Colon cancer is in third place in the system of all cancer diseases of the body. Bowel cancer can develop in people of any age. It is very difficult to identify it at an early stage. Today, intestinal tumor markers are used for this purpose. Such a tumor marker makes it possible to determine whether there is a malignant tumor in the body before the first signs of the disease appear.
What are tumor markers?
A tumor biomarker is a substance (usually a protein molecule) produced either by the cancer cells themselves or by other cells of the body in response to cancer pathology.
Sometimes healthy cells in the body are able to produce such substances in response to certain benign (non-cancerous) conditions. Tumor markers provide information about a tumor, such as how aggressive it is, whether it can be treated with targeted therapy, and how well the tumor responds to treatment.
Tumor markers are proteins or other substances that are produced by cancer cells in higher quantities than normal cells. These biomarkers are found in blood, urine, stool, or other body tissues or fluids.
For some types of neoplasia, the level of a tumor marker reflects the stage (extent) of the disease and/or the patient's prognosis (the likely outcome or course of the disease). An example of this type of tumor marker is α-fetoprotein, which is measured in the blood to assess the stage, prognosis, and track response to treatment of germ cell tumors.
However, increasingly, genomic indicators such as mutated forms of genes, tumor gene expression patterns, or nongenetic changes in the DNA of cancer cells are being used as tumor markers.
Currently, about 60 tumor markers have been characterized in detail and are in clinical use. Some of them are associated with only one type of tumor (specific tumor markers), while others are associated with several types of cancer pathology (nonspecific tumor markers).
This number does not include tumor markers, which are tested using immunophenotyping and immunohistochemistry, which help diagnose cancer and distinguish between tumor types.
At the same time, a “universal” tumor marker that could detect the presence of any type of cancer pathology has not yet been discovered.
When are tests taken?
To determine the level of tumor markers, the patient donates blood as the biomaterial being studied. The sample collection procedure is carried out early in the morning. It is important that the patient does not eat for eight hours prior to the test. To get clear answers, doctors recommend refraining from drinking coffee, tea and juices, replacing them with water.
The results of the study will be ready a day or two after the collection of biological material. Tests to determine the level of protein antigen CA 72-4 are carried out taking into account the possible intake of biotin, since a daily dosage exceeding 5 mg will violate the veracity of the results. It is then recommended to postpone the study for 8 hours until the drug is removed from the body.
The level of Tu M2-RK is determined by analyzing stool. The specificity of this procedure is the prohibition of extracting biomaterial by enema or with the assistance of laxatives. Feces are obtained exclusively naturally. Results are issued after 7 days.
What are tumor markers?
There are two main types of tumor markers that are used in different ways in oncology—circulating tumor markers and tumor tissue markers.
Circulating tumor markers may be found in the blood, urine, stool, or other body fluids of some patients. Circulating tumor markers are used to:
- forecast assessments;
- detection of tumor that remains after treatment (residual disease) or recurrence;
- assessing response to treatment;
- control the development of tumor resistance to treatment.
Although elevated levels of a circulating tumor marker may indicate the presence of a tumor, this alone is not sufficient for diagnosis. For example, non-cancerous diseases can sometimes cause elevated levels of certain tumor markers. Additionally, not everyone with a certain type of cancer will have higher levels of a tumor marker associated with that cancer.
Therefore, measurements of circulating tumor markers are always combined with the results of other tests, such as biopsy or imaging, to diagnose cancer.
Tumor markers may also be measured periodically during cancer treatment. For example, a decrease in the level of a circulating tumor marker may indicate that the tumor is responding to treatment, whereas an increase or unchanged level may indicate that the tumor is not responding.
Circulating tumor markers can also be measured after treatment ends to check for recurrence.
Examples of circulating tumor markers.
- Calcitonin (measured in the blood), which is used to assess response to treatment, screen for recurrence, and assess prognosis in medullary thyroid cancer.
- CA-125 (measured in the blood) to monitor how well treatment is working for ovarian cancer.
- Beta-2 microglobulin (measured in blood, urine, or cerebrospinal fluid) to assess prognosis and monitor response to treatment for multiple myeloma, chronic lymphocytic leukemia, and some lymphomas.
Tumor tissue markers are found in the tumors themselves, usually in a sample of the tumor that is removed during a biopsy.
