Is the malignancy visible during endoscopy?
The importance of fibrogastroduodenoscopy for diagnosing gastric cancer cannot be overestimated. It is this study that allows us to identify or confirm the diagnosis of malignant neoplasm , as well as determine the type of process. This became possible thanks to a biopsy of suspicious areas of the gastric mucosa. The collected tissues are sent for cytological examination, which within 5-10 days gives a conclusion about the type of tissue.
Important International recommendations recommend performing FGDS in all patients over 45 years of age in whom the doctor, after questioning and examination, suspects the presence of stomach cancer.
This makes it possible to identify the disease at an early stage, when the overall prognosis is more positive and the average life expectancy is 10 years or more.
Symptoms
Gastric cancer is a malignant neoplasm that develops from the epithelium of the mucous membrane of the organ. According to official statistics, this disease ranks second in frequency of detection among cancer pathologies in Russia . It has been established that stomach cancer is more common in men over 45 years of age.
The problem with this disease is that it is diagnosed late. Only in 7% of cases (often by chance) can stomach cancer be detected at the first or second stage, when there are no distant metastases yet, and more than half of the patients completely recover.
In other patients, the oncological process manages to metastasize to the lymph nodes, abdominal organs, bones or brain. Then the effectiveness of even the most modern treatment is much lower, and only 5-22% of patients live another 5 years after diagnosis.
4 stages of stomach cancer
The clinical picture of stomach cancer is often subtle (especially in the first stages). The following symptoms come to the fore:
- decreased appetite;
- feeling of heaviness in the upper abdomen;
- periodic aching stomach pain;
- nausea after eating;
- belching;
- one-time vomiting;
- weight loss;
- weakness, decreased performance.
Often the disease is discovered only when surgical complications occur (eg, perforation of the stomach wall, gastric bleeding, hepatic vein thrombosis).
Sometimes situations occur when distant metastases are first detected (for example, in the liver during ultrasound). Then additional examinations are performed (FGDS, computed tomography or magnetic resonance imaging) to detect the primary tumor.
Diagnostic features
Fibrogastroduodenoscopy is performed in the endoscopy room on the patient’s “empty” stomach . Most often, local anesthesia is given to the posterior wall of the oropharynx, or a drug is administered for sedation (medicated sleep). FGDS is performed under anesthesia or sedation in a situation where emergency surgery is planned immediately after completion of the procedure.
The probe is inserted through the oral cavity, passes through the esophagus and enters the lumen of the stomach. If an oncological pathology is suspected, the doctor conducts a thorough examination of the mucous membrane. Normally, it should be pink, without defects, signs of inflammation or growth.
How is gastroscopy done?
To avoid complications after the FGDS procedure, it is important not only to prepare for it, but also to follow a diet after its completion. This article will help you cope with the insertion of the probe more easily.
Classification of the disease
Modern recommendations distinguish three types of early gastric cancer, which have different growth patterns:
- Polypous. Upon examination, a small polyp (proliferation of the mucous membrane) is discovered. It is impossible to visually determine its malignancy.
- Superficial cancer. Characterized by differences in the color of the mucous membrane, slight swelling or a reactive inflammatory reaction is possible.
- Ulcerative type. With FGDS, the neoplasm resembles an erosion or ulcer (defect of the mucous membrane). Differential diagnosis with peptic ulcer is necessary.
Visual picture
If the research is carried out at a later stage, then according to Borrman’s classification, 4 options for the visual picture are possible:
- Polypoid neoplasm. The endoscopist sees the growth of the mucous membrane on a wide stalk. Found in a third of cases.
- Polypoid cancer with ulceration. The neoplasm has a trough-shaped shape. From the edge one can see the proliferation of epithelium with erosion or a zone of necrosis, which are located in the center of the tumor. Occurs in approximately 30% of patients.
- Infiltrative-ulcerative variant. The growth of the oncological process occurs mainly in the thickness of the stomach wall. On the surface you can observe an ulcer (sometimes up to 2-3 cm in diameter), a local inflammatory process and swelling without clear boundaries.
