine), which was introduced into clinical practice in 1942. Sulfasalazine consists of two components linked by a nitrogen bond - sulfapyridine sulfanilamide and 5-ASA. It has been proven that only 5-ASA has an anti-inflammatory effect. Sulfapyridine was forced into the composition of the sulfasalazine molecule, since “pure” 5-ASA is well absorbed in the small intestine, and in the mucous membrane it turns into an inactive metabolite - N-acetyl - 5-ASA. In sulfasalazine, sulfapyridine acts exclusively as a “carrier” that allows delivery of 5-ASA to the affected areas of the colon. Under the influence of colonic microflora, the nitrogen bond is destroyed. Sulfapyridine is absorbed in the colon, undergoes detoxification in the liver through acetylation and is excreted in the urine, and 5-ASA, in contact with the mucous membrane, has an anti-inflammatory effect.
The mechanisms by which 5-ASA exerts its anti-inflammatory effect are not fully understood. Nevertheless, numerous effects are known due to which mesalazine inhibits the development of inflammation. Thus, by inhibiting cyclooxygenase, mesalazine inhibits the formation of prostaglandins. The lipoxygenase pathway of arachidonic acid metabolism is also suppressed, and the release of leukotriene B4 and leukotriene sulfopeptide is inhibited.
At high concentrations, mesalazine can inhibit certain functions of neutrophil granulocytes in humans (eg, migration, degranulation, phagocytosis, and the formation of toxic oxygen free radicals). In addition, mesalazine inhibits the synthesis of platelet activating factor (PAF). Due to its antioxidant properties, mesalazine is able to scavenge free oxygen radicals.
Mesalazine effectively inhibits the formation of cytokines - interleukin-1 and interleukin-6 (IL-1, IL-6) in the intestinal mucosa, and also suppresses the formation of interleukin-2 receptors. Thus, mesalazine interferes directly with immune processes.
It has been shown that the “ballast” component sulfapyridine is mainly responsible for the entire incidence of side effects of sulfasalazine. Literature data on the frequency of side effects caused by sulfasalazine range from 5 to 55%, averaging 21%. In addition to nausea, headache, male infertility, anorexia, dyspeptic disorders, hematological reactions (leukopenia and hemolytic anemia) and hypersensitivity reactions with multiple organ lesions occur.
In order to maintain the anti-inflammatory activity inherent in sulfasalazine, while at the same time avoiding the side effects associated with the sulfapyridine component, preparations containing “pure” 5-ASA have been developed in recent years. An example of a new generation of aminosalicylates is the drug Salofalk, developed by the German pharmaceutical company Doctor Falk Pharma. The drug is available in three dosage forms - tablets, suppositories and microenemas. In tablets, mesalazine is protected from contact with gastric contents using a special acid-resistant polymer shell, which dissolves at pH levels above 6.5. These pH values are usually recorded in the lumen of the ileum. After dissolution of the membrane, a high concentration of the active anti-inflammatory component (mesalazine) is created in the ileum and colon. The choice of a specific dosage form of Salofalk is determined by the extent of the zone of inflammation in the colon. For proctitis, it is advisable to use suppositories, for left-sided lesions - microenemas, and for total colitis - tablets.
The daily dose of aminosalicylates is determined by the severity of the UC attack and the nature of the clinical response to the drug. To relieve acute and moderate attacks of UC, 3-4 g of sulfasalazine or 1.5-2 g of mesalazine are usually prescribed daily, divided into 3-4 doses. In the absence of a good clinical response, the daily dose of mesalazine can be doubled, but it is usually not possible to increase the daily dose of sulfasalazine above 4 g due to the development of severe side effects.
Sulfasalazine blocks the conjugation of folic acid in the brush border of the jejunum, inhibits the transport of this vitamin, and inhibits the activity of the enzymatic systems associated with it in the liver. Therefore, the treatment complex for patients with UC receiving treatment with sulfasalazine must include folic acid in a dose of 0.002 x 3 times a day.
