The drug "Karsil" is an effective and inexpensive remedy for the treatment of chronic liver inflammation. The composition includes only a natural component - spotted milk thistle extract. Thanks to this, the results of treatment become noticeable within a month - some patients experience cases of complete recovery. There are not many side effects of this drug, and they are rare.
What is Karsil?
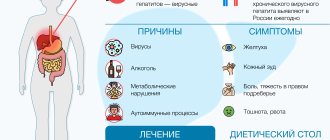
Karsil is a drug from the group of hepatoprotectors. The active component of the drug is silymarin - a substance contained in milk thistle.
Silymarin has an anti-inflammatory effect that helps reduce liver damage associated with viral infections by mitigating the inflammatory cascade and modulating the immune system.
Carsil has an antioxidant effect, which is detected by inhibiting the penetration of toxins into the liver. Hazardous substances are neutralized physiologically, since the active substance interacts with free radicals, interrupts the process of lipid peroxidation and prevents cell damage.
The drug produces the synthesis of proteins and phospholipids in areas of the liver that have lesions. This way it normalizes lipid metabolism, stabilizes and promotes cell membrane regeneration.
What you need to know before you start taking Karsil
Indications for taking Karsil:
- liver damage due to toxins;
- viral hepatitis;
- pancreatitis;
- hyperlipidemia;
- prevention of atherosclerosis;
- dyskinesia (impaired motility) of the biliary tract.
Thanks to the properties of the medicine and proper therapy, the patient’s well-being improves, complaints of weakness, a feeling of heaviness in the right hypochondrium, loss of appetite and vomiting disappear. Long-term use increases the survival rate of people with cirrhosis.
Contraindications for the use of Karsil:
- hypersensitivity to silymarin or additional components in the composition;
- acute poisoning of any origin.
Silymarin has an estrogen-like effect, so it should only be used as prescribed by a doctor if you have hormonal disorders.
Karsil contains a negligible amount of wheat starch and is considered safe for patients with gluten intolerance.
Also among the auxiliary components are lactose monohydrate and sucrase. Therefore, you should not take Karsil if you have the following conditions:
- hereditary intolerance to galactose and fructose;
- Lapp lactase deficiency;
- glucose-galactose malabsorption syndrome;
- sucrase-isomaltase deficiency.
Indications and contraindications
The drug is indicated for use in the presence of the following disorders:
- chronic liver inflammation;
- liver damage due to poisoning;
- alcohol intoxication;
- cirrhosis of the liver.
The drug is prohibited for use by children under 5 years of age inclusive, as well as in a state of acute poisoning (for various reasons). Also, use is excluded if you have an individual intolerance to silymarin or other substances included in the tablets.
How to take Karsil?
When treating liver diseases, you should stick to a diet and not drink alcohol. Use during pregnancy and lactation.
At the moment, there are no reliable studies on the effect of the drug on intrauterine development of the fetus or lactation. The need for treatment with this drug can only be determined by a doctor after a complete examination. The use of the drug Karsil can only be determined by the fact that the benefit from such therapy for the mother will be higher than the potential risk for the child.
Note!
If you have problems with the functioning of the vestibular apparatus, then take the drug with caution.
Instructions Karsil
Registration number: P N014839/01
Trade name of the drug: Karsil ®
International nonproprietary name or generic name: milk thistle fruit extract
Dosage form: pills.
Composition of 1 dragee contains:
Active ingredient: milk thistle fruit extract dry (equivalent to silymarin) - 35,000 mg. Kernel excipients: lactose mono-hydrate (55.380 mg), wheat starch (55.380 mg), povidone (Kolidon 25) (1.650 mg), microcrystalline cellulose (67.000 mg), magnesium stearate (2.500 mg), talc (7.000 mg), dextrose monohydrate (20.600 mg), sorbitol (4.130 mg), sodium bicarbonate (1.360 mg). Shell excipients: cellacephate (0.840 mg), diethyl phthalate (0.600 mg), sucrose (162.190 mg), acacia gum (1.562 mg), gelatin (0.860 mg), talc (26.718 mg), titanium dioxide (4.820 mg), macrogol ( PEG 6000) (0.130 mg), Brown Opalux dye (sucrose, red iron oxide, black iron oxide, methyl and propyl parahydroxybenzoate, purified water) (2.250 mg), glycerol (0.030 mg).
