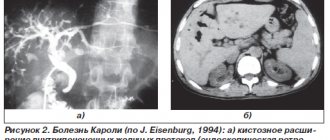
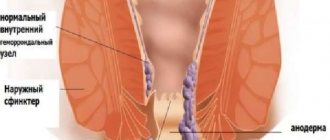
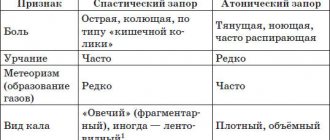
Intestinal motor function is normal and in chronic constipation
At the All-Russian Internet Congress of Specialists in Internal Diseases, Doctor of Medical Sciences Trukhmanov A.S. I read an article about the features and problems of chronic constipation.
00:00
Vladimir Trofimovich Ivashkin , academician of the Russian Academy of Medical Sciences, Doctor of Medical Sciences:
- Now we move on to the next topic. This topic will be presented by Professor Alexander Sergeevich Trukhmanov. “Intestinal motor function is normal and in chronic constipation.
Alexander Sergeevich Trukhmanov , professor, doctor of medical sciences:
— Good afternoon, dear colleagues.
Today we are looking at this issue in an applied sense. How can a doctor diagnose certain disorders of intestinal motility in a patient with constipation? How can certain research methods help us not only in making a diagnosis, but also in selecting the most effective treatment for such a common condition as constipation?
I will just briefly recall the main provisions, including the fact that the regulation of intestinal motor function includes myogenic, nervous and humoral. Without dwelling on it for too long, I want to emphasize that, of course, this function is extremely complex and is subject to numerous influences.
As before, we remember and use in our practical activities in the treatment of these patients the opportunity to influence motor function. In particular, the release of neurotransmitters. Including acetylcholine.
We should also remember that the colon is influenced not only by extraorgan mechanisms, but also includes complex mechanisms of self-regulation of motor function. This component is extremely important.
Seeing how the gut regulates itself is very difficult. There are very subtle diagnostic methods. We will talk about them.
Types of contractile activity of the colon include those that are responsible for the formation of feces and the advancement of the colon in a histal direction. Violation of each of these types of contractile activity can lead to the appearance of both disorders in the form of constipation and the appearance of accelerated transit.
Non-propulsive peristalsis, which involves contraction of the circular layer of muscle, is responsible for producing stool of the consistency we often ask our patients about. This peristalsis is largely regulated by intraorgan mechanisms.
Propulsive peristalsis is responsible for the movement of intestinal contents and for the implementation of the act of defecation. It is regulated not only by intraorgan but also extraorgan mechanisms. This is where the prospects for pathogenetic treatment of constipation lie.
When we have the opportunity to stimulate (and as close as possible to physiological conditions) propulsive peristalsis, then many of our patients with that type of constipation, which is called constipation due to delayed bowel movement, will be supervised very effectively by us.
04:10
Let's move on to an extremely practical problem. How can we diagnose certain types of intestinal motility disorders in real conditions? There are subtle methods. But it is quite obvious that in real conditions we are limited to techniques that do not require special expensive equipment. We strive to diagnose movement disorders using the tools available to us.
Of course, the clinical diagnosis always comes first - the diagnosis that in the vast majority of cases allows us to identify the type of disorder and prescribe pathogenetic treatment.
Diagnostic steps for severe treatment-resistant constipation, which include careful questioning of the patient. Including using self-questioning, that is, keeping a diary by the patient for seven days detailing aspects that will later allow us to establish the exact mechanism of development of the pathology.
The importance of an objective examination of patients, including the anorectal region, has already been discussed in the previous lecture. In cases of severe constipation resistant to treatment (such occur), we often need to make a differential diagnosis at the first stage between constipation with impaired transit through the colon and with impaired evacuation from the rectum (colagenic and rectogenic constipation).
Fecal transit time studies using radiopaque markers are now recommended as a method that can be implemented in real-life practice. This study does not require additional equipment. It is carried out using X-ray equipment.
But there is also a problem. The use of the method to study the transit time of feces in a patient with constipation is due to the fact that the technique itself may still not be completely standardized and therefore has not become widespread.
07:03
(Slide show).
However, according to the methodological recommendations that exist from our colleagues in other countries, we can conclude that visualization of the violation of transit of radiopaque markers in the right side of the abdominal cavity can allow us to say that in this particular case there is a slowdown in the progress of transit through the colon.
In patients with impaired evacuation from the rectum, all radiopaque markers (on the slide in the form of rings) will be localized in the left part of the intestine. Moreover, they can be seen in the rectum.
This is a technique that allows us to tentatively conclude what the main mechanism for the development of constipation is in a given patient.
In addition, the so-called “balloon expulsion test” can be used in practice. However, it requires equipment to study motor function and perform monometry. I will talk about this below.
In specialized institutions, which can be equipped with special equipment for carrying out, for example, manometric monometry, high-resolution monometry, sphincter electromyography, a more accurate diagnosis of very severe resistant and hitherto unclear cases can be carried out.
08:57
What is the significance of the integrative indicator of chyme transit through the gastrointestinal tract in patients with constipation? There are well-known correlations that indicate that patients complaining of constipation have an increased transit time. Although there are no absolutely accurate criteria that can tell us that before such and such a time is the norm, after such and such a time is pathology.
Average values indicate that transit time is prolonged in patients with constipation. After prescribing the drug “Prukaloprid”, this time decreases. This correlates with the patient's assessment of his condition and with an increase in the number of bowel movements per week.
In conditions that do not require special equipment, the function of the pelvic floor muscles can be studied. A defecogram is a method that only requires additional qualifications of a radiologist. It allows us to see abnormalities in anatomy and function that are the basis for the development of rectal evacuation disorders. Those types of constipation that do not require the prescription of therapeutic treatment, but the participation of fellow surgeons and proctologists in solving the problems underlying this condition.
Manometric examination of the intestine requires special equipment. With the development of instrumental diagnostics, this becomes possible in specialized centers. We must know the types of colonic motor function that can be studied. It is very important for us to imagine that using this method we can verify, among other things, the effectiveness of therapeutic effects in patients with constipation associated with impaired intestinal evacuation.
These types of motor activity are represented by segmental propulsive contractions. Numerical criteria for assessing the effectiveness of such reductions are also known.
(Slide show).
This graph shows the segmental motor activity of the colon, which is responsible for the formation of feces. A graph that demonstrates the progression of contractions hystally, starting from the transverse colon, through the descending colon to the sigmoid colon. Increase in amplitude.