It usually takes 3 to 6 weeks to stop an attack of UC. After this, anti-relapse treatment is carried out with sulfasalazine - 2 g per day or mesalazine - 1.5 g per day.
Of the modern drugs for the treatment of proctosigmoiditis and left-sided colitis, the Salofalk suspension is most often used. Disposable containers contain 4 g of mesalazine in 60 ml of suspension or 2 g of mesalazine in 30 ml of suspension. The drug is administered into the rectum 1 - 2 times a day. The daily dose is 2 - 4 g, depending on the severity of the process in the intestine. If the length of the inflammatory process in the rectum is no more than 12 from the edge of the anus, it is advisable to use Salofalk suppositories. The usual daily dose in these cases is 1.5 - 2 g.
When using aminosalicylates, it is possible to achieve remission in 75 - 80% of cases of UC.
The most effective anti-inflammatory drugs in the treatment of UC remain steroid hormones, which in severe forms of the disease are superior in activity to aminosalicylates. Corticosteroids accumulate in inflammatory tissue and block the release of arachidonic acid, preventing the formation of prostaglandins and leukotrienes, which cause the inflammatory process. By blocking chemotaxis, steroid hormones indirectly exhibit an immunomodulatory effect. The effect on tissue fibrinolysis leads to a decrease in bleeding.
Indications for steroid therapy are:
- acute severe and moderate forms of the disease and the presence of extraintestinal complications;
— left-sided and total forms of ulcerative colitis with severe and moderate course in the presence of III degree of activity of inflammatory changes in the intestine (according to endoscopic examination);
- lack of effect from other treatment methods for chronic forms of UC.
The table shows the treatment regimen for UC depending on the severity of the disease and the extent of the process in the intestine (Table 1).
Table 1 Drug treatment for ulcerative colitis
Prevalence of lesions
| Severe form | Moderate form | Light form | |
| Total defeat | Hydrocortisone intravenously, intramuscularly 500 mg/day (125 mg 4 times) for 5-7 days, then Prednisolone 1-1.5 mg/kg body weight per day | Hydrocortisone intramuscularly 250-375 mg/day (125 mg 2-3 times) or Prednisolone 1-1.5 mg/kg body weight per day | Salofalk 1.5-2g per day (in persistent cases - 3-4g) or Sulfasalazine 4 - 6g per day; if there is no effect - Prednisolone 0.5-1.0 mg/kg body weight per day |
| Left-sided lesion | Hydrocortisone intramuscularly 250-375 mg/day (125 mg 2-3 times) for 5-7 days or Prednisolone 1-1.5 mg/kg body weight daily | Prednisolone 1-1.5 mg/kg body weight per day, or Salofalk 4g per day orally or rectally (simultaneous administration of tablets and rectal administration of suspension or suppositories is possible) | Salofalk suspension rectally – 30-60ml (2-4g Mesalazine), or Sulfasalzine 4g per day + Hydrocortisone rectally 65-250 mg/day |
| Distal lesion (proctitis, proctosigmoiditis) | Prednisolone 0.5-1.0 mg/kg body weight daily, | Rectally Salofalk suspension - 30-60 ml (2-4 g of Mesalazine), or Salofalk suppositories 3-4 g (if the extent of the process in the rectum is up to 12 cm); sulfasalzine 4-6 g per day + hydrocortisone rectally 65 mg 2 times a day. | Rectally Salofalk suspension - 30-60 ml (2-4 g of Mesalazine) or Salofalk suppositories 1.5-2 g (with the length of the process in the rectum up to 12 cm); Hydrocortisone rectally 125 mg at night or suppositories with Prednisolone 5 mg 2 times a day. |
In acute severe form of UC or severe attack of chronic forms of the disease, treatment should begin with intravenous administration of hydrocortisone at least 500 mg per day, evenly distributed over 4 - 6 injections with simultaneous correction of water and electrolyte disturbances, administration of blood and blood substitutes and, if possible, hemosorption with in order to quickly eliminate endotoxemia. Hydrocortisone suspension can also be administered intramuscularly, however, the duration of such administration is limited to 5-7 days due to the possible development of abscesses at the injection sites with longer administration and possible fluid retention. After 5 - 7 days, you should switch to oral Prednisolone. During this time, gastroscopy is performed to exclude gastric and duodenal ulcers. In case of a moderate form and the absence of clinical signs and anamnestic indications of gastroduodenal ulcers, treatment should immediately begin with oral Prednisolone. Typically, Prednisolone is prescribed at a dose of 1-1.5 mg/kg body weight per day. A dose of 100 mg should be considered the maximum.