Tumor tissue markers are used to:
- diagnose, stage and/or classify a neoplasm;
- evaluate the forecast;
- determine treatment options (eg, treatment with targeted therapy;
Tumor markers can be measured before treatment to help doctors plan appropriate therapy. For example, some tests, called companion diagnostics, that were developed in conjunction with the corresponding targeted therapy drug, are used to determine whether a particular targeted therapy is appropriate for treatment. Some of these tests measure how much of a tumor marker is present, others simply detect the presence of a specific marker, such as a gene mutation.
Tumor tissue markers are often targets for specific targeted therapy.
Examples of tumor tissue markers
- Estrogen and progesterone receptors (breast cancer), used to determine whether treatment with hormone therapy will be appropriate;
- Analysis of EGFR (non-small cell lung cancer) gene mutations to help guide treatment and assess prognosis;
- PD-L1 (many types of malignant neoplasia) to determine whether treatment with immune checkpoint inhibitors would be appropriate.
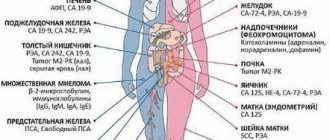
A study of the concentration of the main markers of colon cancer, which is used for diagnosis, assessment of prognosis and monitoring of treatment of this disease.
Synonyms Russian
Blood tests for colon cancer, tumor markers for colon cancer
English synonyms
CA 19-9, CA 242, Gastrointestinal Cancer Antigen, GICA, Carcinoembryonic antigen, CEA, CD66e, CEACAM5.
What biomaterial can be used for research?
Venous blood.
How to properly prepare for research?
- Do not eat for 8 hours before the test; you can drink clean still water.
- Avoid physical and emotional stress for 30 minutes before the test.
- Do not smoke for 24 hours before the test.
General information about the study
Colon cancer (CC) is the most common malignant disease of the gastrointestinal tract. Diagnosis of cancer is complex, with laboratory research methods playing a central role. The study of tumor markers provides valuable diagnostic and prognostic information. The most well studied tumor markers are CA 19-9 and cancer embryonic antigen.
Carcinoembryonic antigen (CEA) is a large glycoprotein that is structurally similar to immunoglobulins. It is synthesized by cells of the colon and probably mediates cell-to-cell interactions. CEA has been shown to play a role in stimulating tumor growth, invasion and metastasis.
An increase in the level of CEA is characteristic of RTC. At the same time, high levels of CEA are more often observed in late stages of the disease and/or in the presence of a large tumor. In the early stages of the disease, CEA levels are usually normal. It should also be noted that in approximately 15% of cases of large RTC, the CEA level also remains normal. There is no relationship between the level of CEA and the histological type of the tumor. An increase in CEA can also be observed in some non-oncological diseases of the gastrointestinal tract (hepatitis, ulcerative colitis and Crohn's disease, pancreatitis), oncological diseases of other organs (breast, ovarian, kidney cancer) and non-oncological diseases of other organs (chronic obstructive pulmonary disease, endometriosis).
The CEA level is also used to assess the prognosis of the disease. It has been shown that a high level of CEA in the preoperative period is an unfavorable prognostic sign. An increase in CEA levels in the postoperative period may indicate a relapse of the disease.
Tumor marker CA 19-9 (carbohydrate antigen) also belongs to glycoproteins in structure. Like CEA, CA 19-9 is found in elevated concentrations in the blood of patients with RTC and some other diseases (pancreatitis, liver disease). As with CEA, the CA 19-9 level does not reflect the histological type of tumor. The sensitivity of CA 19-9 for RTK is lower than the sensitivity of CEA. A joint study of tumor markers CA 19-9 and CEA makes it possible to achieve higher sensitivity. Some authors suggest using the CA 19-9 tumor marker in combination with CEA to assess the prognosis of cancer.
Cancer antigen CA 242 is a high molecular weight glycoprotein, which, like the tumor marker CA 19-9, is produced by epithelial cells of the gastrointestinal tract, but has more pronounced sensitivity and specificity to malignant tumors. CA-242 levels are elevated in almost all patients with gastrointestinal tumors, especially in pancreatic, colon, and rectal cancer. CA-242 is produced by tumor cells and enters the bloodstream, making it an effective tumor marker that allows diagnosing the disease at an early stage and monitoring its progress.
The main feature of the CA 242 tumor marker is its low expression in benign diseases of the gastrointestinal tract, which makes it possible to use it for the differential diagnosis of benign and malignant tumors.