Low-amplitude colonic propulsive motor activity, along with high-amplitude colonic propulsive motor activity, occurs in amplitude and frequency of occurrence. Low amplitude occurs frequently. High-amplitude occurs much less frequently.
These are precisely the activities of the colon that are disrupted in patients with constipation. When we visualize certain disorders of motor activity, we can conclude that this particular patient needs to be prescribed a drug that models this function.
12:59
The number of propulsive contractions of the colon in patients with constipation is significantly less than in healthy volunteers (in the control group). Using the data from this study, we can talk about the need to modify the disorders. We must apply a method that will increase the number of high-amplitude propulsive contractions and thus influence the clinical picture.
Research with Prucalopride has demonstrated that it statistically significantly increases the total number of high-amplitude propagating contractions. This drug, which will be discussed in more detail in the next lecture, affects the motor function of the intestines.
There is a complex of propulsive peristalsis of the colon, which also affects the clinical picture. This drug integratively increases the frequency of contractions of the colon. This is an intestinal prokinetic drug.
We must also talk about the fact that the intestines function differently both during the interdigestive period and during the act of defecation. We can register an increase in intestinal motility during bowel movements. High-amplitude activity is also the object of our influence.
When studying motor skills, we are faced with a large number of methodological and technical problems. In our opinion, this is a technique that allows us to demonstrate in real time that in a number of patients (we are not talking about all patients with constipation - this is natural) in whom constipation is severe and is not modified at the first stage, such studies are indicated .
15:31
In addition to indicators such as the number and total number of contractions, using studies of motor function and monometry, we can study the effective degree of contractions and the pressure that the intestinal wall exerts on the contents. This is also an integrative indicator.
(Slide show).
New generation prokinetics statistically significantly increase blood pressure. The pressure indicator is the area under the “curve” along the ordinate axis.
A domestic device that can be used to conduct these studies. Specialized centers may take advantage of the opportunity to study indicators. This is the activity of the sphincter, reflexes that arise in response to certain stimuli in the intestine, a test for visceral sensitivity, and rectal distensibility.
I will now show you the results of a study that was conducted. I would like to inform the audience that domestically produced equipment allows one to study, for example, the recto-anal inhibitory reflex. This reflex is inhibited primarily in patients with impaired rectal evacuation. This can be seen using this research method.
Or, for example, studying the threshold of visceral sensitivity in patients with irritable bowel syndrome. A decrease in the threshold of visceral sensitivity is a characteristic symptom in them and allows us to penetrate much deeper into the nature of suffering and prescribe pathogenetic therapy.
In addition, specialized institutions conduct studies such as sphincter profilometry. In patients with pelvic floor dysfunction, these data also change. This method is not so complicated, although it looks very exotic. In specialized institutions, primarily proctology hospitals, this method is widely used.
In the future, monometry will most likely be all in the form of high-resolution monometry. This is not some new principle for recording changes in motor function. This is a new type of results analysis and data presentation. This is a new opportunity to see what we have previously recorded with solid-state and open catheter sensors.
(Slide show).
This slide very colorfully presents the characteristics of patients with normal pelvic floor function, relaxation of the internal and external anal sphincters, and with dysfunction of the pelvic floor, where sphincters contract in response to straining. This leads to painful constipation in some patients and requires surgical intervention.
19:29
Electromyographic study. It's traditional. Known to domestic doctors. Does not require additional expensive equipment. But in some cases it makes it possible to visualize dysfunction of the muscles that underlie the development of constipation in patients.
Thus, we can conclude that colonic motility disorders do not need to be studied in all patients with constipation. It is obvious. However, in order to treat patients with this severe suffering, in some cases we must conduct these studies.
Of course, the study of intestinal motor function allows us to verify that new drugs that affect motor function (the so-called intestinal prokinetic agent “Proculapride”), ultimately, by stimulating peristalsis, lead to a normalization of the number of bowel movements. To the treatment of constipation, which is the main task in this case.
Thank you for your attention.
20:52
Ref. material / DIGESTION / 13. FINE MOTOR ACTIVITIES
14.7.4. MOTOR FUNCTION OF THE SMALL INTESTINE
The motor activity of the small intestine ensures further mechanical processing of chyme, its grinding, mixing with alkaline digestive secretions, movement along the intestine in the distal direction, changing the layer of chyme near the mucous membrane, and increasing intracavitary pressure. In addition, the strictly coordinated contractile activity of the muscles of the small intestine determines the duration of retention of contents in each of its sections, which is optimal for the digestion of food substrates with the formation of the required amount of nutrients and their transport into the blood and lymph. Thus, the motor function of the small intestine increases the efficiency of cavity and parietal digestion and promotes the absorption of nutrients.
The ability of smooth muscle cells to be automatic underlies all types of contractions of the small intestine.
The main types of motility of the small intestine are the following.
Rhythmic segmentation
manifests itself in the form of simultaneous contractions of circular muscles in several neighboring areas of the intestine, dividing it into segments, due to which the chyme moves short distances in both directions from the places of narrowing of the intestinal lumen. With the next contraction of the circular muscles, each segment is divided into two parts, and the previously contracted sections of the intestine relax. The contents of each new intestinal segment consists of the chyme of the two halves of the former segments. Rhythmic segmentation ensures mixing of the chyme and its slight displacement in the distal direction.
Pendulum contractions
arise as a result of rhythmic contractions of mainly the longitudinal muscle layer with the participation of circular muscles, leading to the movement of chyme back and forth. They provide mixing of intestinal contents and its weak progressive
advancement in the distal direction. The frequency of pendulum-like contractions and rhythmic segmentation in the same section of the intestine is the same. Alternating rhythmic segmentation and pendulum-like contractions promote thorough mixing of the chyme.
Peristaltic contractions
represent contractions of circular muscles that spread in waves throughout the intestine, preceded by a wave of relaxation. They ensure the movement of contents through the intestine in the proximodistal direction. A peristaltic wave occurs as a result of narrowing of the intestinal lumen with contraction of the circular muscles above the lump of chyme and expansion of the intestinal cavity with contraction of the muscles of the longitudinal layer below the lump. The resulting proxy-modistal pressure gradient is the direct cause of the movement of chyme through the intestine.
Peristaltic contractions can vary in strength and speed of propagation. Sufficiently strong peristaltic contractions move chyme distally over long distances. Such peristaltic movements are called propulsive.
The speed of propagation of peristaltic waves through the small intestine in a healthy person is usually 1-2 cm/s. In the proximal parts of the small intestine it is higher than in its middle part, and in the terminal section of the ileum with rapid peristalsis it reaches 7-21 cm/s. This type of peristaltic contraction occurs at the end of the digestive period.
