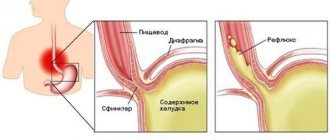
Dysbacteriosis in newborns is quite common. A baby is born and immediately encounters countless microorganisms. His health largely depends on which bacteria will first occupy a place in his mouth, intestines, and genitourinary tract.
It’s good if doctors put the baby to the mother’s breast in the first minutes after birth - then, along with the first drops of colostrum, the newborn receives beneficial bacteria and antibodies to protect against infections. Close contact with the mother immediately after birth contributes to the colonization of the child’s body with the mother’s microflora. And it is very important that the mother’s microflora is in a healthy state.
What is dysbiosis
Dysbacteriosis is a disruption of the normal intestinal microflora, in which there is a decrease in the number of beneficial bacteria (bifidobacteria and lactobacilli) and an increase in the number of pathogenic and opportunistic microorganisms (staphylococci, fungi, Klebsiella, etc.).
Dysbacteriosis can cause poor absorption of food in a newborn, slow weight gain, insufficient absorption of vitamins, the development of allergies, and decreased immunity.
The causes of dysbiosis in a baby are a violation of the mother’s microflora, birth by cesarean section, lack of breastfeeding, the use of antibiotics in the first months of the child’s life, etc.
Etiology. Causes of dysbacteriosis
Dysbiosis can result from exposure to a variety of environmental factors, including diet, toxins, medications, and pathogens. Of these, intestinal pathogens have the greatest potential to cause microbial dysbiosis. The appearance of dysbiosis is influenced by:
- excessive or incorrect use of antibiotics,
- excessive alcohol consumption,
- increased consumption of sugar or protein,
- frequent use of antacids,
- chronic stress,
- poor oral hygiene.
In some cases, dysbiosis is associated with the birth of a child through cesarean section. Along with environmental factors, genetic components are also strongly associated with the development of dysbiosis.
Treatment of dysbiosis in newborns
The intestinal microflora of an infant can be restored with the help of preparations containing beneficial bacteria. Bifidum BAG is well suited for these purposes. This is a probiotic with a high titer of bifidobacteria (1012), which is approved for use from the first days of a child’s life.
"Bifidum BAG" contains bacteria in liquid form, which increases the effectiveness of the drug (compared to dry probiotics).
"Bifidum BAG" can be applied to the nipple and areola of the breast before feeding (for breastfeeding) or added to a bottle with the mixture (for artificial feeding). The probiotic has a pleasant sour milk taste and is usually liked by children.
The effect of “Bifidum BAG” on the body of a newborn:
- eliminates dysbacteriosis;
- increases the absorption of vitamins and minerals;
- reduces the body's allergic mood;
- helps get rid of colic;
- normalizes stool;
- increases immunity.
Atopic dermatitis and dysbiosis
The problem of atopic dermatitis (AD) has become of increasing medical and social importance in recent years, as the prevalence of the disease is steadily increasing. According to modern concepts, AD is a multifactorial disease, the development of which is closely related to genetic defects in the immune response and the negative effects of adverse environmental influences. It has been established that the effect of these factors determines the rate of development of blood pressure, especially in young children. A significant risk factor for AD is the pathology of the gastrointestinal tract, especially intestinal dysbiosis, which is detected in 89–94.1% of children with AD [2, 3, 11]. Undoubtedly, the intestinal microflora, bearing a large functional load, cannot but participate in the occurrence and maintenance of pathological disorders in AD.
This is evidenced by, firstly, the relationship between the severity of dysbacteriosis and the severity of clinical manifestations of AD, and secondly, a targeted effect on the intestinal microflora leads to an increase in the effectiveness of treatment of the underlying disease [2, 8, 14]. In addition, the increase in the incidence of AD occurs in parallel with the widespread prevalence of intestinal dysbiosis during the neonatal period. This may be due to the fact that the same factors contribute to disruption of the intestinal microecology and the occurrence of allergic reactions. Among them, a significant place is occupied by the deprivation of breast milk from the first days of life, early transfer to mixed and artificial feeding, expansion of the range of medicinal and vaccine preparations, widespread introduction of chemicals into everyday life, unfavorable environment, etc. [8]. Analysis of literature data mainly reflects information about the functional state of the gastrointestinal tract and its role in the formation and chronicization of allergic reactions from the first days of a child’s life. However, despite the relative clarity of the existing pattern of “atopic dermatitis + intestinal dysbiosis,” there is no clear explanation of the reason for the formation of this phenomenon and the pathogenetic relationship of these diseases.