If hormonal drugs are well tolerated, it is recommended to take the prescribed dose until a lasting positive result is obtained - within 6 - 8 weeks. After this, a reduction is carried out according to the so-called “fast stepwise” scheme: by 10 mg every 3 to 5 days. Starting from 30 - 40 mg, a single dose of Prednisolone in the morning is recommended, which practically does not cause serious complications. At the same time, Salofalk or Sulfasalazine are included in the treatment regimen, which should be taken until the hormones are completely withdrawn. Starting from 30 mg, Prednisolone is withdrawn more slowly - 5 mg per week for 2 - 2.5 months. Thus, the full course of hormonal therapy lasts from 8 to 16 weeks, depending on the form of UC.
In distal forms of the lesion and I - II degrees of process activity according to sigmoidoscopy data, Hydrocortisone should be prescribed rectally by drip or in microenemas. Moreover, if patients do not hold large volumes well, then the administration of Hydrocortisone (65 - 125 mg) should be started in 50 ml of saline and, as the inflammation subsides and the frequency of false urges decreases, gradually increase the volume to 200 - 250 ml per therapeutic enema. The drug is usually administered after bowel movements in the morning or before bed.
For ulcerative proctitis and sphincteritis, suppositories with Prednisolone (5 mg), administered 3-4 times a day, have a fairly good effect. In more severe distal forms, accompanied by fever, general weakness, anemia and III - IV degree of activity according to rectoscopy, in cases of no effect from Sulfasalazine or Salofalk, treatment with Prednisolone orally at a dose of 30 - 50 mg per day is indicated.
In middle-aged and elderly patients, the dose of Prednisolone should not exceed 60 mg, since they are characterized by the presence of concomitant diseases: atherosclerosis, hypertension, diabetes mellitus, etc. In cases where UC occurs against the background of atherosclerotic lesions of the mesenteric arteries, the treatment complex Vascular drugs should be administered: Trental, Prodectin, etc.
Hormone therapy is associated with the development of side effects: retention of fluid, chlorides and sodium in tissues (edema is possible), arterial hypertension, hypokalemia, calcium loss, osteoporosis, various autonomic disorders, carbohydrate metabolism disorders, adrenal insufficiency, stomach ulcers, gastrointestinal bleeding . In these cases, it is recommended to prescribe adequate symptomatic therapy: antihypertensive drugs, diuretics, calcium supplements, antacids. If carbohydrate metabolism is disturbed, a diet with limited carbohydrates is necessary; according to indications, fractional administration of insulin (according to glycemia) or oral antidiabetic medications are prescribed. To prevent the development of thrombosis in patients with severe forms of UC receiving hormonal treatment, constant monitoring of the blood coagulation system should be carried out and at the same time antiplatelet agents should be prescribed: Curantil, Prodectin, etc.
ACTH-zinc phosphate is effective only in the acute form of UC, since its effect is mediated by the preserved function of the own adrenal glands. The drug is administered intramuscularly at a dose of 20–40 mg, depending on the severity of the attack.