Peristaltic waves can occur in any part of the small intestine. Most often they begin in the duodenum at the time of evacuation of gastric chyme. At the same time, several such wave-like contractions pass through the intestines, which give the intestinal movements a resemblance to the movement of a worm. This is where their name comes from - worm-shaped, or peristaltic, contractions.
Tonic contractions
may be local in nature or move through the intestine at low speed. Rhythmic and peristaltic waves are superimposed on tonic waves. Basal pressure in the cavity of the small intestine is determined not only by the tone of its muscular wall, but also by intra-abdominal pressure and in humans is 8-9 cm of water column. The value of intracavitary pressure in the intestine increases significantly with
the appearance of peristalsis. Tonic contractions underlie the motor activity of smooth muscle sphincters.
Micromovements of intestinal villi
promote mixing of chyme. The frequency of rhythmic contractions of the villi decreases from the proximal to the distal parts of the small intestine. The intestinal hormone vallikinin, produced in the mucous membrane of the small intestine, has a stimulating effect on their motor activity.
14.7.5. REGULATION OF MOTOR ACTIVITY OF THE SMALL INTESTINE
The motility of the small intestine is regulated by myogenic, nervous and humoral mechanisms.
A. Myogenic mechanism of regulation.
The motor activity of the small intestine is based on the properties of smooth muscle cells to spontaneously contract and respond to contraction when stretched.
Spontaneous activity of smooth muscles, manifested in the form of rhythmic generation of slow electrical waves, bursts of action potentials and phasic contractions of the small intestine in the absence of external stimulation, is provided by the myogenic mechanism. The frequency of generation of slow electrical waves is constant for each section of the small intestine and depends on the level of metabolism. A local decrease in temperature in the pacemaker area leads to a decrease in the frequency of generation of slow waves and rhythmic contractions of the smooth muscles of the small intestine and the speed of their propagation. Separation of the pacemaker from the underlying segments of the intestine by completely transecting the intestine or only the longitudinal muscular layer while preserving the external nerves reduces the frequency of intestinal contractions distal to the transection by 20-30%.
Myogenic mechanisms for regulating small intestinal motility also include the contractile response of smooth muscles to stretch. Contraction of the muscles of the longitudinal muscular layer of the intestine provides stretching of the circular muscles sufficient to cause their contraction.
B. Intramural nervous mechanisms of regulation.
The motor activity of the small intestine is regulated by the enteric nervous system - a complex of microganglionic formations, including a full set of neurons (sensory, endogenous,
cillators, interneurons, tonic and efferent neurons), giving it the features of true autonomy (A.D. Nozdrachev). The enteric nervous system exerts descending inhibitory tonic influences on the myogenic rhythm of intestinal smooth muscle. The endogenous oscillator of the intraganglionic ensemble is cholinergic, it causes excitation of the efferent peptidergic neuron, in the endings of which the inhibitory mediators VIP, ATP are released, causes hyperpolarization of the smooth muscle cell membrane, which leads to a decrease in the amplitude of slow electrical waves, cessation of the generation of peak potentials and inhibition of motor activity. intestinal activity. The enteric system, based on incoming sensory information received from receptors, programs and coordinates the motor activity of the small intestine.
The irritant that triggers and maintains intestinal movements is the stretching of its wall. Local irritation of the intestine after transection of extraorgan nerves causes a myenteric reflex,
manifested in muscle contraction above and relaxation below the site of irritation.
The reflex arc of the myenteric reflex closes in the intramural ganglia. Even more pronounced is the “mucous” local reflex,
which occurs when mechanical and chemical stimuli act on the intestinal mucosa, manifested in the contraction of circular muscles proximal to the chyme and their relaxation distal to the intestinal contents. Excitation of stretch receptors or chemoreceptors located in the mucous membrane is transmitted through sensory neurons of the submucosal plexus to interneurons of the intermuscular plexus, which leads to excitation of the cholinergic motor neuron and contraction of the circular muscles of the proximal intestine and activation of the peptidergic inhibitory neuron (ATP, VIP mediators) , causing relaxation of distally located circular muscles.
B. Central influences.
The cerebral cortex, the structures of the limbic system, and the hypothalamus play an important role in the regulation of intestinal motor activity.
Electrical stimulation of the anterior sigmoid gyrus of the cortex stimulates the motility of the small intestine, and on the contrary, it inhibits the orbital gyrus. Irritation of the anterior cingulate cortex (limbic cortex) and amygdala complex
causes both inhibitory and stimulatory effects depending on the initial functional state of the small intestine. Irritation of the nuclei of the anterior and middle parts of the hypothalamus primarily stimulates, and the posterior - inhibits the motility of the small intestine. However, in general, the effect of the central nervous system on the motility of the small intestine is predominantly inhibitory (Yu.M. Galperin).
The influence of the central nervous system on the motility of the small intestine is realized through sympathetic (adrenergic), parasympathetic (cholinergic) and, apparently, serotonergic nerve fibers. Excitation of parasympathetic fibers of the vagus nerves has a predominantly stimulating effect on the motility of the small intestine due to acetylcholine released in their endings. However, inhibitory effects may also occur. The mechanism of the inhibitory effect of the vagus nerve on intestinal motility has not been sufficiently studied. It is believed that its implementation is carried out through the activation of M-cholinergic receptors of the sympathetic terminals and the release of catecholamines by them. The inhibitory effect is better detected against the background of strong contractions of the intestine. Excitation of the sympathetic fibers of the splanchnic nerves has a depressing effect on the motor activity of the small intestine (Fig. 14.14, A). Evidence has been obtained that the splanchnic nerves contain serotonergic fibers, the excitation of which stimulates the motility of the small intestine (Fig. 14.14, B).
Reflexogenic zones and reflexes.
The basic law of reflex regulation of motor activity of the gastrointestinal tract is universal. Its effect is clearly demonstrated by the example of reflex regulation of small intestinal motility in the form of motor and inhibitory intestinal reflexes.
To motor reflexes
intestines include esophageal-intestinal, gastrointestinal and enteric-intestinal reflexes.
Esophageal-intestinal
the motor reflex occurs when the mechanoreceptors of the esophagus are irritated against the background of rest or weak contractions of the small intestine and manifests itself in the form of an increase in its tone and the amplitude of peristaltic waves. The reflex arc of this reflex closes in the medulla oblongata, and the efferent exciting influences on the motility of the small intestine are transmitted through the vagus nerves.