First of all, it is necessary to fully appreciate the significant role that intestinal flora plays in the human body. The mass of all microbes living in the intestines of one person is about two kilograms. The flora of the contents of the large intestine includes anaerobic and aerobic bacteria. Normal microflora consists of 95% anaerobic species of bacteria, the main of which are bifidobacteria and lactobacilli. Aerobic bacteria, represented by Escherichia coli, enterococci, etc., make up the accompanying microflora. Residual microflora include staphylococci, clostridia, proteus, and fungi. The most numerous and irreplaceable representatives of beneficial microflora are bifidobacteria.
Bifidobacteria stimulate peristalsis, preventing stool disorders, increase the body's immunity, decompose some carcinogens and produce vitamins. Typical waste products of bifidobacteria are lactic, acetic, formic and succinic acids, amino acids and proteins, vitamins B1, B2, K, nicotinic, pantothenic and folic acids, pyridoxine, cyanocobalamin. By producing lactic and acetic acid, they prevent the proliferation of pathogenic microorganisms. Bifidobacteria stimulate the human lymphoid apparatus and participate in the synthesis of immunoglobulins. The cell wall of these bacteria contains a large amount of muramyl dipeptide, which activates the formation of B and T lymphocytes and macrophages. These bacteria are natural biosorbents and are capable of accumulating significant amounts of heavy metal compounds, phenols, formaldehydes and other toxic substances that enter the host’s body from the environment and affect a decrease in immunity [1, 2].
Another group of beneficial microorganisms is lactobacilli, without whose participation it is impossible to imagine the normal functioning of the body. Lactobacilli colonize the body of a newborn child in the early postnatal period. The habitat of lactobacilli is various parts of the gastrointestinal tract, from the oral cavity to the colon. For example, lactobacilli (Lactobacillus acitophilus) ensure timely bowel movements.
An interesting fact is that people following a strict vegetarian diet have very high levels of lactobacilli [7]. It is lactobacilli that most likely are the link in the pathogenesis of “atopic dermatitis - dysbacteriosis”. These bacteria stimulate the formation of IgA, which, especially in early childhood, neutralize food allergens and reduce their absorption in the intestine [1, 12, 18]. In addition to the direct effect of lactobacilli on sensitization in AD, an indirect effect of intestinal dysbiosis on the composition of the skin microflora in AD has now been proven: a decrease in the content of lactobacilli in the intestine leads to an increase in the level of Staphylococcus epidermidis on the skin, which is an additional source of allergization of the body [16].
Escherichia coli and enterococci can be classified as neutral microflora, since there is no evidence yet of their beneficial effects on the body. It is likely that these microorganisms are responsible for antiviral immunity. According to some authors, thanks to the phenomenon of molecular mimicry and the presence of receptors acquired from the host epithelium, microflora acquires the ability to intercept and eliminate certain viruses [1, 2]. A number of bacteria are known that have high nitrate reductase activity (propionibacteria, peptococci, veillonella, gram-negative enterobacteria and others), which prevent the development of methemoglobinemia at high nitrate levels. This is especially important in young children who have a high proportion of fetal hemoglobin [9].
In Western countries and in the International Classification of Diseases there is no such thing as “dysbacteriosis”. In foreign literature, the term “Bacterial overgrowth syndrome” is used, which includes a change in the quantitative and species composition of microorganisms characteristic of a biotope. Therefore, dysbiosis is essentially not the name of a disease, but a microbiological manifestation of diseases of the gastrointestinal tract. Dysbiotic conditions lead to changes in the quantitative and qualitative composition of human normal flora, which creates conditions for the implementation of the virulent effect of opportunistic microorganisms, usually found in the intestinal contents in small quantities.
Putrefactive or fermentative flora, fungi, mainly of the genus Candida, develop abundantly; microorganisms that are normally uncharacteristic for it can be found in the intestine. They are not able to perform many physiological functions inherent in normal microflora, and, in particular, the ability to inactivate toxic products of intestinal contents is lost, and the absorption capacity of the intestine is impaired [1, 2]. Thus, intestinal dysbiosis in patients with AD disrupts the enzymatic status of the digestive tract, creating conditions for the development of pathology of cavity, parietal and membrane digestion and absorption. There is an increased intake of bacterial and infectious allergens into the child’s body. Significantly increased antigenic stimulation by under-digested macromolecules of nutrients and bacterial allergens, with a weak immune response and the body’s inability to eliminate antigen-antibody complexes, leads to aggravation of blood pressure.