- Diffuse-infiltrative. Immediate signs may not be detected during FGDS, since the tumor is almost entirely located in the wall of the stomach. However, the presence of compaction of the mucous membrane, rigidity and unnaturalness of the sweet tooth, and impaired motor skills are noteworthy. Detection rate – 30%. Characterized by early metastasis and poor prognosis.
A separate clinical variant of gastric malignancy is lymphoma . As you know, the stomach wall has a large amount of lymphatic tissue (MALT system).
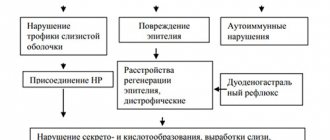
Help Chronic Helicobacter pylori infection plays an important role in the development of the disease, so a rapid test for its presence is almost always positive.
It is necessary to highlight the following characteristics:
- the most common location is the antrum of the stomach;
- the mucous membrane may be little changed, but local redness, surface unevenness and smoothness of folds can be detected;
- proliferation of the mucous membrane;
- swelling without a clear boundary of the stomach wall.
When describing a neoplasm, the endoscopist must determine the nature of tumor growth, the presence of a defect in the mucous membrane, the consistency of the altered tissue, the location and size of the neoplasm.
This article will help you decipher the doctor’s conclusion after FGDS for common pathologies and find out what the protocol normally looks like.
How is a biopsy done?
During FGDS, if areas that resemble a malignant process are detected, a diagnostic biopsy is necessarily performed .
In this case, tissue sampling is done several times. For example, if there is an ulcer, then 3-4 samples are taken from its marginal walls and bottom. During the infiltration process, the deep-seated altered tissues are carefully collected.
After collection, tissue samples are sent for cytological examination, which should determine their type. A biopsy makes it possible to make a final diagnosis of a malignant tumor of the stomach, or to refute it. However, it alone is not enough for a complete diagnosis.
In 85% of cases of gastric cancer, metastases are found in regional lymph nodes or distant organs. Therefore, all kinds of contrast-enhanced imaging methods are used to detect them (CT, MRI, PET).
Forms of oncology
According to WHO recommendations, several histological (by origin and tissue type) forms of gastric cancer can be distinguished :
- papillary adenocarcinoma (from glandular tissue);
- tubular adenocarcinoma (from glandular tissue);
- squamous cell carcinoma (from the epithelium);
- carcinosarcoma (from connective tissue);
- mucinous adenocarcinoma (from glandular tissue, characterized by active mucus production);
- choriocarcinoma (sprouting of embryonic tissue);
- undifferentiated cancer (due to degradation of the tumor cell structure, it is impossible to determine its type);
- poorly differentiated adenocarcinoma.
A common problem in domestic laboratories is the inaccuracy of diagnostics due to the low level of qualifications of personnel (especially in small provincial hospitals) or problems with equipment.
Please note: Sometimes false positive or false negative results occur. To avoid this, it is advisable to conduct a cytological examination in a specialized oncology clinic.
The results of fibrogastroduodenoscopy are delivered to the patient within a few minutes after completion of the examination. They indicate the nature of the mucous membrane, acidity indicators and detected deviations. The results of the cytological examination are ready in 5-10 days.
Despite a steady decline in morbidity and mortality, gastric cancer still remains a pressing problem throughout the world. Around one million new cases of gastric cancer are diagnosed annually worldwide (952,000 according to 2012 data) [1]. It ranks 5th among the most common cancers and the third leading cause of death from malignant neoplasms in the world. The prognosis depends on the stage of the disease: the 5-year survival rate of patients with stomach cancer is 25-30%, but this figure for patients with early stomach cancer after surgical treatment reaches 95%. Unfortunately, more than 40% of all stomach cancer cases are still diagnosed at stage IV. Early stomach cancer accounts for only 5-10% of the total number of patients with stomach cancer in Europe, the USA and Russia, while in Japan this figure reaches 50%. Recently, the incidence of this disease in developed countries has been gradually decreasing. According to the International Agency for Research on Cancer (GLOBOCAN 2012 project), the highest morbidity and mortality rates continue to be reported in East Asian countries. In contrast, in the United States and Western Europe these rates are noticeably lower. Russia occupies an intermediate position in the structure of global morbidity and mortality. In 2012, 37,369 new patients with stomach cancer were registered in Russia, mostly men, which determined the third ranking place in the morbidity structure (8.8% in men and 5.7% in women) [2].
