Introduction
In the Russian Federation, octreotide is considered a mandatory drug in the treatment of acute pancreatitis [1, 3]. The protocols for the treatment of pancreatitis in Japan, Great Britain, the International Association of Pancreatology and the American Association of Pancreatology do not recommend its use [7, 10-12]. This is justified by the results of randomized studies that did not reveal any improvement in patient treatment outcomes when using the drug [5, 6, 8, 9]. The most significant and largest (302 patients) of these studies was a multicenter (32 centers in Europe), randomized, double-blind study performed by W. Uhl et al. [9].
However, the study has a number of shortcomings that allow us to consider the results presented by the authors to be not entirely adequate. Firstly, the analysis was carried out on a general population of patients, extremely heterogeneous in the severity of their condition. This approach to the study may not have revealed a differentiated response of individual groups of patients to the use of the drug. Secondly, it seems incorrect to compare the treatment results of the group receiving octreotide at a dose of 100 and 200 mcg 3 times a day, since the severity of the condition of patients in the latter was 2 points higher according to APACE II (7 points and 9 points, respectively). Thirdly, the most significant shortcoming was the statistical analysis of the data obtained during the study. It was based on a univariate approach without taking into account the effect of potential predictors other than octreotide (severity factors and treatment factors) on disease outcome. All these shortcomings of the most important study on the problem under study allow us to assert that the assessment of the effectiveness of octreotide in the treatment of patients with pancreatic necrosis remains relevant.
We conducted a study, the purpose of which was to assess the effect of octreotide on the dynamics of mortality and the prevalence of necrosis of the pancreas and parapancreatic tissue in initially severe destructive pancreatitis (9 or more points on the APACHE II scale).
The selection of the category of patients for the study was based on the high frequency of their unfavorable disease outcomes.
Side effects of the drug Octreotide
Possible pain, itching or burning sensation, redness and swelling at the injection site, anorexia, nausea, vomiting, cramping abdominal pain, bloating, flatulence, loose stools, diarrhea and steatorrhea, phenomena resembling acute intestinal obstruction (progressive bloating, severe pain in the epigastric region, muscle protection), formation of gallstones (with long-term use in 10–20% of patients), acute pancreatitis, hair loss, liver dysfunction, including acute hepatitis without cholestasis, hyperbilirubinemia, accompanied by increased alkaline levels phosphatases, γ-glutamyltransferases and, to a lesser extent, transaminases, decreased glucose tolerance, persistent hyperglycemia or hypoglycemia (with long-term use).
Material and methods
The multicenter study included 204 patients with severe pancreatic necrosis who were treated in the surgical departments of the Yaroslavl Regional Clinical Hospital (from 1996 to 2011), City Hospital No. 1 of Vologda (from 2005 to 2010), and City Hospital No. 2 of Kostroma (from 2008). to 2009) and city hospital No. 1 of Rybinsk (from 2008 to 2009).
The criteria for inclusion of patients in the study were the following: 1) detection of necrosis of the pancreas and parapancreatic tissue during surgery; 2) the presence of necrosis of the pancreas and parapancreatic tissue at the autopsy of deceased non-operated patients; 3) the presence of fluid formation with sequestration in non-operated patients according to the results of ultrasound, computed tomography and magnetic resonance imaging; 4) severity of the condition upon admission, 9 or more points on the APACHE II scale.
The criteria for excluding patients from the study were: 1) the presence of a malignant neoplasm; 2) absence of necrosis of the pancreas during surgery, during autopsies of dead bodies, during ultrasound examination, MRI, CT; 3) the severity of the condition at admission is less than 9 points on the APACHE II scale.
An important condition for the study was a complete sample of patients with severe pancreatic necrosis for the entire calendar year in each of the indicated hospitals, which actually made it possible to consider it prospective based on the condition at a point in time in the past [2].
During the study, we used the international classification of acute pancreatitis, according to which the predicted severity of the disease was determined [4].
At the beginning of the study, a traditional analysis approach was used, similar to that used by W. Uhl et al. [9]. The patients were divided into two groups: main ( n
=114), in which octreotide was used in standard doses (100 mcg 3 times a day subcutaneously and intramuscularly), and a control group (
n
=90), in which the drug was not used. Mortality rates and the prevalence of necrosis in the study and control groups were compared using univariate statistical methods. To correctly interpret the results obtained, the groups were additionally (unlike the study by W. Uhl et al.) compared with respect to other potential predictors (severity factors upon admission to hospital and treatment factors), which could also influence the outcome of the disease.