In recent years, in the treatment of inflammatory bowel diseases, especially CD, drugs containing the glucocorticosteroid (GCS) budesonide as an active component have been actively used. Unlike traditional corticosteroids, budesonide has a very high affinity for receptors and high metabolism in the liver during the “first pass” (about 90%). Due to this, it has a very powerful local anti-inflammatory effect with a minimum number of systemic side effects. As an alternative to Prednisolone and Hydrocortisone, the drug Budenofalk can be recommended. When developing the structure of Budenofalk, the physiological characteristics of the gastrointestinal tract were taken into account. Each capsule of Budenofalk contains about 350 microspheres consisting of budesonide, coated with a polymer shell that is resistant to the action of gastric juice. The release of budesonide from microspheres occurs in the ileum and colon at pH values > 6.4. Budenofalk is used to treat mild and moderate exacerbations of UC. The recommended daily dose is one capsule of Budenofalk containing 3 mg of budesonide, 3 times a day.
For the treatment of extremely severe forms of UC that are responsive to corticosteroid therapy, the immunosuppressant Cyclosporin A has recently been successfully used, which is administered intravenously (daily dose 4 mg/kg body weight). Therapy with Cyclosporine A avoids colectomy in 40 - 50% of patients. Widespread use of the drug is limited by its high cost and significant number of side effects.
In chronic continuous course of UC and in cases of hormonal dependence, it is advisable to use Azathioprine (1.5 – 2.5 mg/kg body weight per day). It has been shown that the use of azathioprine or 6-mercaptopurine in these clinical situations achieved a positive effect (remission, dose reduction or complete withdrawal of corticosteroids) in almost 60% of patients.
Violation of the barrier functions of the colon in UC may cause the development of toxemia syndrome. To correct it, it is necessary to prescribe an appropriate complex, restore eubiosis, antibacterial therapy, hemosorption, and ultraviolet irradiation of autologous blood.
Considering the pronounced metabolic disorders and catabolic effect of steroid hormones, parenteral administration of protein preparations is advisable: serum albumin, plasma protein, essential amino acids.
To improve the processes of microcirculation and transcapillary exchange, the administration of Reopoliglucin, Hemodez (in normal dosages) is indicated.
In case of anemia (hemoglobin 90 g/l and below), which is a sign of a severe attack of UC, it is recommended to carry out blood transfusion of 250 ml of single-group blood with an interval of 3 - 4 days. When the level of iron in the blood serum decreases, it is necessary to include iron supplements in the treatment complex.
Considering the immunological disorders in UC, immunomodulators, Levamisole, Timalin, etc., are used in the treatment of the disease. However, their role in UC is not completely clear, the therapeutic effect of their use is short-term, so the activity of these drugs as basic agents is questionable.
It is advisable to recommend the use of immunomodulators in combination with basic anti-inflammatory treatment.
Vitamins of group B, C, A, D, K are prescribed, which also help restore eubiosis in the intestines.
The treatment complex includes psychotropic drugs in usual dosages, focusing on individual tolerance.
Exacerbation of UC in some cases is accompanied by irritable bowel syndrome, most often manifested by constipation. In this case, the prescription of wheat bran or patented preparations containing ballast substances (Mukofalk, etc.), which help normalize stool and at the same time act as enterosorbents, is justified.
Inpatient treatment ends when clinical-endoscopic remission is achieved, after which the patient is subject to dispensary observation in a clinic with a general practitioner, gastroenterologist or proctologist.
Anti-relapse treatment for UC. The question of the nature and duration of anti-relapse treatment for UC remains unresolved. According to one point of view, anti-relapse treatment is recommended for life, however, given the high cost of drugs (Table 2) and the risk of side effects with their long-term use, the gastroenterology department and State Research Center for Coloproctology adhere to the following tactics. After stopping the attack of UC, a maintenance dose of aminosalicylates is recommended for a period of 6 months. If during this period of time there were no clinical signs of exacerbation of the disease, and a control endoscopic examination after six months shows remission, anti-relapse treatment can be discontinued. If during the course of anti-relapse therapy the patient’s condition was unstable, sometimes it was necessary to increase the dose of aminosalicylates to eliminate the symptoms of exacerbation, and control endoscopy revealed signs of active inflammation, anti-relapse treatment must be extended for another six months. Patients with chronic continuous course of UC require long-term continuous treatment, usually with high doses of aminosalicylates, but this therapy is not “anti-relapse” in the full sense of the word. It is more of a restraining anti-inflammatory treatment. In this category of patients, cytostatics (azathioprine or 6-mercaptopurine) and intermittent corticosteroid regimens are also widely used.