Diagnosis and timely treatment of precancerous pathology is the main way to reduce morbidity and mortality, and detection of gastric cancer in the early stages and its adequate treatment improves the prognosis. Since carcinogenesis is a long process, this makes it possible to detect early forms of cancer through screening. According to the WHO definition, screening is the identification of a disease or defect using test studies that can be carried out quickly and on a mass scale. One of the most famous gastric cancer screening programs is conducted in Japan. It has been a nationwide program since the 80s of the last century and consists of three stages: large-frame fluorography, endoscopic examination, and morphological examination. The results of this program are impressive: 16 detected cases of gastric cancer per 1000 asymptomatic population, and 65% of them are early cancer [3]. The most studied method of screening for gastric cancer is photofluorography with double contrast. The research methodology is as follows: the initial (“indirect study”) consists of 8 small-frame radiographs of the stomach using double contrast; If any changes are detected, a more detailed examination (“direct examination”) is performed, consisting of 11 consecutive radiographs. According to numerous studies, the sensitivity and specificity of the double-contrast photofluorography method was 89 and 92%, respectively (Table 1) [4-8]. The use of this method can reduce mortality by 40-60%, and therefore it can be recommended for population screening.
Table 1. Sensitivity and specificity indicators for gastric cancer screening using double contrast photofluorography [4–8]
However, the detection rate of gastric cancer during endoscopic examination is 2.7-4.6 times higher compared to fluorography, and the use of new techniques can further increase the sensitivity of the method. Currently, the endoscopic method is increasingly used in screening programs. According to A. Tashiro et al. [9], endoscopic examination revealed early gastric cancer in 0.87% of cases, which was 2.7 times higher than the number of early forms detected during fluorography. In addition, the cost of identifying one patient was several times lower with endoscopic screening compared to X-ray screening. However, on the one hand, the endoscopic method requires highly qualified physicians, on the other hand, there is currently no reliable data on the effect of this method on mortality. Thus, gastroscopy can be recommended for individual screening programs.
A promising non-invasive screening method is the study of the level of serum pepsinogens, which alone or in combination with the determination of antibodies to Helicobacter pylori
may be applicable to identify risk groups [10]. Studies have shown that a decrease in the level of serum pepsinogens and a decrease in the pepsinogen I/pepsinogen II ratio are reliable signs of atrophic changes in the gastric mucosa [11]. According to a number of publications, the sensitivity and specificity of the laboratory method in the diagnosis of atrophic gastritis are 15-75 and 92.2-97.8%, respectively [12-14].
Endoscopic examination with biopsy is the “gold standard” for diagnosing structural changes in the gastric mucosa. In clinical practice, various endoscopic techniques are used to search for early gastric cancer and clarify the diagnosis of neoplastic changes, making it possible to determine their nature and boundaries, as well as assess the depth of the lesion. Chromoscopy, staining the mucous membrane with dyes, is a basic technique for diagnosing precancerous changes and early stomach cancer. Currently, the most widely used solution is 0.2% indigo carmine, which allows contrasting relief and minor changes in the mucous membrane and is an effective and inexpensive way to assess the boundaries of pathological areas [15]. Narrow band imaging (NBI) endoscopy is an optical diagnostic technique based on the use of special optical filters that narrow the spectrum of the light wave. Conventional endoscopic systems use almost the entire visible light spectrum from 400 to 800 nm (Fig. 1) [16]. The narrow-spectrum system takes advantage of two light waves with a length of 415 and 445 nm in the diagnosis of vascular structures of the mucous membrane of the digestive tract, since these light waves are well absorbed by hemoglobin [17]. This allows you to obtain a detailed image of the vascular pattern of the tissue, its changes characteristic of pathological areas of inflammatory origin, as well as precancerous diseases and early forms of cancer. In addition, this endoscopic system increases image contrast, which creates the effect of “virtual chromoscopy”, which can be used for accurate assessment of the boundaries of a pathological formation [18].
Rice. 1. Narrow-spectrum endoscopic system.