The question is still being debated: what comes first - atopic dermatitis or intestinal dysbiosis? Many researchers have tried to answer this question in their observations, but contradictions exist to this day. For example, infants with AD are more likely than healthy children to have increased levels of bacteroides and decreased levels of bifidobacteria in their feces. The fact that differences in the microflora in a healthy child and those with allergies are determined at the preclinical stage indicates that changes in the microflora are primary and not secondary to allergies [5].
This circumstance led to the assumption that prophylactic administration of a probiotic to pregnant women could prevent the development of atopy in the first two years of a child’s life. This theory was confirmed by domestic researchers who found that children born to mothers who received probiotics in the form of primary prevention of allergic diseases tend to have less frequent occurrence of allergic rashes in the first year of life and are less likely to be diagnosed with AD [5]. However, a diametrically opposite opinion exists among foreign authors. Karla Gale (2010) conducted an extensive study of 415 pregnant women who received probiotic milk or placebo from 36 weeks of pregnancy to three months of postnatal breastfeeding. At the age of two years, children born to such mothers were examined for the presence of atopy (BP, bronchial asthma and allergic rhinoconjunctivitis). Statistical analysis did not reveal a significant difference between the comparison groups; therefore, according to the author, the use of probiotics by pregnant and nursing mothers does not make any sense to prevent the development of atopy in children [19].
Most researchers are inclined to believe that the development of dysbacteriosis involves artificial feeding, malnutrition of the nursing mother, as well as the early introduction of complementary foods and products that are not appropriate for the child’s age, which are subsequently trigger factors in the development of a genetic defect in the immune response in AD [6, 8, 10 , 15, 21]. The formation of a child’s microbial biocenosis begins from the first stages of life. During childbirth, when swallowed, the microflora of the mother's birth canal enters and the vaginal flora colonizes in the child's digestive system, which prevents the development of dysbiosis in the newborn. Starting from the 4th day of life, lactobacilli, Escherichia, streptococci, and staphylococci are detected in the newborn’s colon. The flora of a child in the first year of life is directly dependent on the nature of feeding. In children receiving artificial feeding, bacteroides and veillonella appear more often and in higher titers. With an excessive amount of the latter, increased gas formation and the development of dyspeptic manifestations may be observed [7].
It is possible that the autonomic nervous system also takes part in the development of the “atopic dermatitis + intestinal dysbiosis” phenomenon. In school-age children, blood pressure in two thirds of cases is accompanied by vegetative dystonia syndrome [13]. Other authors also point to the presence of a neurological (non-immune) component in children suffering from AD [3, 12]. The above makes us think about a broader understanding of the pathogenesis of dysbiosis in patients with AD. As a rule, with pronounced vegetovisceral changes, there are functional disorders of the gastrointestinal tract (more frequent stools, increased intestinal motility), then secondary enzyme deficiency, malabsorption, and nutritional disorders are layered, which, in turn, worsens the course of the disease. A frequent companion to vegetative visceral dysfunction is the syndrome of increased neuro-reflex excitability, which is supported by persistent itching. Thus, a vicious circle is created in a child with AD: hypersensitization - itching - neuro-reflex excitability - vegetovisceral disorders - gastrointestinal dysfunction - hypersensitization [4].
Summarizing the diverse and contradictory literature data, the question of the cause-and-effect relationship of the development of dysbiosis in children with AD still remains open. We can assume several reasons that may be direct or indirect circumstances of the death of beneficial bacteria in such patients:
- artificial feeding, malnutrition of the nursing mother, as well as early introduction of complementary foods and foods that are not appropriate for the child’s age;
- a lack of digestive enzymes leads to the fact that undigested food remains undergo fermentation and serve as a substrate for the growth of pathogenic microbes;
- decreased tone or spasms of intestinal smooth muscles (vegetovisceral disorders, dyskinesia, vegetative-vascular dystonia);
- a lack of substances in the diet that serve as a substrate for the growth of beneficial microbes or the presence of certain foods that contribute to their death. A deficiency of fermented milk products and plant fiber in the human diet deprives the beneficial flora of the nutrient medium;
- the presence of parasites (worms, protozoa) in the intestines that have a detrimental effect on beneficial microflora;
- taking modern medications that reduce gastric secretion (H2-histamine receptor blockers), which are also capable, albeit indirectly, of reducing the resistance of the natural intestinal microflora;
- the use of cytostatics, glucocorticoids, which reduce the body’s immunoresistance.