table 2
| Name of the drug | Manufacturer | Cost (in rubles) |
| Sulfasalazine | "Slovenia" | Tablets 50pcs*500mg = 195 rub. — 359 rub. |
| Salofalk | "Doctor Falk" (Germany) | Tablets 100 pcs * 250 mg = 580 rub. 00 kopecks – 980 rub. 00kop |
| — “ — | — “ — | Suspension 2 g = 500 rub. 1036 rub. 4 years -= 1300 rub. 2179 rub. |
| Folic acid | Russia | Tablets 100 pcs * 400 mcg = 155 rub. |
| Hydrocortisone | Gideon Richter (Hungary) | Injections 2.5% (2 ml) 5*2=109 rub. – 123 rub. |
| Prednisolone | Hafslund | Tablets 30pcs*5 mg = 40 rub. – 70 rub. 00 kop. |
| Glucocorticoid (budesonide) | Poznan-FZ-Polfo | Aerosol 1 pc*0.2 mg (10 ml)= 195 rub. – 372 rub. |
| Reopoliglyukin | Belarus | Injections = 51 rub. — 80 rub. |
| Hemodez Mukofalk | Russia "Doctor Falk" Germany | Injections 200 ml – 24 rub. 400 ml. – 59 rub. 150 g - 191 rub. –212 rub. |
Source of publication: www.webmed.ru
Crohn's disease: causes and symptoms
Chronic inflammatory Crohn's disease is characterized by the fact that it affects the entire gastrointestinal tract, starting from the oral cavity and ending with the anus. In this case, as a rule, inflammation begins in the ileum, and then spreads to the rest of the intestine.
Symptoms of inflammation of the ileum are identical to those of acute appendicitis. Therefore, often, when doctors begin to operate on a patient, they discover Crohn’s disease and make the correct diagnosis.
As for the causes of this disease, doctors are inclined to believe that it is infectious in nature, since antibiotics cope well with it.
Predisposing factors:
- transmission of measles
- food allergy
- stress
- smoking
- heredity
The clinical picture includes local, general and extraintestinal symptoms of Crohn's disease.
Clinical case
Patient L., born in 1981. The disease debuted at the age of 14 years with diarrhea mixed with blood 5-6 times a day. With suspicion of UC, she was observed by a pediatric gastroenterologist. At the age of 16 years, the diagnosis was verified in the gastroenterology department of the Republican Clinical Hospital in Cheboksary. The colonoscopy protocol (CS) from 1998 concluded: “The mucous membrane of the rectum is edematous, brightly hyperemic, with a white coating. The mucous membrane of the sigmoid colon is brightly hyperemic and granular. At a distance of 25-30 cm from the anus, a group of erosions measuring 0.1x0.2x0.4 cm, some with fibrin deposits. Proximal to the splenic angle of the colon, the mucous membrane is edematous, hyperemic, eroded, and moderate contact bleeding is noted.” A blood test taken one day before the CS revealed mild normochromic anemia (hemoglobin - 115 g/l), neutrophilic leukocytosis (12.6·109/l), ESR accelerated to 30 mm/h and a twofold increase in the level of C- reactive protein.
Based on the given clinical and laboratory data and anamnesis data (the patient had more than six-month periods of remission), the following clinical diagnosis was formulated: “Ulcerative colitis, chronic relapsing course, total (according to the Montreal classification), moderate attack (according to the Truelove-Witts criteria ), moderate endoscopic activity (according to Schroeder)."