Magnifying endoscopy allows you to examine the pathological area with an optical magnification of more than 100 times and evaluate in detail its structure and exact boundaries. The combined use of magnifying and narrow-spectrum endoscopy makes it possible to evaluate in more detail the pitting and vascular pattern of the area under study. The high specificity and sensitivity of these methods [19, 20] in diagnosing structural changes in tissue in early forms of cancer and precancerous changes in the gastric epithelium allow us to consider these methods as “optical biopsy” [21] (Table 2).
Table 2. Sensitivity and specificity indicators of narrow-spectrum endoscopy (in%) compared with standard examination in white light in detecting early gastric cancer [19, 20]
During an endoscopic examination with magnification of the gastric mucosa, two main components are assessed [22]: the microvascular pattern and the microstructure of the epithelial surface, by the nature of changes in which the histological structure of the pathological area can be predicted. According to K. Akisato [23], with the development of cancer, there is a significant restructuring of the microvascular bed in the form of changes in the concentration of vessels, type of branching, size of vessels, and the appearance of vessels specific to gastric cancer. Studies show that when analyzing the morphometric parameters of gastric cancer microvessels using magnifying endoscopy, they have more connecting points compared to the surrounding mucosa, more end points and branching points [24]. Therefore, one of the most characteristic signs of the neoplastic process is a change in the microvascular pattern. In accordance with a study by Japanese specialists [25], there are four categories of pathological changes in microvessels that are reliably associated with gastric cancer:
1. Dilation: the presence of microvessels with a caliber 2 times larger compared to the vessels of the surrounding area.
2. Abrupt caliber alteration: the presence of microvessels whose caliber is either half as large or twice as large compared to the original size. Destruction of the microvascular network is also included in this concept.
3. Tortuosity: the presence of microvessels that are variably tortuous or twisted.
4. Heterogeneity in shape: the presence of microvessels, the shape of which is individual and variable.
According to a prospective study by M. Kato et al. [26], the sensitivity and specificity of such signs as dilatation and heterogeneity of the shape of microvessels (in combination with the disappearance of the surface structure) in the diagnosis of cancer is 92.9 and 94.7%, respectively, and is significantly higher than similar indicators when using standard white light endoscopy ( 42.9 and 61.0% respectively).
Based on the analysis of the microvascular pattern using magnifying endoscopy, it is possible to predict the histological structure of the tumor, which is necessary for further selection of the method of tumor resection (endoscopic resection or surgery). T. Nakayoshi et al. [27] identified two main types of vascular pattern in early gastric cancer:
1. Fine network pattern: a large number of microvessels interconnected in the form of a fine network. This type of vascular pattern was determined in 68.7% of cases of differentiated adenocarcinoma (intestinal type).
2. Corkscrew pattern: isolated, convoluted microvessels shaped like a “corkscrew”. This type of vascular pattern was detected in 85.3% of cases of undifferentiated cancer.
With the development of neoplastic transformation, changes occur in the microstructure of the pits and grooves. In a study by Y. Otsuka et al. [28] in the case of differentiated adenocarcinoma, the density of the gastric glands was significantly higher compared to the normal mucosa. As shown by studies by K. Yagi et al. [29], areas of neoplasia tend to increase the density of the glands and, as a consequence, reduce the intermediate part between the mouths of the glands, which makes the so-called “white zone”, representing the marginal pit epithelium, indistinct or invisible. For the same reason, M. Kaise et al. [30] changes in the surface structure in gastric cancer were characterized by heterogeneity, irregularity of epithelial structures or its absence (complete or partial). The sensitivity, specificity and accuracy of these signs as a diagnostic criterion for early cancer were 100, 89.7 and 90.4%, respectively [31].