Gastrointestinal
motor reflexes (gastroduodenal, gastrojejunal and gastrointestinal)
structural) are observed when the mechanoreceptors of the stomach are irritated or filled with food, which leads to the appearance or intensification of existing contractions of the small intestine. Excitation to the small intestine during stomach irritation is transmitted in two ways: along the wall of the digestive tract - with the help of local reflexes that close in the ganglia of the enteric nervous system; reflexively - through the vagus nerves, with the closure of the reflex arc in the central nervous system.
Enteric
the motor reflex occurs with adequate mechanical and chemical stimulation of the small intestine and is manifested by increased contractions of the underlying sections of the intestine. Excitation from the proximal to the distal parts of the intestine is transmitted using local reflexes (Fig. 14.15), which are closed in the intramural ganglia, as well as central reflexes, which realize their stimulating effect on intestinal motility through the vagus nerves.
To inhibitory reflexes
intestines include reflex inhibition (relaxation) of the upper parts of the small intestine during food intake; enteric inhibitory reflex and rectoenteric reflex.
Reflex inhibition of small intestinal motility during the act of eating is manifested by a decrease in the tone and amplitude of peristaltic contractions of the proximal intestine, followed by an increase in its motor activity. This phenomenon is called perceptual inhibition
(receptive relaxation) of the intestine. Affe-
of the small intestine, chyme inhibits the entry into its cavity of subsequent portions from the proximal parts and enhances the motor-evacuation activity of this and lower segments of the intestine. When, as a result of hydrolysis, absorption and movement of chyme, its quantity in a given segment decreases, the inhibitory effect on the overlying sections of the intestine decreases. As a result, the motility of the proximal parts of the intestine increases and chyme moves down the intestine, entering the intestinal segment that is freed from contents, where the processes of hydrolytic breakdown of nutrients and the absorption of the products of their digestion into the blood and lymph continue.
D. Humoral regulation.
Motilin, gastrin, CCK, histamine, serotonin, substance P, bradykinin, vasopressin and oxytocin, acting on myocytes and neurons of the enteric nervous system, enhance, and secretin, VIP, GIP inhibit the motility of the small intestine.
the rental path of the reflex arc of this reflex begins with the receptors of the root of the tongue and pharynx, and the efferent link is represented by adrenergic fibers of the splanchnic nerve.
Enteric
the inhibitory reflex is caused by strong irritation of the mechano-receptors of any part of the gastrointestinal tract, which leads to a weakening of the motor activity of other parts, including the small intestine, with the exception of the ileocecal sphincter. The reflex closure occurs in the spinal cord below ThV|. The most important role in the implementation of this reflex belongs to the adrenergic fibers of the splanchnic nerve.
Rectointestinal
the reflex occurs as a result of irritation of the mechanoreceptors of the rectum and the sphincters of its ampulla. It is manifested by inhibition of motility of the small and large intestine. The closure of this reflex occurs in the spinal cord. The transmission of inhibitory influences from the rectum to the motor activity of the small intestine is carried out through adrenergic fibers of the splanchnic nerves.
The motor and inhibitory reflexes of the intestine that arise during the digestion process ensure the optimal rate of digestion of nutrients and absorption of hydrolysis products in each part of the small intestine. Overflow of any department
Mechano- and chemoreceptors of the colon and the possibility of drug effects on them
Physical stimuli. Mechanical stimuli play an important role in maintaining peristalsis: an increase in intraluminal pressure during the passage of gases or the flow of feces. Stretching the muscle sheath leads to depolarization of smooth muscle cells (SMCs) and response contraction. Depolarization in response to stretch is mediated by activation of mechanosensory, stretch-dependent potassium channels (stretch-dependent K+ (SDK) channels) [22]. In addition, when stretched, molecules associated with the cell cytoskeleton are activated, and this leads to the activation of processes that maintain the cell’s life cycle. It can be assumed that mechanical stimulation is necessary for the normal growth and differentiation of muscle cells, enteric nerves and blood vessels [23]. According to modern concepts, the most important role in the regulation of intestinal motor activity and sensitivity to mechanical stimuli belongs to the interstitial cells of Cajal, located between the circular and longitudinal muscle layers and constituting the syncytium with the SMC. The network of Cajal cells begins in the proximal parts of the stomach, and their accumulation in the body of the stomach is considered to be the pacemaker of the upper parts of the digestive system. Cajal cells contact the varicose terminals of the axons of nerve cells and the membranes of the SMC, modulating the transmission of nerve impulses to muscle fibers. At the same time, they have been shown to be sensitive to mechanical stimuli [27]. Stretching the walls of the stomach during food intake causes depolarization of the membrane and the appearance of slow-wave activity of the pylorus. Apparently, this underlies the so-called gastro-colytic reflex, which is expressed in the appearance of the urge to defecate after 20–30 minutes. after breakfast [3]. Interestingly, the generator function of Cajal cells depends on the activity of cyclooxygenase-2: when this enzyme is suppressed, they become insensitive to stretching. Apparently, eicosanoids, products of cyclooxygenase-2, are involved in the perception of stretching [27]. In turn, SMC contractility and the function of mechanosensory potassium channels are influenced by humoral factors, intestinal hormones and the central nervous system. Type C natriuretic peptide secreted by the endothelium, which has a paracrine effect on gastrointestinal tract SMCs, stimulates the production of cyclic guanosine monophosphate (cGMP) and causes relaxation of gastrointestinal SMCs. Experiments have shown that along the gastrointestinal tract there are areas with high sensitivity to the action of natriuretic peptide, which are “responsible” for slowing down transit. In mice, such sensitivity is shown for the pylorus, colon and rectum. In the future, receptor targeting strategies may be used to treat ileus and pyloric stenosis [26]. Glucagon-like peptide-1 is found in the mucosa, stratum circularis, and myenteric plexus of the colon. It suppresses the contractility of the circular muscle layer without having a noticeable effect on the longitudinal one. The expression of this peptide has been shown to be significantly increased in irritable bowel syndrome (IBS) with constipation [6]. In recent years, much attention has been paid to imbalance in the state of the parasympathetic and sympathetic nervous system during constipation and other conditions, in particular, with bronchial obstruction, gastroesophageal reflux, rhinosinusitis, and cardiovascular diseases. In IBS with constipation, disturbances in parasympathetic nervous regulation are noted [1, 5]. Chemical stimuli. The perception of chemical stimuli - chemoreception - involves the interaction of molecules with proteins on the cell membrane, which leads to the activation of ion channels and depolarization. A variety of chemoreceptors have been found in the colon, and it is likely that many have not yet been discovered. This indicates a high degree of its interaction with the external