Taking into account the total localization of UC of III degree of activity and moderate severity, therapy with sulfasalazine was started at a dose of 3.0 g/day in combination with glucocorticoids (GCS) 60 mg/day. Clinical improvement was achieved, and GCS was gradually completely discontinued. Until 2004, she regularly took sulfasalazine 2.0 g/day or salofalk 3.0 g/day and topical salofalk suppositories 2 times a day. GCS was periodically added to treatment at a starting dose of 60 mg/day with complete withdrawal when clinical remission was achieved.
The endoscopic picture in 2004 was described as follows: “The mucous membrane of the rectum, sigmoid and colon is moderately edematous, granular, with foci of hyperemia. The Bauginian valve is pink, elastic. The mouth of the appendix has no signs of inflammation. The mucous membrane of the cecum is edematous, with an enhanced vascular pattern.”
In 2004, the patient became pregnant. At 11 weeks, an exacerbation of the disease developed: diarrhea with blood appeared up to 10 times a day. Oral prednisolone at a dose of 40 mg and intravenous infusion of fresh frozen plasma were prescribed. The condition was relatively stabilized, but at 28 weeks an attack of urolithiasis occurred, for which ureteral catheterization was performed. Subsequently, the threat of miscarriage remained, and premature birth occurred at 34 weeks.
In accordance with the recommendations of the Russian Gastroenterological Association and the Russian Association of Coloproctologists for the diagnosis and treatment of adult patients with UC, pregnancy should be planned during the period of remission. However, most drugs for the treatment of UC are associated with a low risk of adverse effects on the fetus (with the exception of methotrexate). Side effects of GCS can be reduced by concomitant calcium intake, proton pump inhibitors, and monitoring of blood glucose levels [4].
Until 2008, the patient felt satisfactory and remained in clinical remission. In 2009, another exacerbation developed: diarrhea up to 12 times a day mixed with blood, pus, mucus, and abdominal pain. The dose of prednisolone was increased to 60 mg/day, metronidazole 1.0 g/day was added. When trying to reduce the dose of GCS to the daily dose (10 mg), all symptoms of the disease returned within a few days. Thus, the patient developed hormone dependence. To overcome it, azathioprine was added to treatment at a dose of 150 mg/day.
The literature provides information about an increase in the risk of developing opportunistic infections during the use of GCS by 2.2 times, immunosuppressive therapy by 3.4 times, and with their combined use by 17.5 times [5, 6].
During therapy in 2009, the patient developed infiltrative tuberculosis of the apex of the left lung, which required discontinuation of the cytostatic drug and anti-tuberculosis treatment according to a multicomponent regimen with rifampicin for 1 year. However, it was not possible to completely cancel the GCS, and the patient continued to receive prednisolone 10 mg/day until 2014. In June 2014, she was consulted at the Federal State Autonomous Educational Institution of Higher Education “Russian National Research Medical University named after. N.I. Pirogov" (Moscow). The previously established diagnosis was confirmed, and it was recommended to resume therapy with azathioprine 100 mg/day in combination with methylprednisolone 16 mg/day. It was possible to achieve some clinical improvement in the course of the disease, but complications of the therapy began to arise (boil of the facial skin, boil of the labia majora). Since October 2014, aching pain along the colon has resumed, most pronounced in the left iliac region, decreasing after the passage of gas and defecation, rumbling and transfusion in the abdomen, increased gas formation. Loose stools were noted up to 7 times a day mixed with pus, blood, mucus, with the remains of undigested food, and there were false urges and tenesmus. In addition, the patient noted severe general weakness (she lost 5 kg in 2 months) and arthralgia in the large joints of the lower extremities. The patient independently stopped taking azathioprine, which led to a severe exacerbation of the disease. Over the next year, she was hospitalized several times. At that time, laboratory data showed hypochromic anemia (hemoglobin - 105 g/l), neutrophilic leukocytosis (14.6·109/l), ESR - 36 mm/h and a twofold increase in the level of C-reactive protein. An endoscopic examination revealed destructive changes in the mucous membrane, purulent plaque, many erosions and ulcers up to 4-5 mm in size, moderate contact and spontaneous bleeding. Prednisolone 125 mg was administered intravenously for 7 days, followed by a transition to oral administration at a dose of 75 mg with a gradual reduction to a daily dose of 40 mg and the addition of sulfasalazine 2.0 g/day. Infusions of metronidazole 500 mg/day were performed and electrolyte disturbances were corrected. Treatment was continued on an outpatient basis with aminosalicylic acid at a dose of 3.0 g/day in combination with budesonide at a dose of 9 mg/day. When a relatively stable state is achieved, rifaximin 800 mg/day for 7 days in combination with bifiform is recommended.