Thus, disturbances in microbiocenosis in AD may be due to various reasons. Most general practitioners very often treat “dysbacteriosis” without taking into account (or simply not diagnosing) either the underlying pathology that led to its development or the true nature of the change in microbiocenosis. The selection of therapy is carried out purely empirically, which leads to low effectiveness of treatment and brings to the fore the pharmacoeconomic aspect of the treatment.
First of all, this syndrome cannot be exaggerated, but it is necessary to verify the gastrointestinal pathology that led to its disorders. Prescribing an adequate diagnostic program will allow you to correctly assess the situation and carry out both etiopathogenetic treatment of the underlying pathology and correction of microecological disorders. This will not only increase the effectiveness of treatment, but will also reduce the cost of treatment and avoid polypharmacy [1]. Laboratory diagnosis of dysbiosis is most often based on microbiological analysis of feces. Microbiological criteria include a decrease in bifidolactobacteria, a decrease or increase in Escherichia, the appearance of strains with altered properties, an increase in the number of cocci, detection of opportunistic gram-negative bacilli, as well as fungi and clostridia more than 103 CFU/l. In analyses, various combinations of these shifts are possible [7].
Modern principles of therapeutic correction of dysbiotic changes and restoration of eubiosis include a wider arsenal of measures than just the prescription of pharmacological drugs. Particular attention should be paid to the nutritional regimen and diet of a sick child, since with the normalization of the environment, metabolism is activated and the number of obligate microflora increases. Proof of this is the unexpected result of a study conducted by O. V. Usova (2005). According to the author, children living in conditions of strict regime and dietary control (orphanage), despite an extremely unfavorable premorbid background, suffer from intestinal dysbiosis much less often and in a milder form than socially prosperous children [15].
The diet usually allows you to restore normal digestion within two months. Considering that AD in young children in 73% of cases develops due to sensitization to cow's milk proteins, the use of adapted dietary products with a change in protein sources and structure is promising: goat's milk protein, hydrolyzed cow's milk protein, soy protein, etc. [10]. In older children, the diet during treatment should contain a minimum of carbohydrate foods. Functional food with a large amount of ballast substances (dietary fiber, bran), products enriched with live cultures of bacteria (kefir, fermented milk mixtures, yoghurts, etc.). To completely overcome dysbiosis, you need to eat more plant foods. A diet that includes foods that suppress putrefactive processes in the intestines is very useful: apricots, black currants, rowan berries, cranberries, cumin, provided that the child with AD does not have food allergies to these foods.
In case of fungal dysbacteriosis, it is necessary to give up everything that contains yeast cultures - grapes, raisins, kvass, fresh bread, cheese, fungal kefir. Systematic consumption of refined, canned foods, yeast, and smoked meats has an unfavorable effect on the intestinal flora. The greatest successes in this direction have been achieved by Japanese researchers, who consider the development of the functional food industry to be the most promising direction in solving the problem of eliminating dysbiosis. Now in Japan, which has come out on top in terms of average life expectancy, 30% of food products are fermented or enriched with live bifidobacteria.
In the treatment of intestinal dysbiosis, probiotics currently occupy a key position - preparations containing microorganisms that normally do not live in the intestines, but create conditions for the proliferation of beneficial bacteria and have a positive effect on the intestinal microbiocenosis. The name probiotics has been in use since the 1960s and comes from the Latin pro bio, for life, originally used to refer to certain foods containing live bacteria. The first scientist to conduct research on the possibility of restoring intestinal microflora using lactic acid bacillus was the famous Russian scientist and Nobel Prize laureate Ilya Mechnikov. Already in 1907, Ilya Ilyich Mechnikov put forward a theory of longevity, based on which a significant role was assigned to the normal microflora of the human body. He also proposed a practical way to improve health, prolong and improve the quality of life - the use of lactic acid products. Essentially, this was the beginning of the probiotic era.