Necrosis involving one of the sections of the pancreas or a significant volume of parapancreatic tissue was considered widespread. The prevalence of necrosis was determined during surgery or (in non-operated patients) based on autopsy results. In addition, pancreatic necrosis was considered focal in patients whose cure did not require surgical intervention.
Factors of severity of the condition were age (number of years), gender (1 - male, 0 - female), severity of organ dysfunction on the APACHE II scale (in points), body temperature (°C), pulse rate (per 1 min), mean arterial pressure (in mm Hg), respiratory rate (in 1 min), level of consciousness according to the Glasgow scale (in points), number of blood leukocytes (thousand 109/l), band neutrophils (in %), level in blood creatinine (in mg/dl), potassium (in mmol/l), sodium (in mmol/l), glucose (in mmol/l), partial blood oxygen tension (in mmHg), blood pH values . These parameters were assessed upon admission.
Of the treatment predictors, methods of antibacterial prophylaxis were assessed and compared (aminoglycosides, penicillins and first generation cephalosporins - code 1; third and fourth generation cephalosporins - code 2; fluoroquinolones and carbopenems - code 3), frequency of use of quamatel (1 - used, 0 - not used ), anti-enzyme therapy (1 - used, 0 - not used), early nutritional support (1 - used, 0 - not used), nutritional support for purulent complications (1 - used, 0 - not used).
The results of treatment of patients in the main and control groups were compared only if there were no significant differences between these groups in the above-mentioned factors of severity of the condition and treatment factors. Differences were considered significant if the p
for the criterion used was below 0.05.
We used t
-test for a normal distribution of the attribute value,
U
-test when comparing groups with a non-normal distribution of the attribute value, and the χ2 test when comparing groups according to a qualitative attribute.
At the second stage of the study, an alternative version of the analysis was used, which did not require the formation of comparison groups and their randomization, as in the study by W. Uhl et al. - determining the combined influence of factors on the outcome of treatment. The result was a model for predicting the probability of an event occurring (a change in the outcome of treatment) based on the existing factors of the severity of the condition upon admission to the hospital and the factors of the treatment provided.
To assess the dynamics of mortality, the Cox proportional hazards model was used. Its peculiarity was the assessment of the influence of these parameters in each case of completed observation of the life of patients, i.e. until the moment of death.
The cumulative influence of factors on the prevalence of necrosis was assessed using logistic regression.
When selecting variables in the model for regression analysis, the backward elimination method was used [2].
The influence of the predictors included in the model and the accuracy of the entire model as a whole were considered significant if the p
χ2 criteria were less than the critical value (0.05) for all included factors and for the model as a whole.
The degree of influence of the predictor was determined by the value of the regression coefficient. Its larger numerical values indicated a greater power of influence. The direction of influence was assessed by the sign of the coefficient. With a negative value of the regression coefficient, the influence of the parameter of the severity of the condition or treatment was considered inversely dependent, i.e., its increase reduced the probability of death; with a positive value of the coefficient, an increase in the effect of the factor indicated an increase in the probability of death.
Statistical processing of the material was carried out using the StatSoft, Inc. program. (2007), Statistica version 8.0 and MedCalc version 10.5.0.0.
Special instructions for the use of the drug Octreotide
In case of a pituitary tumor that secretes growth hormone, strict medical supervision of patients receiving octreoid is necessary, since it is possible that the size of the tumors may increase with the development of such a serious complication as a narrowing of the visual fields. When treating endocrine tumors of the digestive tract and pancreas with octreotide, in rare cases a sudden relapse of the disease may occur. In patients with insulinoma during treatment with octreoid, an increase in the severity and duration of hypoglycemia may be observed. In diabetic patients receiving insulin, Octreoid may reduce the need for insulin. There is no experience with the use of Octreoid during pregnancy and lactation; during this period the drug is prescribed only for absolute indications.
Results and discussion
Already at the stage of univariate analysis, i.e., with a simple comparison of groups, a significantly lower mortality rate was revealed in the group of patients receiving octreotide compared with the group that did not receive it (52% versus 72%; χ2 test; p
=0.003).