In May 2022, the condition worsened again: loose stools 14 times a day in small volumes with mucus and blood, more often at night, bloating, tenesmus, periodic aching or spasmodic pain of low intensity in the left half of the abdomen, sometimes worsening after defecation, arthralgia, weakness, lack of appetite, thirst, insomnia. Low-grade body temperature persisted, anemia increased (hemoglobin - 95 g/l), neutrophilic leukocytosis (15.6·109/l), ESR - 53 mm/h, the level of C-reactive protein increased threefold. In addition, there was an increase in pancreatic amylase to 51 U/l (reference values - up to 50 U/l) and type 1 elastase to 26 μg/l (reference values - up to 3.5 μg/l). The level of fecal calprotectin exceeded the norm by almost 20 times and reached 980 mcg/g.
During colonoscopy, the mucous membrane in all parts of the colon is sharply swollen with an abundance of acute ulcers of varying sizes (some of the ulcers with traces of ongoing bleeding) and acute erosions, numerous submucosal hemorrhages. Severe contact bleeding, multiple polypoid formations in the sigmoid, rectum, and transverse colon.
For differential diagnosis with Crohn's disease, a study was carried out for antineutrophil cytoplasmic antibodies (pANCA), IgG/IgA, antibodies to Saccharomyces cerevisiae (ASCA), IgG/IgA, antibodies to intestinal goblet cells, antibodies to the exocrine pancreas. These antibodies were not detected in the patient's blood.
Complex therapy was prescribed (GCS, metronidazole, sulfasalazine), but it was not possible to achieve remission and overcome hormonal dependence. Since August 2022, azathioprine was resumed, but the clinical and endoscopic activity of the process remained. In accordance with clinical recommendations, in case of relapse that occurs during maintenance therapy with thiopurines, re-prescription of corticosteroids is indicated, however, in order to avoid the formation of steroid dependence, the prescription of biological drugs is considered more appropriate [4].
Considering the ineffectiveness of previous therapy with immunosuppressants, the high degree of inflammatory activity of the disease, and the formation of hormone dependence, it was decided to begin selecting a biological drug for the patient. Before starting anti-cytokine therapy, it is necessary to screen for the presence of tuberculosis infection, which includes: a thorough medical history, x-ray (not fluorography!) of the chest in two projections, a Mantoux test with intradermal administration of 2 tuberculin units, and, if necessary, a quantiferon test or Diaskintest [7].
Biological drugs - tumor necrosis factor alpha (TNF-α) blockers - infliximab, adalimumab, golimumab have a fairly wide range of indications [8]. In addition to inflammatory bowel diseases, they are used for rheumatoid and psoriatic arthritis, ankylosing spondylitis, non-infectious uveitis, and psoriasis [9–12]. Preparations containing monoclonal antibodies to TNF-α lead to a certain decrease in the level of the body’s immune defense and increase its susceptibility to various infections, including tuberculosis, and increase the risk of destruction of tuberculous granuloma and reactivation of latent tuberculosis [4]. The patient already had a history of infiltrative tuberculosis, so it was decided to refrain from using this group of drugs.