Of great interest is the development of a new direction in the treatment of dysbiosis - prebiotic therapy. Drugs in this group are isolated from natural sources, have certain regulatory functions and will soon compete in the market with many drugs. Unlike probiotics, which introduce probiotic bacteria from the outside, prebiotics act as a nutrient medium for restoring the body’s own beneficial microflora. Studies on volunteers show that automicroorganisms provide a more rapid restoration of the normal state of intestinal microflora than those administered externally [20]. The basis of prebiotics are preparations containing bifidogenic factors that stimulate the growth and development of beneficial bacteria (lactulose, soy oligosaccharide, xylobiose, etc.); from the practical side, preparations containing lactulose have proven themselves well. The ideal combination is medicines containing prebiotics and adsorbents.
Adsorbents remove toxic substances from the body and reduce flatulence, usually observed with dysbacteriosis. In particular, these conditions are met by the drug Lactofiltrum, which contains a prebiotic and sorbent. Lactulose, which is part of the drug Lactofiltrum, is a synthetic stereoisomer of milk sugar - lactose. The products of bacterial metabolism of lactulose shift the pH of the environment in the large intestine to the acidic side, thereby inhibiting the proliferation of pathogenic microorganisms and putrefaction processes. Secondly, being a food substrate for bacteria, lactulose stimulates the growth of bifidobacteria and lactobacilli. The second component of Lactofiltrum is lignin, which is a complex natural organic compound, a product of hydrolysis of wood. Lignin, due to its large surface area and developed pore system, has a high sorption capacity and, thus, is able to remove toxins, allergens and pathogenic microflora from the body.
To treat dysbiosis, drugs that improve digestion are also used (for example, the enzyme preparation Mikrasim, etc.).
To normalize the contractility of the intestinal wall, acupuncture, special massage systems and self-massage of the abdomen are used [17]. Simultaneously with the treatment of dysbiosis, digestive disorders and helminthic infestations are treated.
Despite the fact that to date there are many unanswered questions about the etiology and pathogenesis of the development of dysbiosis in patients with AD, it is clear that the intestinal microflora, having a large functional load, is involved in the occurrence and maintenance of pathological disorders in AD. This fact obliges doctors to correct these disorders taking into account the diet and nature of nutrition, using modern combined products (Lactofiltrum) containing prebiotics and enterosorbents.
Literature
- Ardatskaya M.D., Minushkin O.N. Modern principles of diagnostics and pharmacological correction // Gastroenterology, supplement to the journal Consilium Medicum. 2006. T. 8. No. 2.
- Gushchina N. S. Improving the treatment and rehabilitation of children of primary school age with atopic dermatitis in a sanatorium-forest school. Abstract of dissertation. for the academic degree of Ph.D. M., 1996. 30 p.
- Zhadambaa Soel-Erdene. Batbaatar G. Gorshkova G. V. Main factors influencing the course of atopic dermatitis. IV Annual Meeting of the Mongolian Society of allergology and International Educational Exchange Program American Academy of Allergy, Asthma and immunology. Ulaanbaatar. 2006. P. 15–16.
- Healthy skin: A manual for doctors / Ed. ed. A. N. Razumova. M.: Ministry of Internal Affairs, 2007. 60 p.
- Kotegova O. M. Risk of allergic pathology in children of women with obvious and latent sensitization. In the book: The health of the child is the health of the nation. Kirov, 2006. pp. 37–38.
- Mazankova L.N., Ilyina N.O., Kondrakova O.A., Begiashvili L.V. Modern aspects of rational diagnosis and correction of intestinal dysbiosis in children // Bulletin of pediatric pharmacology and nutrition. 2007, vol. 4, 2, p. 24–29.
- Mukhina Yu. G. Diagnosis and correction of dysbiosis in children // Breast Cancer. 1999, vol. 7, no. 11.
- Plaksina I. A. Prevalence and clinical and immunological features of the course of atopic dermatitis accompanied by intestinal dysbiosis. Author's abstract. diss. ... Ph.D. Krasnodar, 2007. P. 21.
- Prevention of allergic diseases in children at risk. Methodical manual, ed. L. F. Kaznacheeva. Novosibirsk, 2009. 45 pp.
- Sentsova T. B., Denisova S. N., Belitskaya M. Yu., Arsenyeva N. A. Clinical and immunological manifestations of food allergy in young children // Issues of modern pediatrics. 2004, vol. 3, p. 380.
- Smirnova G.I. Modern principles of pathogenetic therapy of atopic dermatitis in children // Issues of modern pediatrics. 2006, vol. 5, no. 2, p. 50–56.
- Suvorova K. N., Kuklin V. T., Rukavishnikova V. M. Pediatric dermatovenerology. Kazan, 1996. 441 p.