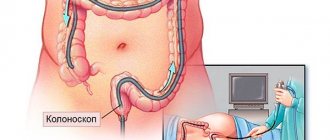
Currently, the study of the concentration of these tumor markers is not used as an independent test for the diagnosis of cancer, but is complemented by other tests (for example, a fecal occult blood test) and instrumental studies (for example, colonoscopy). The result of the tumor marker study is assessed taking into account all relevant information about the patient.
What is the research used for?
- For the diagnosis of colon cancer (CC);
- to assess the RTC forecast;
- for early detection of RTC recurrence.
When is the study scheduled?
- If colon cancer is suspected in the presence of unmotivated weakness, weight loss, decreased appetite, nausea, stool abnormalities, blood in the stool, abdominal pain;
- periodically when monitoring the patient in the postoperative period.
What do the results mean?
Reference values
For each indicator included in the complex:
- [08-006] CA 19-9
- [08-042] Carcinoembryonic antigen (CEA)
- [08-105] CA 242
| Reasons for the increase | Reasons for the downgrade: | |
| REA |
|
|
| SA 19-9 |
|
|
| CA 242 |
|
|
What can influence the result?
- Presence of concomitant diseases;
- relapse of the disease and the appearance of metastases;
- start of chemotherapy.
Nonspecific markers
Nonspecific markers are determined in various malignant tumors, and often in non-cancerous pathologies.
In colorectal cancer, several nonspecific tumor markers are more often used. Determining each of them has its own task - testing for sensitivity to a specific therapy, diagnosing relapses, monitoring the effectiveness of treatment
Examples of markers used in colorectal cancer.
Mutation of the DPD gene in the blood. Types of neoplasms: stomach, pancreatic, breast and colorectal cancer. Used to predict the risk of toxic reactions to 5-fluorouracil therapy.
BRAF V600 mutations in tumors. Types of neoplasms: cutaneous melanoma, Erdheim-Chester disease, colorectal cancer and non-small cell lung cancer. Used to select patients who are most likely to benefit from treatment with specific targeted therapies.
Mutation of the KRAS gene - in the tumor. Types of neoplasms: colorectal cancer and non-small cell lung cancer. Used to determine whether a particular type of targeted therapy is appropriate for treatment.
Preparation for the analysis of tumor markers of the gastrointestinal tract
Preparation for analysis for tumor markers for cancer:
- two days before diagnosis, stop drinking alcohol and fatty foods;
- the day before the examination, limit physical and emotional stress;
- eight hours before the procedure, refrain from eating any food;
- Immediately before the analysis, you should not worry and remain calm.
By following these simple rules, a patient suspected of having a malignant tumor can significantly increase the reliability of diagnostic results based on blood tests.
Specific markers
The presence of these tumor markers or a significant increase in their concentration is typical for tumors of a strictly defined type or a specific organ.
For intestinal cancer, such a highly specific indicator will be UGT1A1 (28 homozygosity variant). It is specific for rectal and sigmoid cancer.
This oncotest for intestinal cancer is determined by scraping from the mucous membrane of the cheeks, and sometimes in a mask of blood analysis. It is used to predict the effectiveness of irinotecan therapy. But many authors question its value.
These are, perhaps, all the currently known specific tumor markers for rectal cancer, as well as tumor markers for sigmoid colon cancer.
How are biomarkers taken?
Tumor markers for bowel cancer are measured differently and require different tests. Some markers are found in blood or urine, so this will require you to provide a small amount of blood or urine sample.
Other biomarkers, such as those involved in the fecal occult blood test (FOBT), can be isolated from fecal matter and require a stool sample.
Tissue samples may also contain tumor biomarkers and may include a tumor biopsy. This is a more invasive procedure than collecting urine or stool. After the doctor takes the sample, it is sent to a laboratory for testing using various methods to determine biomarker levels.
The diet and composition of the diet before taking tests may be important. The attending physician gives the patient individual recommendations. For example, specific foods, such as red meat or fruits and vegetables, can interfere with the test and lead to false positive results, even if they were consumed a day or two before the test.
Where to get tested for intestinal tumor markers
We offer to undergo such an examination at our City Medical Center. Modern equipment, qualified staff and reasonable prices for our services are the advantages thanks to which patients return to the clinic when necessary. The analysis results will be ready within one day. They are sent by email, which saves the patient’s time because there is no need to come to the clinic for the results. If you do not have the opportunity to visit our laboratory, you can call a nurse to your home and receive the final results by email.