environment and the plasticity and adaptability of the intestine to external conditions. A very interesting fact is that chemoreceptors similar to taste buds have been identified throughout the gastrointestinal tract and in the colon [4]. Bile acids. Bile acids play a critical role in the regulation of motility and secretion in the colon. The main part of bile acids (95%) is absorbed in the terminal ileum, enters the portal vein and is transported to the liver. Enteric plexus neurons and colonic endocrine cells express the G protein-coupled bile acid receptor TGR5 (or GPBAR1). The interaction of bile acids with this receptor leads to increased motility, as well as the secretion of chlorides into the lumen. A change in the flow of bile acids into the colon due to pathology or as a result of side effects of drugs leads to diarrhea or constipation. It is possible that individuals with reduced TGR5 receptor density are prone to constipation. This assumption, from our point of view, is supported by the results of the work of F. Alemi et al. In a mouse study, the authors clearly demonstrated the importance of TGR5 in regulating transit time, fecal water content, and bowel movement frequency. This work compared wild-type mice (with normal TGR5 gene function) and mice with the TGR5 gene knocked out. Exposure to bile acids caused colonic peristaltic contractions through the release of 5-hydroxytryptamine and calcitonin gene-associated peptide by primary afferent neurons and enterochromaffin cells. Contractions induced by mechanical stimuli or other transmitters were independent of bile acid receptor activation. In mice with the TGR5 gene knocked out, the total duration of intestinal transit was 1.4 times longer than in wild-type mice. In the absence of the TGR5 receptor, defecation occurred 2.6 times less often, and the water content in the stool was low (37% compared to 62% in mice with an intact receptor) [2]. Bile acid preparations are currently being developed to treat constipation. Food components. Fruits, berries and, to a lesser extent, raw vegetables have a laxative effect due to the high content of plant glycosides that stimulate intestinal chemoreceptors. In some fruits of plant origin, the content of glycoside-like substances is especially high: these are ripe plums, buckthorns, figs, cherries, grapes, and gooseberries. In recent years, interesting information has emerged that some foods contribute to constipation due to the high content of substances that inhibit peristalsis. Opioid-like substances have been found in wheat products (bread, pasta, baked goods, confectionery, and products containing whole grains) and dairy products (milk, cheese, yogurt). Some milk opioids are 100 times more potent than morphine. The review article by J. Coleman examines the facts and theoretical principles about the influence of products - wheat, milk and meat from cows that received feed enriched with opioids on the state of the nervous system [8]. The author points out that wheat gluten contains up to 15 opioid sequences; During the digestion process, these exorphins are released and become active [11, 12]. Human digestive enzymes are not able to fully hydrolyze exorphins; Perhaps, like gluten, they are able to survive in the intestinal wall for many months. Inhibition of peristalsis can also be caused by plants rich in alkaloids - β-carbolines, and protein-rich foods (meat, fish, beans, cereals, soybeans) subjected to heat treatment, during which similar substances are formed. β-carbolines have a local anesthetic effect and reduce the sensitivity of intestinal chemo- and mechanoreceptors and slow down peristalsis. Microflora. The intestinal microflora produces short-chain fatty acids (SCFA). Although SCFAs exhibit an antidiarrheal effect by improving water absorption and reducing mucus secretion, in general, lactic acid bacteria have a stimulating effect on peristaltic activity. Experiments have shown that acetic acid enhances ileal motility, stimulates the ileocecal reflex, and improves blood supply to the colon. Butyric and valeric acids stimulate the motility of the middle and distal part of the colon. [24]. It is believed that the propulsive effect of SCFA is realized through its effect on chemoreceptors, the intestinal nerve plexus, and also through direct stimulation of SMCs [7]. Nature wisely ordered that irritants pass into their active form already at the level of the colon - under the influence of microflora. Thus, some plant glycosides become active in the process of hydrolysis by the microflora of the colon, and thus have virtually no irritating effect in the overlying sections where active digestion occurs. Apparently, the same applies to the action of bile acids, which undergo transformations under the influence of microflora. In IBS, an increase in the population of sulfate-oxidizing microorganisms, bacteria of the Enterobacteriaceae family, and a decrease in the content of bifidobacteria were noted [14]. Perhaps, through its effect on intestinal chemoreceptors, this is associated with the clinical manifestations of the disease. Recently, a study in gnotobiotic rats was published showing that transfer of fecal microflora from a patient with IBS can induce intestinal distension hypersensitivity [9]. The ability to influence the motility and secretion of the colon by influencing chemo- and mechanoreceptors is widely used in practice: dietary recommendations and the effect of antidiarrheal and laxatives are based on this (Table 1). Contact laxatives. The principle of action of contact (stimulating, local) laxatives is also based on irritation of intestinal chemoreceptors. Laxatives that increase the volume of intestinal contents and cause activation of mechanoreceptors in the muscle layer are referred to as “bulk laxatives”; however, they may partially have a stimulating and emollient effect, especially if they contain plant components. In particular, psyllium-based preparations contain the iridoid glycoside aucubin. Aucubin undergoes hydrolysis to form glucose and aucubigenin; the latter irritates chemoreceptors and activates peristalsis. Suppositories with glycerin also have a partially stimulating effect. Laxatives with a classic stimulant effect are represented by drugs based on senna, bisacodyl and sodium picosulfate; Castor oil and misoprostol are used much less frequently. Cassia leaves, also known as “Alexandria leaf,” are used as pharmaceutical raw materials for the manufacture of senna preparations. The active substances contained in the plant are represented by “sennosides” - anthraglycosides and anthraquinones rhein, aloe-emodin, etc. These compounds irritate the chemoreceptors of the colon and cause increased peristaltic activity. Some senna alkaloids also exhibit anticholinesterase activity [25]. Senna is often included in herbal combined laxatives, incl. dietary supplements. When using them, the possibility of potentiating the effects of different components should be taken into account. Long-term use of senna preparations can provoke the development of pseudomelanosis of the colon - pigment deposits in the macrophages of the submucosal layer; with long-term use, pigment deposition in the renal tubules is also possible. Available data do not suggest that preparations containing senna extract or sennozoides have genotoxic potential [19, 20]. By their chemical structure, bisacodyl and sodium picosulfate are diphenylmethane derivatives. In the lumen of the large intestine, bisacodyl undergoes hydrolysis under the action of enzymes of the mucous membrane, and sodium picosulfate - under the action of enzymes of the normal microflora of the large intestine. The active substance formed as a result of hydrolysis, biphenol, interacts with the chemoreceptors of the intestinal wall, affects the calcium channels of the SMC, enhancing the natural high-amplitude contractions of the colon