Magnifying and narrow-spectrum endoscopy requires the specialist to have knowledge not only in the field of gastroenterology, oncology and morphology, but also in new highly specialized disciplines, such as the microanatomy of the mucous membrane of the digestive system. In the era of computerization, automated systems that allow analysis of the epithelial surface pattern and microvascular pattern can help specialists in interpreting the data obtained, predicting histological changes, and, as a result, making clinical decisions when performing diagnostic endoscopy [32]. Computer systems that make it possible to predict pathological conditions and thereby increase the accuracy of diagnosis are called computer-aided decision support systems (CADSSs) [33]. The use of automated decision-making systems in modern endoscopy has created a new direction in endoscopic diagnostics - computer-aided endoscopy. As part of the joint project of the international research laboratory “Discrete and Computational Geometry named after. B.N. Delaunay" Yaroslavl State University. P.G. Demidov and the endoscopic department of the Yaroslavl Regional Clinical Oncology Hospital have developed a comprehensive decision support environment when using narrow-spectrum and magnifying endoscopy in the stomach. The created system allows you to recognize and classify the processed image, and, according to testing, the average value of the proportion of correctly classified points was 79%.
Another new optical technique for endoscopic diagnosis is autofluorescence endoscopy (Autofluorescence imaging - AFI), based on the phenomenon of natural fluorescence (autofluorescence) of endogenous fluorophore substances of the mucous membrane of the digestive system. The human body contains a large number of molecules that exhibit the phenomenon of autofluorescence. In terms of the application of this phenomenon in the endoscopic diagnosis of tumors of the digestive tract, the most important compounds are: collagen, elastin, reduced form of nicotinamide dinucleotide phosphate, flavin adenine dinucleotide-riboflavin-adenosine diphosphate, porphyrins. When this method is applied to normal mucosa, exciting light with a short wavelength reaches the submucosal layer and causes a natural fluorescent glow of endogenous fluorophores (primarily collagen, which has a green glow), which is perceived by the endoscope matrix without significant absorption by the mucous membrane. Areas with thickened mucous membranes, primarily tumor tissue, largely absorb natural fluorescence, resulting in a purple or violet coloration of pathological areas. In-depth areas with thinned mucous membrane, on the contrary, increase autofluorescence due to a decrease in the thickness of the barrier layer, as a result of which the green color of such formations appears (Fig. 2) [34]. However, autofluorescence endoscopy has very low specificity due to the large number of false-positive examination results. Despite this, 23% more flat-elevated early cancers were detected with this technique compared with white light examination, thereby establishing the place of autofluorescence endoscopy as a “red flag” technology for screening the surface of the gastric mucosa to detect pathological areas of suspicious regarding neoplasia [35].
Rice. 2. The principle of obtaining an image during examination in autofluorescence mode. a — raised pathological area: absorption of autofluorescence, appearance of a violet glow; b — deepened pathological area: increased autofluorescence, the appearance of a green glow.
Trimodal endoscopy allows you to combine white light examination for routine examination, autofluorescence mode for searching for pathological foci and narrow-spectrum mode for detailed study of the changes found. Examination in various modes allows for a comprehensive assessment of various mucosal characteristics and serves as a convenient tool for endoscopic screening for gastric cancer (Fig. 3).
Rice. 3. Various endoscopic techniques in the diagnosis of early cancer. a — routine endoscopy in white light: flat-raised area of the cardia of the stomach; b — chromoscopy with indigo carmine; c — autofluorescence endoscopy: purple area on a green background; d — narrow-spectrum endoscopy with magnification: destroyed pattern of the epithelial surface with the formation of a fine network of pathological microvessels; d — analysis of the endoscopic image using an automated decision support system: the brown zone corresponds to gastric cancer with a high probability; histological verification: well-differentiated adenocarcinoma.
see fig. 4
Rice. 4. Indications for endoscopic mucosal resection (ERM) and expanded indications for endoscopic dissection in the submucosal layer (EDPS) (adapted from Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer 2007; 10 (1): 1-11).
see fig. 5
Rice. 5. Stages of endoscopic dissection in the submucosal layer (diagram). a — marking the boundaries of the tumor; b — injection of hyaluronic acid solution; c — making a circular incision; d, e, f - dissection of the pathological area using an electrosurgical knife in the submucosal layer (adapted from Asano M. Endoscopic submucosal dissection and surgical treatment for gastrointestinal cancer. World J Gastrointest Endosc 2012; 4 (10): 438-447).
