Classification
The main characteristics of UC, according to the classification, are considered to be the form of the disease (type of course), the extent of inflammation in the colon, the activity of inflammation and the severity of the course.
In accordance with these categories, the following clinical variants of UC are defined [1, 4]:
• acute form – the first attack of the disease lasting up to 6 months; can manifest itself in two variants: in a fulminant form, characterized by a rapid increase in clinical symptoms and a severe course, and in a form with a gradual onset and a mild clinical picture;
• relapsing form with a cyclical intermittent course with alternating exacerbations and remissions;
• continuous form with a persistent, usually sluggish course, moderate inflammatory activity in the mucous membrane and the absence of endoscopic remission for 6–8 months, subject to adequate therapy.
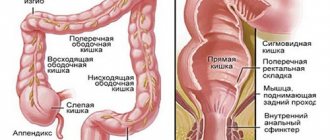
According to the extent of the inflammatory process in the colon with UC, in accordance with the Montreal classification, they are distinguished [5]:
• distal limited colitis – proctitis;
• left-sided colitis – includes proctosigmoiditis or more extensive diffuse damage to the colon from the anus to the splenic angle;
• widespread colitis – damage to the colon extending more than to the splenic angle, including total UC.
Clinical picture
In world practice, the severity of an attack (exacerbation) of UC is assessed using the disease activity index. There are several indices of UC activity (Rakhmilevich, Mayo, Oxford index, Swedish index, Truelov and Witts severity rating system, Montreal classification). There is no consensus among experts regarding the preferability of using any particular index, since all of them are not sufficiently validated. Currently, there is a tendency to additionally use the level of C-reactive protein (CRP) to assess activity, which is reflected in the European consensus (ECCO-consensus) on the diagnosis and treatment of UC. This document discusses all indices, with experts making the following conclusion: currently, to assess the severity of UC, it is necessary to take into account all parameters of the disease - clinical, laboratory, instrumental and histological [4]. The Expert Council of the Russian Group for the Study of Inflammatory Bowel Diseases (IBD) proposes a set of criteria that includes easily determined parameters for exacerbation of UC, including the level of CRP. This complex is broader than what is usually used when assessing activity indices, and therefore is more informative. The set of assessed disease parameters, to a greater extent than any indices (section 2.2.2.), corresponds to the expert assessment of the ECCO-consensus and takes into account both clinical and laboratory manifestations of the disease. This scoring system is an expanded modification of the UC severity criteria proposed by Truelov and Witts [6]. In accordance with the severity of clinical and laboratory symptoms, mild, moderate and severe UC are distinguished (see table).
The clinical picture of UC includes several groups of symptoms [1]:
• intestinal (blood in the stool, diarrhea, tenesmus);
• systemic extraintestinal autoimmune manifestations (arthropathy, skin lesions, aphthous stomatitis, eye lesions, primary sclerosing cholangitis, etc.);
• endotoxemia syndrome (general intoxication, febrile fever, tachycardia, anemia, increased ESR, leukocytosis with a shift in the leukocyte formula to immature forms, toxic granularity of neutrophils, increased levels of acute-phase proteins - CRP, seromucoid, fibrinogen);
• metabolic disorders (weight loss, dehydration, hypoproteinemia, hypoalbuminemia, hypokalemia and other electrolyte disturbances).