and the secretion of water and electrolytes into the lumen of the colon [17]. Modern dosage forms of bisacodyl - Dulcolax® - are represented by tablets coated with a special pH-sensitive coating and rectal suppositories. The shell of tablets for oral administration is made on the basis of Eudragit L 100 and Eudragit S 100 polymers, which cause its dissolution in an alkaline medium with pH>7.0, which is characteristic only of the large intestine, which ensures the release of the active substance selectively in the initial parts of the large intestine. This avoids the effect of bisacodyl on the small intestine, interference with the digestive processes and the entry of the drug into the enterohepatic circulation. The results of large randomized studies of the effectiveness and safety of bisacodyl when administered as a course were obtained specifically for the dosage form with a pH-sensitive shell [15, 16]. It is advisable to take Dulcolax® tablets at night so that the effect of the drug (after 6–12 hours) coincides with the appearance of morning peristaltic contractions after waking up. The effect of rectal suppositories appears on average after 30 minutes. Dulcolax® in tablets or rectal suppositories can be prescribed occasionally and in courses lasting up to 4 weeks. The effectiveness and favorable safety profile of Dulcolax® have been proven by numerous international placebo-controlled randomized clinical trials, which have shown a statistically significant increase in stool frequency, improvement in stool consistency and no need to strain in patients with chronic constipation compared to placebo; at the same time in 4 weeks. There was no addiction or effect on electrolyte levels [15, 16, 21]. Bisacodyl (Dulcolax®) is effective for all types of constipation: with normal and delayed transit, episodic and chronic, proctogenic; often used in the form of short courses for painful processes in the anorectal area, in the postpartum period, in patients with limited motor activity (schemes 1, 2) [15, 16]. During simultaneous treatment with antacids, antisecretory agents, and consumption of dairy products, an interval of at least 1 hour should be observed before taking Dulcolax, because in an alkaline environment, release of the active substance and irritating effect of the drug in the upper gastrointestinal tract are possible. Bisacodyl should be taken at least 2 hours before taking digoxin, because it is able to reduce the concentration of glycoside in the blood. Bisacodyl, like many other laxatives, is contraindicated in case of: hypersensitivity to the active or excipients; acute diseases of the abdominal organs, including appendicitis; intestinal obstruction and obstructive bowel diseases; acute inflammatory bowel disease; severe dehydration; severe pain in the abdominal area with nausea and vomiting [28]. Bisacodyl, like other laxatives, should be administered with caution to patients with hepatic and/or renal insufficiency. The safety of bisacodyl in pregnant women has not been studied, so it should only be used after consultation with a specialist if the expected benefit to the mother outweighs the risk to the fetus [28]. Bisacodyl does not pass into breast milk, so the drug can be used during breastfeeding. Dulcolax® tablets are approved for children from 4 years of age, and suppositories - from 10 years of age [28].
References 1. Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J et al. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities // Gastroenterology. 1994. Vol. 106. R. 945–950. 2. Alemi F., Poole DP, Chiu J. et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice // Gastroenterology. 2013 Jan. Vol. 144(1). R. 145–154. 3. Beckett EA, McGeough CA, Sanders KM & Ward SM // J. Physiol. 2003. Vol. 553. R. 545–559. 4. Bezencxon S. le Coutre J., Damak S. Taste-Signaling Proteins Are Coexpressed in Solitary Intestinal Epithelial Cells // Chem. Senses. 2007. No. 32. P. 41–49. 5. Canning BJ Reflex regulation of airway smooth muscle tone // J Appl Physiol. 2006 Sep. Vol. 101(3). R. 971–985. 6. Chen Y., Li Z., Yang Y. et al. Role of glucagon-like peptide-1 in the pathogenesis of experimental irritable bowel syndrome rat models // Int J Mol Med. 2013 Mar. Vol. 31(3). R. 607-613a. 7. Cherbut C. Effects of short-chain fatty acids on gastrointestinal motility. In: Physiological and Clinical Aspects of Short-Chain Fatty Acids, edited by Cummings JH, Rombeau JL and Sakata T. Cambridge, UK: Cambridge Univ. Press, 1995. P. 191. 8. Coleman J. Opioids In Common Food Products - Addictive Peptides In Meat, Dairy and Grains. https://www.karlloren.com/diet/p18.htm. 9. Crouzet L., Gaultier E., Del'Homme C. et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota // Neurogastroenterol Motil. 2013 Apr. Vol. 25 (4). R. 272–282. 10. Di Palma JA, Halpert A. Guideline. Managing: Chronic Constipation. Version 1.0. International Gudelines Center. 2008. www. 11. Fukudome S., Jinsmaa Y., Matsukawa T., Sasaki R. and Yoshikawa M. Release of opioid peptides, gluten exorphins by the action of pancreatic elastase // FEBS letters. 1997. Vol. 412(3). R. 475–479. 12. Fukudome S., Yoshikawa M. Gluten exorphin C. A novel opioid peptide derived from wheat gluten // FEBS Lett. 1993. Vol. 316(1). R. 17–19. 13. Hsieh C. Treatment of constipation in older adults // Am Fam Physician. 2005 Dec 1. Vol. 72 (11). R. 2277–2284. 14. Jia W., Whitehead RN, Griffiths L. et al. Diversity and distribution of sulphate-reducing bacteria in human faeces from healthy subjects and patients with inflammatory bowel disease // FEMS Immunol Med Microbiol. 2012 Jun. Vol. 65(1). R. 55–68. 15. Kamm MA, Mueller-Lissner S, Wald A et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation // Clin Gastroenterol Hepatol. 2011 Jul. Vol. 9 (7). R. 577–583. 16. Kienzle-Horn S., Vix J.M., Schuijt C. et al. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebo-controlled study // Aliment Pharmacol Ther. 2006 May 15. Vol. 23 (10). R. 1479–1488. 17. Manabe N., Cremonini F., Camilleri M. et al. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers // Aliment Pharmacol Ther. 2009 Nov 1. Vol. 30(9). R. 930–936. 18. Menees S., Saad R., Chey W.D. Agents that act luminally to treat diarrhoea and constipation // Nature Reviews Gastroenterology & Hepatology. 2012. No. 9. R. 661–674. 19. Mitchell J.M., Mengs U., McPherson S., Zijlstra J., Dettmar P., Gregson R., Tigner JC. An oral carcinogenicity and toxicity study of senna (Tinnevelly senna fruits) in the rat. Arch Toxicol. 2006 Jan. Vol. 80(1). R. 34–44. 20. Morales MA, Hernández D., Bustamante S., Bachiller I., Rojas A. Is senna laxative use associated with cathartic colon, genotoxicity, or carcinogenicity? // J Toxicol. 2009. 2009. 287247. 21. Mueller-Lissner S, Kamm MA, Wald A et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation // Am J Gastroenterol. 2010 Apr. Vol. 105(4). R. 897–903. 22. Sanders KM, Koh SD Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation // J Physiol. 2006 Jan. 1.Vol. 570 (Pt 1). R. 37–43. 23. Sawada Y. Website https://www.dbs.nus.edu.sg/staff/sawada.html. 24. Scheppach W. Effects of short chain fatty acids on gut morphology and function. // Gut. 1994 Jan. Vol. 35 (1 Suppl). R. 35–38. 25. Serrano MA, Pivatto M., Francisco W., Danuello A., Regasini LO, Lopes EM, Lopes MN, Young MC, Bolzani VS Acetylcholinesterase inhibitory pyridine alkaloids of the leaves of Senna multijuga // J Nat Prod. 2010 Mar 26. Vol. 73(3). R. 482-484. 26. Sogawa C., Wakizaka H., Aung W. et al. C-type natriuretic peptide specifically acts on the pylorus and large intestine in mouse gastrointestinal tract // Am J Pathol. 2013 Jan. Vol. 182(1). R. 172–179. 27. Won K.-J., Sanders KM, Ward SM Interstitial cells of Cajal mediate mechanosensitive responses in the stomach // Proc Natl Acad Sci USA. 2005 Oct. 11.Vol. 102 (41). R. 14913–14918. 28. Instructions for medical use of the drug Dulcolax® tablets.