When dysplastic and neoplastic changes in the mucous membrane are detected, an important therapeutic and diagnostic stage in the management of such patients is endoscopic resection of the pathological focus. In recent years, there has been progress in the development of various endoscopic techniques for resection of the mucous membrane of the gastrointestinal tract [36]. The technique of loop biopsy (strip biopsy) has been known since the 80s of the last century and is performed using a two-channel endoscope. As with many other techniques, a physiological solution with the addition of a small amount of adrenaline at a dilution of 1:1,000,000 is first injected into the submucosal layer. Next, using a gripper previously threaded through an endosurgical loop, the pathological area is lifted, after which the loop is tightened under the base of the formation and resection is performed. Aspiration techniques began to develop in the 90s and have become very popular both in Japan and in Western countries. These include, in particular, a resection technique using a plastic cap fixed to the distal end of the endoscope and a loop located inside the cap. Using this technique, the pathological area is aspirated into the cap and grasped at the base with a loop for further resection. There are also ligation techniques using ligators, which are used to treat varicose veins, and special aspiration mucosectors. A feature of these techniques is that the pathological area is resectioned not along the inner diameter of the aspiration cap, but outside it.
Since the beginning of the 2000s, a new technique has been widely introduced into practice - resection with dissection in the submucosal layer. The advantage of this technique is, first of all, that resection is performed as a single block, even in the case of large sizes and complex location of the pathological area. This significantly increases the radicality of resection. In addition, the size and shape of the resection is controlled, and resection of pathological areas with ulceration is possible. However, compared to conventional resection methods, the operation is technically more complex, takes longer, and has a high risk of complications such as perforation and bleeding [37]. Pathological areas in which the risk of lymphatic metastasis is insignificant are subject to endoscopic removal. According to Japanese guidelines, indications for mucosal resection are differentiated type of adenocarcinoma, protruding type less than 30 mm in diameter, deep type less than 20 mm, without ulceration or signs of invasion. Under such conditions, the risk of metastasis to the lymph nodes is minimal. However, upon further study of more than 5,000 cases of stomach cancer after gastrectomy with extended lymph node dissection, Japanese scientists led by T. Gotoda revealed the absence of metastasis in more advanced forms of cancer. In this regard, so-called expanded indications for resection using the submucosal dissection technique have appeared: intramucosal cancer without ulceration, regardless of size, intramucosal cancer with ulceration <30 mm, cancer with minimal submucosal invasion (<500 µm: sm1) <30 mm [ 38].
Endoscopic resection using the dissection technique in the submucosal layer has the following stages:
1. Marking the boundaries of the pathological formation, which is carried out directly with an electrosurgical knife. In this case, marks are placed using coagulation 0.5 cm outward from the visible edge of the pathological area.
2. Injection of a hyaluronic acid solution into the submucosal layer. This drug, unlike saline solution, maintains the shape of the “cushion” that appears during injection for a long time, which is very important during such long-term operations.
3. Circular cut. Using an electrosurgical knife, an initial incision is first made to the submucosal layer, then a circular incision is made approximately 0.5 cm outward from the marks.
4. Dissection of the pathological area using an electrosurgical knife in the submucosal layer, resulting in the formation of an extensive post-coagulation ulcerative defect, which is completely epithelialized in 1-1.5 months.
A large number of instruments are used as electrosurgical knives (needle knife, IT knife, double knife, etc.), each of which has its own characteristics and advantages. Currently, with the emergence of hybrid technologies that combine several instruments into one, a hybrid water-jet knife (HybridKnife, “ERBE”, Germany) is widely used for endoscopic dissection in the submucosal layer. This knife has a small channel in the central part for supplying saline solution and combines the functions of an injector and an electrosurgical knife, so there is no need to change instruments when performing endoscopic dissection.
The 3- and 5-year survival rates for endoscopic resection of early gastric cancer, according to various sources, are above 95-100% [39, 40], and when using the submucosal dissection technique - up to 100% [41], while the quality of life many times higher than after radical surgical treatment.