Choice of treatment tactics for different clinical variants of Crohn's disease
In the clinical picture of CD with intestinal damage, there are four main syndromes: intestinal syndrome, endotoxemia, extraintestinal manifestations and malabsorption syndrome. Intestinal symptoms during the active phase of the disease include diarrhea and abdominal pain. Diarrhea, the most common symptom, occurs in 70–80% of cases of CD of the small intestine, ileum, or colon. Excretion of blood in the stool is not necessary. This symptom appears only when the process is left-sided and distal in the colon. Despite the absence of visible blood in the stool, CD is characterized by progressive iron deficiency anemia. Abdominal pain of a constant or paroxysmal nature usually corresponds to the site of the lesion and the formation of an abdominal infiltrate, but it can also be diffuse and not localized. In some cases, acute attacks of pain may be the only symptom for several years. The cause of the pain is often not identified. Ultimately, patients are operated on with suspected acute appendicitis. During laparotomy, the phenomena of terminal ileitis or typhlitis are detected. Attacks of pain are usually accompanied by a rise in temperature to 38–39°C. Fever, peripheral blood parameters (leukocytosis, increased ESR, toxigenic granularity of neutrophils), an increase in blood C-reactive protein, seromucoid and fibrinogen indicate acute inflammation and reflect endotoxemia syndrome during the attack of the disease [2]. Episodes of fever without abdominal pain may occur for several years before the first intestinal symptoms appear. Persistent diarrhea, an inflammatory process with exudation of protein into the intestinal lumen and increased protein catabolism lead to significant weight loss, metabolic disorders (dehydration, hypokalemia, hyponatremia, hypoproteinemia, etc.) and the development of malabsorption syndrome. CD is often accompanied by extraintestinal systemic organ damage, reflecting an autoimmune component in the pathogenesis of the disease. CD is characterized by extraintestinal manifestations such as erythema nodosum, pyoderma gangrenosum, aphthous stomatitis, various arthropathy, including HLA B27 associated rheumatoid arthritis and ankylosing spondylitis and sacroiliitis (Bechterew's disease), vitiligo, psoriasis. The frequency of various systemic manifestations of the disease is in the range of 5–20%. According to our data, their overall frequency is 62%, of which almost 25% of patients have arthropathy. Rheumatoid arthritis and ankylosing spondylitis occur in equal proportions with a frequency of 8% [2,3]. The nature of histological changes and the depth of damage to the intestinal wall during transmural inflammation determine the range of complications of CD. The most typical and common complications: inflammatory strictures with subsequent development of intestinal obstruction, abdominal infiltrates and abscesses, interintestinal and external (intestinal-cutaneous) fistulas. When the anorectal area is affected, paraproctitis, perianal and rectovaginal fistulas and deep anal fissures are formed. When assessing the severity of CD, the nature and severity of clinical symptoms and complications are taken into account. It is customary to assess the severity of the disease using the CDAI (Crohn's disease activity index), also called the Best index [3,5]. This index includes a number of clinical signs, each of which, depending on their presence or severity, is assessed with a certain number of points. The index is calculated using a special formula. The CD activity index includes the following criteria: frequency of loose stools per week, presence of abdominal pain and its intensity, general health (good, fair, bad, very bad), systemic manifestations and/or anal manifestations (fissures, fistulas, abscesses) (there are , no), use of opiate antidiarrheals (loperamide), fever (> 37.5°C), hematocrit, degree of weight loss, presence of palpable infiltrate in the abdominal cavity. It is accepted that an index of less than 150 is regarded as remission of CD, an index of 150–220 corresponds to low activity and a mild course of the disease, 221–450 characterizes the moderate severity of CD, and above 450 points corresponds to high inflammatory activity and a severe course [1]. Treatment of patients with CD is a very difficult problem, because even with adequate therapy, in most patients the disease is progressive in nature with the development of complications or the formation of continuous forms of the disease. Treatment should be carried out differentially, taking into account the localization and extent of the inflammatory process, the type and nature of complications and the severity of the disease. The goal of treating CD is to relieve exacerbations and achieve