Description of the drug Karsil
Dragees are biconvex in shape, coated with a brown outer layer and a white inner layer. Fracture appearance: brownish-yellow to light brown.
Pharmacotherapeutic group: hepatoprotective agent
ATC code: [A05BA03] Pharmacological properties Pharmacodynamics: Karsil® belongs to the group of hepatoprotective drugs. Contains milk thistle fruit dry extract (silymarin equivalent), which is a mixture of 4 flavonolignan isomers: silibinin, isosilibinin, silydianin and silicristin. The mechanism of action of the drug is still not well understood. It has been established that the hepatoprotective effect of silymarin is caused by a competitive interaction with toxins on the corresponding receptors in the hepatocyte membrane, thus exhibiting a membrane-stabilizing effect. Silymarin has metabolic and cell-regulatory effects by regulating cell membrane permeability, inhibiting the 5-lipoxygenase pathway, especially leukotriene B4 (LTB4), and also binding to free reactive oxygen radicals. Stimulates the synthesis of proteins (structural and functional) and phospholipids in affected hepatocytes, accelerating regenerative processes. The action of flavonoids, to which silymarin belongs, is also determined by their antioxidant and microcirculation-improving effects. Clinically, these effects are expressed in the improvement of subjective and objective symptoms and in the normalization of indicators of the functional state of the liver (transaminases, gammaglobulin, bilirubin). This leads to an improvement in general condition, a reduction in complaints related to digestion, and in patients with reduced absorption of food due to liver disease, it leads to an increase in appetite and weight gain.
Pharmacokinetics: After oral administration, silymarin is slowly absorbed from the gastrointestinal tract. Subjected to enterohepatic circulation. Does not accumulate. When studying C14-labeled silibinin, the highest concentrations are found in the liver and very small amounts in the kidneys, lungs, heart and other organs. Metabolized in the liver through conjugation. Glucuronides and sulfates are found in bile as metabolites. The half-life is 6 hours. It is excreted mainly in bile (about 80%) in the form of glucuronides and sulfates and to a small extent (about 5%) in urine.
Indications for use of Karsil
— Toxic liver damage; — Conditions after acute hepatitis; — Chronic hepatitis of non-viral etiology; — Liver steatosis (non-alcoholic and alcoholic); — In complex therapy of liver cirrhosis; — Prevention of liver damage due to prolonged use of medications, alcohol, and chronic intoxication (including occupational).
Contraindications Hypersensitivity to the active substance or any of the excipients of the drug. Children under 12 years of age.
Use during pregnancy and breastfeeding It is not recommended to use the drug during pregnancy and breastfeeding.
Method of administration of Karsil and dose
The pills are taken orally before meals with a sufficient amount of water. The course of treatment lasts at least 3 months. Adults and children over 12 years of age: treatment of severe liver damage begins with a daily dose of 420 mg (4 tablets 3 times a day). For milder cases and as maintenance therapy - 1-2 tablets 3 times a day. As a preventive measure, take 2-3 tablets per day.
Side effects The drug is well tolerated. The following side effects are rarely observed: - from the gastrointestinal tract: nausea, dyspepsia, diarrhea. - on the skin: in isolated cases, allergic skin reactions are possible - itching, rash, alopecia. Others: It is rare to see an exacerbation of existing vestibular disorders. Side effects are transient and disappear after stopping the drug.
Overdose There is no evidence of drug overdose. In case of accidental ingestion of a high dose, it is necessary to induce vomiting, perform gastric lavage using activated charcoal and carry out symptomatic treatment if necessary.
Interaction with other drugs Pharmacodynamic drug interactions Silymarin does not have a significant effect on the pharmacodynamics of other drugs. When silymarin is used together with oral contraceptives and drugs used in hormone replacement therapy, the effects of the latter may be reduced.