Currently, minimally invasive surgical interventions at the intersection of laparoscopic surgery and operative endoscopy - transluminal endoscopic operations through natural orifices (NOTES - Natural Orifice Translumenal Endoscopic Surgery) - have received active development. Using these techniques, it is possible to carry out minimally invasive treatment for advanced stages of gastric cancer with a high risk of metastasis to regional lymph nodes. The operation is performed using a combined approach (from the stomach and from the abdominal cavity), combining the capabilities of endoscopy and laparoscopic surgery [42]. Considering the fact that a significant number of standard gastric resections with lymph node dissection are not always necessary (in the absence of metastatic lesions of the lymph nodes), to identify the volume of lymph node metastasis, the so-called lymphatic mapping of gastric cancer and biopsy of the “sentinel” lymph node are used, which determines the minimum required extent of resection [43]. To do this, a special substance is first endoscopically introduced into the tumor, usually a combination of dyes (indocyanine green) and radioisotope markers (colloid-labeled Tc99 particles) [44]. Then, through a transluminal approach, after examination, an excisional biopsy of the “sentinel” lymph node, i.e., the lymph node that has accumulated the injected substance, is performed. In the absence of metastatic lesions, endoscopic full-thickness resection of the tumor is performed under the control of laparoscopic access. Thus, it is possible to perform minimally invasive treatment in the presence of a stomach tumor not only with T1-2N0M0, but also even with T3 tumors, however, in the latter case, the issue of closing the defect of the gastric wall remains problematic.
The evolution of endoscopic technologies allows not only accurate diagnosis of early forms of gastric cancer, but also minimally invasive treatment at more advanced stages of this disease, increasing survival and improving the prognosis of patients after surgery.
Alternative methods for examining the stomach
In addition to FGDS, there are a number of other diagnostic methods that help detect gastric malignancy. Their comparative characteristics are presented in the following table:
| FGDS | Ultrasound | CT | MRI | |
| Description of the procedure | Endoscopic diagnosis with visual examination of the mucosa | Non-invasive technique using ultrasound | X-ray technique with computer processing of the obtained images | Based on the use of magnetic resonance of atomic nuclei |
| Information content for stomach cancer | High (allows you to make a diagnosis and determine the type of cancer) | Low (only large tumors are visualized) | High (detects metastases to lymph nodes and distant organs) | High (detects metastases to lymph nodes and distant organs) |
| Using Contrast | Not required | Not required | Increases the information content of the technique | Increases the information content of the technique |
| Carrying out a biopsy | Held | Not carried out | Not carried out | Not carried out |
| Time spending | 30-40 minutes | 15-20 minutes | 10-15 minutes | 30-50 minutes |
| Side effects | · Traumatization of the gastric mucosa Bleeding Entry of stomach contents into the respiratory tract | None | Frequent use increases the risk of developing tumors. | None |
| Contraindications | · Severe condition of the patient · Blood clotting disorders Acute stage of myocardial infarction or stroke | None | · Pregnancy · Severe obesity | · Presence of metal implants · Severe obesity |
| Price for research in Moscow | 1200-9700 rub. | 700-4800 rub. | 4000-13500 rub. | 7600-21000 rub. |
Early diagnosis in the case of oncology gives a better chance of long-term remission. Want to know if stomach cancer is visible on an ultrasound? Read this article.
What to do if cancer is discovered?
If fibrogastroduodenoscopy was performed in a regular hospital or clinic, then after receiving the results the patient is referred for consultation to an oncologist .
He examines the patient and issues a referral for treatment to a public oncology hospital.
If the patient wishes, then with the results of FGDS and a referral from an oncologist (sometimes with their translation), he can also contact private foreign oncology clinics that have representative offices in Russia. They usually require a repeat endoscopic examination of the stomach with a biopsy.
When a patient enters a specialized medical institution, a full range of diagnostics is required :
- examination by a gynecologist for women and a proctologist for men;
- general blood and urine analysis;
- blood chemistry;
- specific tumor markers (CA 72-4, CEA, Ca 19-9);
- X-ray of the lungs;
- electrocardiogram;
- ultrasound examination or CT scan with contrast of the abdominal organs;
- Ultrasound of the lymph nodes of the supraclavicular region;
- colonoscopy;
- PET-CT (if possible).
If distant metastases are detected and accessible, it is necessary to perform a biopsy under ultrasound guidance.