and maintain remission. Basic pathogenetic therapy for CD includes three groups of drugs: corticosteroid hormones, 5-aminosalicylic acid (5-ASA) drugs and immunosuppressants [1,2]. Currently, therapy aimed at inducing remission in patients with mild or moderate CD traditionally begins with the prescription of 5-ASA drugs - mesalazine or sulfasalazine. In case of insufficient effectiveness of aminosalicylates, metronidazole and/or ciprofloxacin are prescribed. In the absence of an adequate therapeutic response, corticosteroids are used. In severe cases, steroid hormones are used as first-line therapy, and in case of refractory to steroids (steroid resistance or steroid dependence), reserve drugs - immunosuppressants - are used. Such established approaches are not differentiated and do not take into account the evidence-based medicine accumulated to date, which makes it possible to weigh the relationship between the effectiveness, safety and tolerability of drugs and to develop optimal treatment approaches for different courses and different forms of the disease. The aminosalicylates Sulfasalazine and mesalazine are highly effective basic drugs for the treatment of mild to moderate ulcerative colitis. This suggested that the drugs could be equally effective in CD. Today, they have become firmly established in the treatment regimens of CD. However, the results of controlled trials refute the feasibility of this approach. Several placebo-controlled clinical trials, as well as comparative studies with glucocorticoids, have been devoted to studying the effectiveness and safety of sulfasalazine in CD [5–8]. The results of these studies indicate that although the effectiveness of sulfasalazine was higher than placebo, it was nevertheless inferior to even low doses of systemic corticosteroids (0.25 mg/kg/day). Unlike ulcerative colitis, even with mild CD, sulfasalazine exhibits low activity. A positive result of treatment with sulfasalazine was obtained only in patients with predominant damage to the colon, which is explained by its direct activation by enzymes of the colon flora, and only in cases of mild disease [9]. Only patients who did not receive prior hormonal therapy responded to sulfasalazine therapy, while the drug was not effective in those who received corticosteroids [7]. All of the above suggests that sulfasalazine has limited possibilities for use in patients with only mild CD with predominant localization in the colon. The well-known side effects of sulfasalazine, occurring in 10–30% of patients, also limit its use [9,10]. Thus, the proven low effectiveness of sulfasalazine in combination with a wide range of adverse reactions does not allow it to be recommended for the treatment of patients with CD with terminal ileitis or ileocolitis; there are limited indications for the treatment of mild colon CD. Mesalazine does not have the side effects of sulfasalazine and is not inferior to it in effectiveness. Tablet mesalazine preparations produced in different countries are similar in action and effectiveness and are 5-ASA in a protective coating. They differ in the nature of the enteric coating (eudragit, acrylic or ethylcellulose) and, accordingly, in the location and rate of release of 5-ASA in the intestine [2]. The clinical effectiveness of mesalazine correlates with the intraluminal concentration of 5-ASA, so the location of the lesion must be taken into account when prescribing it. Mesalazine is used as first-line therapy in patients with mild to moderate CD by 75% of physicians [11]. Clinical trial results, however, are conflicting. Mesalazine at various doses has been studied in several double-blind, placebo-controlled studies [12,13,15]. In some of them, the drug at a dose of 2–4 g per day was not superior to placebo in its ability to induce remission in patients with CD. However, when analyzing pooled data from 3 studies using a relatively high dose of mesalazine (4–4.5 g/day), a statistically significant difference from placebo was revealed [11]. Mesalazine at a dose of 2 g per day is significantly inferior to steroids (prednisolone, methylprednisolone and budesonide); increasing the dose to 4.5 g gives comparable results [20,23,24,25]. In a comparative study with budesonide (9 mg/day), mesalazine (4 g/day) was inferior to the glucorticoid in its ability to induce remission in patients with mild and moderate forms of CD [16]. Side effects when using mesalazine are quite rare. Thus, not all research results are equally positive regarding mesalazine. The minimum effective dose for mild CD is 4 g per day, although some studies did not have enough patients to draw meaningful conclusions. Given the low incidence of side effects compared with sulfasalazine and systemic steroids, mesalazine can be recommended as an initial trial treatment