Vedolizumab (Entyvio) is the only biological drug registered in the Russian Federation for the treatment of IBD; it selectively blocks inflammation in the intestine [11]. It is a humanized monoclonal IgG antibody that binds to α4β7 integrin on the surface of lymphocytes and selectively blocks its interaction with the cell adhesion molecules MADCAM-1 of the intestinal mucosa. It is these molecules that play a leading role in the migration of T-helper lymphocytes, which cause the chronic inflammatory process characteristic of UC and Crohn’s disease [11].
Safety data over 4,800 patient-years of exposure demonstrate no increased risk of infections, including severe infections, with vedolizumab. The frequency of adverse events when used in standard doses is comparable to placebo, which allows long-term use of the drug [13]. The probability of stable remission after a course of administration of vedolizumab, all other things being equal, is 2-4 times higher than that with the use of drugs from the TNF-α group for moderate and severe UC in case of resistance to standard therapy [14]. The likelihood of vedolizumab discontinuation due to adverse reactions is 4.8-7 times lower than with adalimumab or golimumab therapy during 52-week maintenance therapy in patients receiving treatment with monoclonal antibody drugs for the first time [14].
Clinical trials and post-marketing data showed that the incidence of opportunistic infections and tuberculosis in patients receiving vedolizumab was low. No viral reactivation of hepatitis B or C has been reported [15]. The high selectivity of the immunosuppressive effect of vedolizumab allows it to be prescribed even to patients with an increased risk of developing opportunistic infections or the elderly [16]. A similar trend is observed with regard to the incidence of neoplasms: vedolizumab has been proven to have a favorable safety profile with low incidence of malignant neoplasms over a long period of treatment [17].
This patient had the following indications for the use of this drug: moderate and/or severe active UC, inadequate response and/or treatment failure (or decreased effectiveness), intolerance to one or more drugs of standard therapy [11].
After screening for tuberculosis, yersiniosis, and viral hepatitis, induction therapy with vedolizumab was started in November 2022 and azathioprine was continued at a dose of 150 mg/day.
Vedolizumab (Entyvio) is administered intravenously at a dose of 300 mg over 30 minutes, then at the same dose 2 weeks and 6 weeks after the first administration, then every 8 weeks [11]. According to the literature, the therapeutic effect appears after the first use of vedolizumab [18].
The patient tolerated the drug infusion satisfactorily. Signs of a significant improvement in well-being and regression of symptoms were noted after 3 weeks (after the second injection): tenesmus disappeared, body temperature normalized, the frequency of stool and the amount of pathological impurities in it decreased, and weakness became significantly less disturbing.
From the fourth week of therapy, the patient noted a significant improvement: stools were without pathological impurities, with a frequency of no more than three times a day, sleep and appetite normalized, and weakness decreased. Clinical improvement was confirmed by laboratory data: the hemoglobin level increased to 110 g/l, the severity of leukocytosis decreased to 9.0 × 109/l, ESR (13 mm/h), levels of pancreatic amylase and C-reactive protein normalized. Fecal calprotectin decreased from 890 to 90 mcg/g (reference values - up to 50 mcg/g). Endoscopically: the rectum is straightened, there is cloudy mucus on the walls, the mucous membrane is swollen, with areas of atrophy and scars.
To date, the patient has received 7 infusions of Entyvio 300 mg every 8 weeks. Clinical response and remission were assessed according to generally accepted standards widely used in IBD clinical trials. Clinical response was defined as a decrease of ≥3 points in the Harvey-Bradshaw Index (HBI) and an absolute Simple Clinical Colitis Activity Index (SCCAI) score of ≤2 at follow-up compared with baseline. Clinical remission was defined as SCCAI ≤2. No adverse events associated with receiving the drug were noted; the development of opportunistic infections, tuberculosis, and oncological processes was not registered. The patient's condition is assessed as good. Complete clinical endoscopic remission was achieved.