How to examine the esophagus in St. Petersburg: the advantages of contacting ICLINIC
The initial symptoms of esophageal cancer are almost subtle, so preventive fibrogastroscopy is necessary for early diagnosis of the tumor. However, in order to obtain a competent examination, you need to contact only time-tested clinics. ICLINIC provides an individual approach to each patient, working exclusively in his interests. Even subtle changes are not missed during fibrogastroscopy, but are carefully analyzed not only visually, but also through biopsy.
The expert level of equipment allows ICLINIC doctors to notice the smallest defects of the mucous membrane, measuring just over 1 mm. If esophageal cancer is detected, an immediate targeted biopsy is performed, which saves the patient’s time before starting antitumor treatment. Only doctors with the highest category and clinical experience are allowed to work with patients at the ICLINIC clinic. If even minor dysphagic disorders appear, it is necessary to perform fibrogastroscopy as soon as possible. The research carried out in our clinic is completely safe and will prolong a person’s life, even if symptoms of esophageal cancer have not yet appeared. The ease of registration and the kindness of the staff complete the picture of excellent service at the ICLINIC clinic.
Choice of treatment tactics
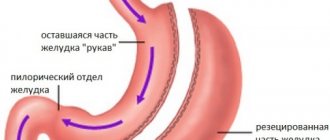
According to national recommendations of oncologists, the choice of treatment tactics for stomach cancer depends on the extent of the tumor . If the disease is detected early, endoscopic resection of the mucosa with or without submucosal layer is performed.
Open abdominal surgery is also possible. It is carried out if there are no distant metastases, and the malignant process does not spread beyond the regional lymph nodes.
Radical tumor removal is recommended in the absence of distant metastases. Plastic surgery of the remaining part of the stomach is required.
In the terminal (last) stage of cancer, surgical intervention is necessary only if complications arise that can threaten the patient’s life (bleeding, narrowing of the lumen, disruption of the integrity of the stomach with the development of peritonitis). Radical removal of the organ then does not improve the prognosis, since distant metastases exist.
Please note National recommendations advise prescribing chemotherapy to all patients after (and in certain situations before) radical or endoscopic intervention, in the last stage of the disease.
The most commonly prescribed drugs are epirubicin, cisplatin and oxaliplatin. Only in the presence of serious concomitant diseases is chemotherapy not carried out, but symptomatic treatment is prescribed. Usually 6-8 courses are carried out until clinical remission is achieved.
When is EGDS (gastroscopy) necessary?
There are diseases that are considered precancerous and require periodic examination regardless of the presence of symptoms. These include:
- chronic atrophic gastritis with the so-called intestinal metaplasia, especially in the presence of Helicobacter pylori infection;
- stomach ulcer;
- Barrett's esophagus;
- vitamin B12 deficiency (pernicious anemia);
- adenomatous polyps of the stomach;
- hypertrophic gastropathy.
Stomach operations due to benign tumors, undergone more than 10 years ago, and family history are also situations that require close monitoring.
You should be regularly monitored with mandatory regular endoscopy if your relatives have been diagnosed with:
- cancer of the gastrointestinal tract,
- familial adenomatous polyposis of the colon,
- Gardner syndrome,
- Peutz-Jeghers syndrome,
- familial juvenile polyposis,
- Li Fraumeni syndrome.
With the development of genetics, certain genes have become known, certain mutations of which, under special conditions, lead to stomach cancer.
There are also regions in which, apparently as a result of nutritional or environmental characteristics, the incidence is significantly higher than average (Japan, some European countries, Scandinavia, South and Central America, China, Korea, countries of the former USSR). People who live in these areas for a long time need to be more attentive to their health and periodically undergo endoscopy. A signal for mandatory examination can be an indicator such as the concentration of pepsinogen in the blood serum (normally less than 70 ng/ml) and the ratio of its fractions (PG1/PG2).
If it is impossible to conduct endoscopy, for example, due to concomitant diseases, polyposition radiography with double contrast with barium suspension and air can be performed. This method, however, is much less sensitive in the early stages of the disease and does not allow morphological verification. Additionally, MSCT and ultrasound are used, however, the accuracy of the latter study, even with an experienced researcher, is very dependent on the technical capabilities of the device, body type and correct preparation of the patient.
Get a treatment program