for mild CD, but the benefit rate is not high enough [4,11]. The overall effectiveness of 5-ASA drugs does not exceed 30%. The feasibility of their use as anti-relapse therapy has not been proven. Systemic glucocorticosteroids Glucocorticosteroids have been used in the treatment of inflammatory bowel diseases for 50 years and have traditionally been first-line drugs for severe CD [1,2,4]. However, in the context of the above, steroids should be recommended for moderate to severe disease and in case of ineffectiveness of 5-ASA in mild cases [4,11]. The effectiveness of corticosteroids and the severity of side effects depend on the rate of their metabolism in the liver, bioavailability, affinity for receptors and other parameters. Prednisolone and its methylated analogs (methylprednisolone) are considered the drug of choice. The average oral dose of prednisolone during a CD attack can be 40–60 mg, but a dose of 1 mg/kg body weight per day should be considered more effective, and in very severe cases the dose can be increased to 1.5–2 mg/kg per day. The use of high-dose oral steroids for a severe attack of CD competes in effectiveness with intravenous high-dose hormonal therapy (250–500 mg methylprednisolone or hydrocortisone per day for 7 days). Use of prednisolone in a minimum and average dose of 0.25–0.75 mg kg/day. led to improvement in 60% of patients, compared with 30% in the placebo group [7]. More convincing results were obtained with 6-methylprednisolone at a dose of 48 mg [8]. Other studies have confirmed that systemic steroids are more active in CD than placebo, sulfasalazine, antibiotics, and azathioprine, but are associated with a high incidence of side effects [13,15,17,18–23]. Tolerability of corticosteroids largely depends on the route of administration, dose, duration of therapy, gender and individual sensitivity to drugs. Common side effects include moon face, steroid diabetes, osteoporosis, mood changes, insomnia, swelling, weight gain, stretch marks. Thus, despite the proven high effectiveness of systemic steroids in inducing remission in CD, their long-term use is limited by frequent and varied side effects. For the same reason, it is impossible to use steroids to maintain remission. Topical steroids The need to use predominantly steroid hormones in the treatment of CD and the limitations caused by their side effects have led to the creation of a safe alternative to systemic steroids that are not inferior to them in effectiveness. The new group of drugs is called “topical” steroids. These are local hormones that create a high concentration of the drug in the affected area (in the intestine) and have virtually no side effects [11,24–26]. In Russia, in 2004, one of the most effective topical steroids for the treatment of CD was registered - budesonide. Budesonide demonstrated similar activity to systemic corticosteroids, with better tolerability and less influence on the hypothalamic-pituitary-adrenal system. The advantages of budesonide over systemic steroids lie in the peculiarities of its pharmacodynamics. First of all, budesonide has a high affinity for glucocorticoid receptors in target tissues (in this case, receptors of the intestinal mucosa), many times higher than the affinity of traditional drugs: prednisolone and hydrocortisone. Thus, the affinity of budesonide for steroid receptors is 100 times higher than that of hydrocortisone, 50 times higher than that of prednisolone and approximately 18 times higher than that of methylprednisolone. In addition, budesonide has minimal absorption from the gastrointestinal tract, low systemic bioavailability (no more than 10%) compared to prednisolone and hydrocortisone, and high first-pass metabolism. The latter quality ensures the absence of toxic metabolites in the blood after the first passage of the drug through the liver. These properties of budesonide make it possible to certain extent to increase the effectiveness of treatment of patients in need of hormonal therapy. The activity of the drug is enhanced due to its high affinity for steroid receptors, microgranular galenic form with the release of high concentrations in the terminal ileum and colon. The absence of side effects characteristic of systemic steroids is due to low absorption from the intestinal lumen in combination with high first-pass metabolism and low systemic bioavailability. The attractive features of budesonide make it possible to take the drug for a long time to stop an attack of CD without the risk of developing side effects. The use of budesonide for anti-relapse therapy of this disease is debated [27,28]. Randomized clinical trials have demonstrated similar efficacy of systemic steroids and budesonide with significantly fewer side effects of the latter [20,23,29,30]. The optimal dose of budesonide was 9 mg