- Suprun I.M., Makarova V.I., Plaksina N.Yu. Autonomic homeostasis and functional state of the digestive tract in school-age children with atopic dermatitis. Cherepovets: Publishing house "Cherepovets", 2007. 32 pp.
- Tuchkov D. Yu. Diarrhea syndrome in atopic dermatitis in young children. Author's abstract. diss. ... Ph.D. Orenburg, 2004. P. 20.
- Usova O. V. Comparative analysis of risk factors for the development of intestinal dysbiosis in children in various social conditions / “Lomonosov 2005”, section “Fundamental Medicine”, 2005, p. 487–488.
- Fokina R. A. Features of the course of atopic dermatitis in Yakutia in children and adolescents in a comparative aspect // Far Eastern Medical Journal. Appendix 4. 2007, p. 18–19.
- Shamova A. G., Shamov B. A., Denisova S. N. New technologies for the prevention and treatment of food allergies in children // Issues of gynecology, obstetrics and perinatology. 2005, vol. 4, no. 4, p. 98–103.
- Cremonini F. et al. Meta-analysis: the effect //Aliment Pharmacol Ther. 2002; 16:1461–1467.
- Karla Gale. Probiotics in Pregnancy Reduce Eczema in Infants // Br J Dermatol. Published online June 9, 2010.
- Nancy Toedter Williams. Probiotics // American Journal of Health-System Pharmacy. 2010; 67(6):449–458.
- Yang YW, Tsai CL, Lu CY Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of rospective cohort studies // The British Journal of Dermatology. 2009; 161(2):373–383.
Yu. A. Gallyamova, Doctor of Medical Sciences, Professor
RMAPO, Moscow
Contact information about the author for correspondence
Prevention
There are many ways to prevent dysbiosis, some of which are closely related to lifestyle changes, eating healthy foods, and giving up alcohol.
Diet is one of the most important factors influencing the homeostasis of the intestinal microbiota. For years, researchers around the world have found links between diet and diseases associated with dysbiosis, such as obesity, diabetes and allergic disorders. Recent studies have shown that vitamin D is a likely protector and reduces the risk of inflammatory bowel disease and colitis. It is also important not to overuse antibiotics and take them only as prescribed by a doctor. Antibiotics have a destructive effect on the normal microflora of the colon. Your doctor may also prescribe prebiotics and probiotics. The former are healthy plant fibers found in many foods that promote the growth of beneficial bacteria in the colon. The second are live microorganisms that, when taken in sufficient quantities, can benefit the body and help prevent and treat dysbiosis. It is also worth observing good oral hygiene.
How to treat acne from dysbacteriosis correctly?
You should forget about self-medication and turn to specialists. Acne therapy is the prerogative of a dermatologist. If dysbiotic disorders are suspected, the doctor refers the patient for examination to a gastroenterologist.
For mild to moderate acne, a dermatologist may prescribe topical antibiotics, for example, Clindovit® gel6,18. The drug contains clindamycin phosphate; it exhibits antimicrobial activity against propionibacteria6. Clindovit® gel reduces the level of free fatty acids on the skin. To reduce the risk of developing antibiotic resistance, it is recommended to combine the drug with benzoyl peroxide or azelaic acid, for example, Azelik®28 gel.
*acne
Diagnosis of the disease
In order to accurately make a diagnosis, the doctor must take a series of tests to document his assumptions. To make an accurate diagnosis, you will need:
- Undergo bacteriological examination of stool. During this study, it is possible to detect the presence of pathogenic microflora, as well as identify the pathogen
- Do a stool culture for dysbacteriosis. Usually the analysis is done for about a week, since during this time bacteria can multiply and grow, and doctors can conduct their research and find out resistance to antibiotics
- Submit stool for a coprogram, which will show the presence of undigested pieces of food in the stool, as well as detect signs of an inflammatory process in the intestines
- Study of microflora metabolites
And only after receiving all the test results, the specialist will be able to make an accurate diagnosis and agree on the treatment he has chosen with the parents.
Complications
Dysbacteriosis creates conditions for the development of a number of intestinal and extraintestinal diseases, namely:
- Inflammatory bowel disease;
- Irritable bowel syndrome;
- Diabetes;
- Obesity;
- Colorectal cancer;
- Cardiovascular diseases;
- Central nervous system disorders;
- Autism.
When the gut microbiome loses bacterial diversity, the risk of developing various chronic diseases increases.