Small bowel movements
Movements of the small intestines occur as a result of coordinated contractions of transverse and longitudinal muscle fibers. There are two types of intestinal movements: pendular and peristaltic.
Pendulum-like movements are manifested in the fact that in a short section the intestine sometimes shortens, sometimes lengthens, and the contents move in one direction or the other. During pendulum-like movements, alternating rhythmic contractions of the longitudinal and curved muscle fibers of the intestine occur. Contractions of the longitudinal muscles cause shortening and thereby expansion of the intestinal section. Contractions of the circular muscle fibers narrow the intestinal lumen and move the intestinal contents to both sides of the narrowed area. They occur randomly in one or another part of the intestine. The rhythm of pendulum-like contractions reaches 20 contractions per minute in the upper sections and up to 5-10 per minute in the lower sections of the small intestines. Due to the non-simultaneous contractions of different parts of the intestines, the so-called rhythmic segmentation of the intestinal contents occurs, which is either divided into parts (segmented) or joined together again.
The physiological significance of pendulum-like movements is the mixing of intestinal contents with digestive juices.
Another type of intestinal movement is peristalsis; it consists in the fact that above the food bolus, a circular interception is formed due to the contraction of circular fibers, and below, due to the contraction of the longitudinal muscles, an expansion of the intestinal cavity is formed. Thanks to such contractions, the contents of the intestine move to the expanded area. The contraction of the circular muscle fibers then spreads to this area, which narrows; Below, the intestine expands due to contraction of the longitudinal muscles.
Thus, a wave of contraction of circular fibers spreads throughout the intestine, and their contraction in each individual section is preceded by a contraction of longitudinal fibers in the underlying segment. Naturally, this kind of movement of the small intestines moves their contents only in this direction - from top to bottom.
At the same time, several such wave-like contractions pass along the length of the intestine, which give the intestinal movements a resemblance to the movement of a worm. This is where their name comes from - worm-like, or peristaltic, movements.
Rhythmic contractions of the intestinal muscles, whether pendulum-like or peristaltic, occur against the background of a constantly existing tone, i.e. some tension in the intestinal muscles. Its tone, however, is not constant - you can observe its increase or decrease.
Smooth muscle fibers of the intestine have automaticity, which is manifested in their ability to contract rhythmically in the absence of external irritations.
The automation of intestinal muscles and the influence of various salts, poisons and other substances on it can be studied on an isolated section of intestine cut out from the body. Such a segment can be reduced for hours, provided that it is immersed in a Locke or Tyrode solution heated to body temperature and saturated with oxygen.
After removal of the ganglion cells of the Auerbach plexus, the muscles of the intestine, as well as the stomach, continue to perform rhythmic pendulum-like contractions. This gives us the right to believe that rhythmic automation is of myogenic origin, i.e., inherent in the muscular elements of the intestine.
| As for peristaltic movements, which are a rather complex coordinated activity, they are carried out only in the presence of nerve cells of the Auerbach plexus embedded in the intestinal wall. The role of ganglion nerve cells is to coordinate contractions of the longitudinal and circular muscles of the intestine. Contractions of intestinal muscles are regulated by reflex-humoral-chemical agents. Impulses from the central nervous system enter the muscles of the intestinal wall via the vagus and sympathetic nerves. The nature of the influence of these nerves on intestinal motor function is opposite. Their effect can be studied in experiments with electrical stimulation. Irritation of the n.vagi stimulates intestinal movements, i.e., it enhances muscle contractions and increases tone (Fig. 88, A). Irritation of n.splauchici causes inhibition of intestinal contractions and a sharp drop in muscle mass and chemistry (Fig. 88, B). Depending on the strength of irritation, the state of the intestinal muscles, blood chemistry, the nature of metabolic processes and other physiological conditions, the intestinal nerves can cause various effects that do not coincide with those indicated above. Rice. 88. The influence of irritation of the vagus (A) and sympathetic (B) nerves on the motor activity of the small intestine. |
The influence of the nervous system on the motor function of the intestines is clearly manifested when emotional states arise in a person or animal. Emotions of anger, fear, and pain usually lead to inhibition of intestinal contractions due to the fact that during emotional states, excitation of the sympathetic nervous system occurs. With some strong emotions, for example fear, violent intestinal peristalsis (“nervous diarrhea”) is sometimes observed.
When the autonomic nerves innervating the intestines are irritated, chemical transmitters of the nerve impulse - mediators are formed at their endings: acetylcholine when the vagus nerve is irritated, norepinephrine when the sympathetic nerve is irritated.