When to take it
Diagnostic circulating tumor markers for colon cancer are tested for the first time, usually when oncopathology is suspected.
In Europe and in particular in Belgium, the FOBT test with tumor DNA is used for screening tests in the absence of symptoms or primary suspicion of bowel cancer. The fecal test detects mutated KRAS, TP53, APC genes and microsatellite instability (MSI) markers. The average sensitivity level of such a test is in the range of 25-65%.
If there is a reasonable suspicion of intestinal cancer, a blood test is performed for carcinoembryonic antigen, or as it is also called carcinoembryonic antigen.
If the level of this marker is significant, a set of diagnostic studies is indicated to establish a final diagnosis.
What tumor marker indicates bowel cancer?
There is not yet a specific tumor marker that can be used to speak with a reasonable degree of confidence about the presence of a malignant tumor in the intestine. However, a comprehensive analysis with the determination of several different biomarkers can form a reasonable suspicion and become the basis for a serious diagnostic study.
Markers determined by blood tests
Carcinoembryonic antigen (CEA) level is the tumor marker most commonly used in colorectal cancer. Its level can be checked preoperatively for prognosis, used during therapy to assess response to treatment, or after completion of therapy to monitor relapse.
CA 19-9 is a non-tumor blood marker that may be elevated in colorectal cancer.
Chromosome 18q loss of heterozygosity (18qLOH) is a tissue marker often used in patients with stage II or III colorectal cancer. May affect prognosis.
Tumor tissue markers (analysis of stool and biopsy material)
MSI (microsatellite instability) is a way to measure mismatch repair (MMR) deficiency in tumor DNA. MMR deficiency leads to increased mutations in colon cells, which contributes in part to the development of colon cancer.
- MSI can be used to identify early-stage colon cancer that may require more aggressive treatment or to identify patients who should undergo further genetic testing due to the risk of a familial syndrome associated with multiple types of cancer.
- MSI identifies tumors as MSI-high (MSI-H) or MSI-stable and MSI-low.
K-RAS mutations - Specific mutations in the K-RAS gene can predict whether a patient may benefit from treatment with multiple biological treatments.
BRAF mutations - usually associated with the V600E mutation. May be a predictor of prognosis after diagnosis of colorectal cancer.
Main symptoms of bowel cancer
The appearance of more significant signs of an intestinal tumor is noted as the tumor grows:
- the presence of blood is observed in the stool: both individual streaks and completely colored stool;
- the presence of pus and mucus is noted in the stool, causing an unpleasant odor in the stool;
- patients begin to worry about constipation, which is replaced by diarrhea;
- blood pressure decreases, the blanching of the skin disappears, and attacks of cold sweat occur periodically - when the tumor is localized in the cecum;
- development of nausea and vomiting, which does not bring relief and is accompanied by an increase in body temperature;
- the appearance of pain arising in the projection of the neoplasm onto the abdominal wall;
- feeling of incomplete bowel movement after defecation;
- long absence of bowel movements, which can last several weeks. The abdominal wall becomes hard and painful;
- Over time, general oncological symptoms develop and secondary tumors appear.
The manifestations of intestinal cancer depend on a number of factors, primarily on where the malignancy is localized.
Decoding the results
For those biomarkers for which quantitative assessment is used, interpretation of the results often presents certain difficulties. In a healthy person, its level in the blood can be up to 5 ng/ml, and in smokers it can be up to 20 ng/ml. At the same time, an increase to 25-26 ng/ml should already alert the doctor, while in some non-tumor diseases its level can rise to 60-80 ng/ml.
Tumor marker CA 19-9 normally has a concentration of up to 37 U/ml. With hepatitis or cholecystitis, its level can rise to 500 U/ml. Higher levels indicate the likelihood of cancer. A very high concentration - up to 10,000 U/ml - is an indicator of probable tumor metastasis.
Symptoms of small intestine cancer
The formation of cancerous tumors in the small intestine is quite rare, but such cases do occur. In such cases, the disease manifests itself with the following symptoms:
- dyspepsia: nausea, vomiting, intestinal spasms, pain in the epigastric region;
- lack of appetite, aversion to food, weight loss;
- intestinal bleeding, the main manifestation of which is the dark color of stool;
- intestinal obstruction – in the later stages of the disease;
- compression of nearby organs with the subsequent development of many severe symptoms: jaundice, ascites, pancreatitis, peritonitis.