When comparing groups by the prevalence of necrosis of the pancreas and parapancreatic tissue, it was also found that in the main group, widespread necrosis was significantly less common than in the control group (64% versus 77%; χ2 test; p
= 0.051). Thus, evaluation of the effectiveness of octreotide by univariate statistical analysis, used only in patients with a prognostically severe course of the disease, and not on the total number of patients with pancreatitis, as in the study by W. Uhl et al., showed a decrease in mortality and the prevalence of necrosis.
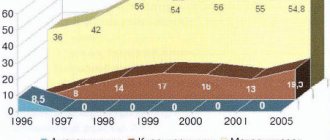
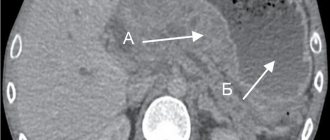
To adequately interpret this result and exclude the possibility of indirect correlation, the groups were compared for other potential predictors, which could also influence mortality and the prevalence of necrosis. Analysis of the severity of the condition of patients in both groups upon admission revealed virtually no differences between them (Table 1). The integral index of organ dysfunction (according to the APACHE II scale) did not differ in both groups (12.0±3.3 points in the main group versus 12.0±3.3 points in the control group; t
-test;
p
=0.959) (see figure).
Table 1. Differences in factors of severity of the condition of patients in the main and control groups
The severity of the patient's condition upon admission in patients with an initially severe course of the disease.
However, differences in treatment factors were significant. To exclude the fact that the detected decrease in mortality and prevalence of necrosis in the group of patients receiving octreotide was due to more frequent use of nutritional support at an early stage of the disease (χ2 test; p
=0.000) and pancreatic antibiotics (
U
-test;
p
=0.000) (Table 2), rather than using octreotide, turned out to be impossible. In this regard, to establish the cumulative effect of octreotide on mortality with other factors, a method of statistical analysis was used - the Cox proportional hazards model.
Table 2. Differences in treatment factors in the main and control groups
Cox analysis (Table 3) made it possible to construct a significant model of treatment factors and severity of the condition affecting mortality ( p
=0.000).
Octreotide was included in the predictor model ( p
= 0.000).
Table 3. Cox regression results
Moreover, the regression coefficient for octreotide turned out to be the largest of all predictors included in the model (β = –0.8). This meant that the strength of its influence on changes in mortality was the greatest among all factors included in the model. The sign of the regression coefficient “–” indicated that the fact of completeness of observation of the patient’s lifespan (corresponding to the fact of death) and the prescription of octreotide are inversely related, i.e., the prescription of octreotide reduced the likelihood of death in patients with destructive pancreatitis.
To determine the effect of octreotide administration on the prevalence of necrosis of the pancreas and parapancreatic tissue, logistic regression analysis was used (Table 4), which made it possible to construct a significant model of the mutual influence of factors ( p
=0.0001).
This model, along with other predictors that mutually influence the development of widespread necrosis, included octreotide ( p
= 0.033) as the factor with the strongest influence, the regression coefficient was –0.875. The sign of the regression coefficient “–” indicated that the change in the prevalence of pancreatic necrosis and the administration of octreotide are inversely related, i.e., the administration of octreotide reduced the likelihood of the prevalence of necrosis in patients with destructive pancreatitis.
Table 4. Results of logistic regression on the prevalence of necrosis
Thus, using the Cox proportional hazards model, a pattern was identified that the use of octreotide has a very strong effect on reducing mortality in initially severe forms of pancreatic necrosis. Using logistic regression, it was found that octreotide is the most significant factor influencing the reduction in the prevalence of necrosis of the pancreas and parapancreatic tissue in patients with severe pancreatic necrosis.
The data from the study allow us to recommend the administration of octreotide to patients with initially severe pancreatic necrosis.
Indications for use of the drug Octreotide
Acromegaly (control of the main manifestations of the disease and a decrease in the level of growth hormone and somatomedin C in the blood plasma in cases where the effect of surgical treatment, radiation therapy and treatment with dopamine agonists is not sufficient); relief of symptoms of endocrine tumors of the digestive tract and pancreas: carcinoid tumors with the presence of carcinoid syndrome; VIPs; glucagonomas; gastrinomas/Zollinger-Ellison syndrome (usually in combination with H2-histamine receptor blockers); insulinomas (to control hypoglycemia in the preoperative period, as well as for maintenance therapy); somatoliberins; refractory diarrhea in patients with AIDS; prevention of complications after pancreatic surgery; stopping bleeding and preventing re-bleeding from esophageal varices in patients with cirrhosis of the liver (in combination with specific therapeutic measures, for example, endoscopic sclerotherapy).