If you prevent the destruction of acetylcholine by cholinesterase and irritate the vagus nerve in a dog whose vessels are connected to the vessels of the second dog, so that a continuous exchange of blood occurs between both animals (cross-circulation), then when irritating the vagus nerve in one dog, a change in intestinal contractions and in the second (Fig. 89). This depends on the fact that acetylcholine, formed in the nerve endings of the vagus nerve and destroyed by cholinesterase, enters the blood and can have an effect at a distance from the organ where it was formed.
| Rice. 89. The influence of irritation of the vagus nerve on the movements of the small intestines of two dogs under conditions of cross circulation (according to K. B. Babsky and others). The midline marks the period of vagus nerve irritation in one dog. Once the stimulation of the vagus nerve ceases in one animal (lower curve), strong intestinal contractions begin in the other. |
Humoral irritants that stimulate intestinal movements are choline and some other substances formed by the mucous membrane of the duodenum and small intestines and entering the blood during digestion - enterocrinin and 5-hydroxytryptamine (serotonin). They are considered as specific hormones - causative agents of intestinal movements. Polypeptides, extractive substances, bile and salts of potassium, calcium, magnesium, which are absorbed in the small intestines, also change the motor activity of the intestine in a humoral way.
Contractions of intestinal smooth muscles occur as a result of mechanical and chemical irritations of the mucous membrane. Thus, stretching of the intestinal wall with food gruel (chyme) coming from the stomach causes peristaltic and pendular movements. The faster the intestine stretches, the stronger the contractions of the intestinal muscles. Mechanical irritation explains the fact that so-called roughage, containing difficult-to-digest substances, such as bran, is a powerful irritant of intestinal movements.
Chemical irritants that cause intestinal movements upon contact with the mucous membrane are acids, alkalis and many salts (the latter in concentrated solutions). Thus, the introduction of gastric juice, weak solutions of acids and alkalis into the intestine significantly enhances intestinal contractions and increases muscle tone. Certain products of digestion of nutrients, such as soap, sharply stimulate the movements of the digestive tract.
The mechanism of influence of all local, i.e., mechanical and chemical stimuli acting on the intestinal mucosa is quite complex. They can influence, firstly, reflexively, stimulating the mechano-chemoreceptors of the mucous membrane, and secondly, stimulate the formation of chemical compounds that, when absorbed into the blood, stimulate intestinal movements.
Motor activity of the small intestine
The motor activity of the small intestine ensures the mixing of food contents with digestive secretions, the movement of chyme through the intestine, the change of the layer of chyme and its mucous membrane, and an increase in intraintestinal pressure, which promotes the filtration of some components of chyme from the intestinal cavity into the blood and lymph.
Contraction of the small intestine occurs as a result of coordinated movements of the longitudinal (external) and transverse (circulatory, i.e. internal) layers of smooth muscle cells. These abbreviations can be of several types.
According to the functional principle, all abbreviations are divided into two groups:
- Local, they provide mixing and rubbing of the contents of the small intestine.
- Aimed at moving intestinal contents.
There are several types of contractions: rhythmic segmentation, pendular, peristaltic (very slow, slow, fast, rapid), antiperistaltic and tonic.
Rhythmic segmentation is provided mainly by contractions of the circulatory layer of muscles. In this case, the contents of the intestine are divided into parts. The next contraction forms a new segment of the intestine, the contents of which consist of parts of the former segment. This achieves mixing of the chyme and increasing pressure in each of the forming segments of the intestine.
Pendulum-like contractions are provided by contractions of the longitudinal muscle layer with the participation of the circulatory layer. With these contractions, chyme moves forward and backward and a slight forward movement occurs.
Peristalsis consists of the fact that above the chyme, due to the contraction of the circular layer of muscles, an interception is formed, and below, as a result of the contraction of the longitudinal muscles, an expansion of the intestinal cavity occurs. These interceptions and expansions move along the intestine, moving a portion of chyme in front of the interception. Several peristaltic waves simultaneously move along the length of the intestine.
During antiperistaltic contractions, the wave moves in the opposite (oral) direction. Normally, the small intestine does not contract antiperistaltically. Tonic contractions can have a very low speed, and sometimes not spread at all, significantly narrowing the intestinal lumen over a large area.
The motility of the small intestines is regulated by nervous and humoral mechanisms; the role of myogenic mechanisms, which are based on the properties of smooth muscle automation, is quite important.
The regulation of small intestinal motility is carried out by the intramural nervous system and the influences of the central nervous system. Intramural neurons provide coordinated contractions of the intestine. Their role is especially great in peristaltic contractions. Intramural mechanisms are influenced by extramural, parasympathetic and sympathetic nervous mechanisms, as well as humoral factors.
Parasympathetic nerve fibers primarily excite, while sympathetic nerve fibers inhibit contractions of the small intestine. These fibers are conductors of reflex regulation of small intestinal motility. The act of eating food conditionally and unconditionally reflexively first briefly inhibits and then enhances intestinal motility.
Irritation of the nuclei of the anterior and intermediate regions of the hypothalamus primarily excites, and the posterior - inhibits the motility of the stomach, small and large intestine. The cerebral cortex influences intestinal motility mainly through the hypothalamus and limbic system.
The important role of the cerebral cortex and the second signaling system in the regulation of intestinal motility is proven by the fact that when talking or even thinking about tasty food, intestinal motility increases, while with a negative attitude towards food, intestinal motility is inhibited. With anger, fear and pain, it is also inhibited. Sometimes, with some strong emotions, such as fear, violent intestinal peristalsis (“nervous diarrhea”) is observed.
Reflexes from various parts of the digestive tract to the motor apparatus of the small intestine are important: esophageal-intestinal (exciting), gastrointestinal (exciting) and intestinal (exciting and inhibitory), rectoenteric (inhibitory). The arcs of these reflexes are closed in the central nervous system, as well as in the ganglia of the autonomic nervous system.
Adequate irritation of any part of the gastrointestinal tract causes excitation in the irritated and underlying areas and increased movement of contents in the caudal direction from the site of irritation; at the same time, it inhibits motility and delays the advancement of chyme in the overlying parts of the gastrointestinal tract.
The motor activity of the intestine depends on the physical and chemical properties of chyme.
Rough foods (brown bread, vegetables, etc.) and fats increase its activity.
Consequently, the activity of any part of the intestine is the total result of the excitatory influence from the proximal and inhibitory influence from the distal (relative to this) parts of the gastrointestinal tract.
Humoral substances change intestinal motility, acting directly on muscle fibers and through receptors on neurons of the intramural nervous system. Vasopressin, oxytocin, bradykinin, serotonin, histamine, gastrin, motilin, cholecystokinin-pancreozymin, substance P and a number of other substances (acids, alkalis, salts, products of digestion of nutrients, especially fats) enhance the motility of the small intestine.