Pharmacokinetic drug interactions Silymarin may enhance the effects of drugs such as diazepam, alprazolam, ketoconazole, lovastatin, vinblastine due to its inhibitory effect on the cytochrome P 450 system. Clinical studies show a negligible risk of possible pharmacokinetic interactions of silymarin as an inhibitor of the CYP3A4 and UGT1A1 isoenzymes, and cytostatics, which are substrates of these enzymes.
Special instructions Prescribe with caution to patients with hormonal disorders (endometriosis, uterine fibroids, breast, ovarian and uterine carcinoma, prostate carcinoma) due to the possible manifestation of the estrogen-like effect of silymarin. The drug contains wheat starch as an excipient, which may pose a risk of worsening the condition in patients with celiac disease (celiac enteropathy), as well as glycerol, which can cause headaches or irritant effects on the gastric mucosa when using high doses.
Release form Dragee 35 mg. 10 tablets per blister made of PVC film and aluminum foil. 8 or 18 blisters along with instructions for use in a cardboard box.
Storage conditions: In a dry place, protected from light, at a temperature not exceeding 25 °C. Keep out of the reach of children!
Shelf life: 2 years. Do not use after the expiration date stated on the package.
Conditions for dispensing from pharmacies Without a prescription.
Manufacturer Sopharma JSC Bulgaria, 1220 Sofia, st. Ilienskoe highway 16 Tel.: (+359 2) 81 34 200; fax: (+359 2) 936 02 86.
Consumer complaints and information about adverse events should be sent to the following address: Representative office of Sopharma JSC (Bulgaria) Moscow Russian Federation, 109429, Moscow, MKAD, 14 km, no. 10. Tel.: (495) 786-2226
Method of application and dosage of Karsil
Karsil is available in the form of dragees (milk thistle content equivalent to 22.5 mg silymarin) and capsules (milk thistle content equivalent to 90 mg silymarin).
Capsules and tablets should be taken orally whole and not chewed, and washed down with plenty of water.
Recommended dosage of Karsil:
| Severity of liver damage | Dosage form | |
| Dragee | Capsules | |
| Light to moderate | 1-2 tablets 3 times a day | 1 capsule 1-2 times a day |
| Heavy | up to 2-4 tablets 3 times a day | starts with 1 capsule 3 times a day |
The course of treatment lasts about 3 months. The exact dose and duration of the course can only be determined by a doctor, depending on your individual indications and the severity of the disease.
Note!
Do not give Karsil to your child if he is under 12 years of age, as this may worsen his condition and put his life at risk.
Composition and description of tablets "Karsil"
"Karsil" refers to antitoxic drugs that clear the liver of toxins (hepatoprotective effect). It is produced only in the form of tablets - there are 80 pieces in one package. The active ingredient is silymarin: it is an extract of the fruit of the spotted milk thistle plant. Each tablet contains 22.5 mg of this component.
The drug is available without a prescription, but it is recommended to consult a doctor before starting therapy. The tablets are stored under normal conditions (room temperature not higher than 25 degrees, moderate humidity). The shelf life is 2 years from the date of production.
What are the side effects of Karsil?
Patients usually respond well to treatment with Karsil.
side effects may occur :
- short-term gastrointestinal disorders (bloating, feeling of fullness or abdominal pain, anorexia, changes in bowel habits, diarrhea, dyspepsia, flatulence, nausea, heartburn);
- headache;
- itching, rash, anaphylactic shock;
- dyspnea;
- increased alopecia (baldness);
- increased diuresis (increased urination).
Side effects disappear after stopping the drug, usually without the use of additional measures.
Interaction of Karsil with other drugs and substances
If you use Karsil, oral contraceptives and at the same time drugs that are used in estrogen replacement treatment, then the effectiveness of the latter drugs may decrease. Check with your doctor before combining these drugs.
Karsil enhances the effect of the following substances:
- fexofenadine;
- clopidogrel;
- warfarin;
- diazepam;
- alprazolam;
- ketoconazole;
- lovastatin;
- atorvastatin;
- vinblastine.
If you have type 2 diabetes, talk to your doctor before taking Karsil, as components of the drug may lower your blood sugar readings.
Karsil increases the amount of Simeprevir (hepatitis C medicine) in the blood plasma.