per day, the duration of treatment was 12–16 weeks, followed by gradual withdrawal. Budesonide proved to be effective mainly in moderate cases of CD in the form of terminal ileitis and ileocolitis and in these cases should be a first-line drug [4,11,26]. From our point of view, it should also be used for mild CD of the same localization. Budesonide is not indicated for CD with systemic autoimmune manifestations. In severe cases of CD, the effectiveness of the drug at the indicated dose is insufficient. Immunosuppressants In the absence of a response to treatment with hormones, in cases of steroid resistance and steroid dependence, reserve drugs are prescribed - immunosuppressants [32–35]. Azathioprine and 6-mercaptopurine, methotrexate and cyclosporine A are used. Data on the effectiveness of immunosuppressants in CD are very contradictory. The rate of achieving clinical effect and entering remission for both azathioprine and methotrexate does not exceed 70% [32]. Cyclosporine is less effective for CD; its effect is more pronounced in patients with ulcerative colitis. The use of immunosuppressants is limited to some extent by a wide range of side effects. For azathioprine, these are nausea, vomiting, diarrhea, flu-like syndrome with fever and myalgia, bone marrow suppression with leukopenia, neutropenia (less often with agranulocytosis), thrombocytopenia. Taking azathioprine may also be accompanied by hepatotoxic reactions in the form of cytolysis and cholestasis, pancreatitis, and the development of polyneuritis. The drug is believed to increase the risk of malignancy, although many dispute this position [36]. Side effects develop with a frequency of 6–20%. Methotrexate is the treatment of choice in patients with CD who have not responded to treatment with steroids and azathioprine [34,37]. The incidence of side effects when taking methotrexate is 10–20% (including nausea, vomiting, diarrhea, ulcerative stomatitis, leukopenia, thrombocytopenia, anemia, hepatotoxicity, pulmonary fibrosis, alopecia). With long-term use, necrosis of hepatocytes and liver fibrosis are possible. The action of azathioprine and methotrexate develops slowly, improvement can be noticeably earlier than 3-4 weeks, a period of 4-6 months is required to obtain the maximum effect, because immunosuppressors cannot be used in acute situations, they are suitable only for treatment chronic sluggish active forms of BC. Azatioprine is also used to maintain remission, but the ability of methotrexate to maintain prolonged remission is considered doubtful. Remission induced by methotrexate persists for one year only in 40% of patients [38]. Thus, the use of immunosuppressors is limited, on the one hand, by side effects and a slowly developing effect, on the other hand, they often do not give the desired result. In practice, an alternative treatment method for patients with resistant CD is surgical treatment. Infliximab a new direction in the treatment of patients with BCs who did not respond to treatment with steroids (both systemic and topical), provides for the selective suppression of the synthesis and activity of the factor of tumor necrosis (FNO). For this purpose, infliximab is widely used, which is chimeric monoclonal antibodies to FNO -A and consisting of 25% of mouse protein and 75% of human immunoglobulin. Infliximab has been used abroad for 8 years, and in Russia, first of all, it was registered for the treatment of two diseases similar in terms of development of inflammation: BC and rheumatoid arthritis (currently also registered for the treatment of ulcerative colitis). Infliximab is recommended with a non -complicated steroid -resistant form of BC and with a fistulous form, and it is active both with a moderate and in severe course of the disease [33,39,40]. In the case of a complicated course for induction of remission, one intravenous infusion is enough at the rate of 5 mg/kg/day. With a fistulous form, 3 -fold administration of the drug with an interval of 2 weeks is used [41,42]. Ifliximab has a prolonged effect. Successful results were obtained in patients with BC with the development of inflammation in the field of the ileoanal reservoir and with perianal localization of BC [43]. In controlled multicenter tests, the frequency of response to infliximab in steroid -resistant forms of BCs ranges in an interval of 50–82%, the frequency of reaching clinical remission is 25–48% after 4 weeks [44–46]. Long -term treatment with infliximab for 44 weeks turned out to be an effective, well -tolerated method of stopping the symptoms of BC and the achievement of remission in patients who did not respond to traditional treatment, and with maintenance therapy, remission was preserved for 54 weeks in 39% of patients [47]. It is noteworthy that in patients who received infliximab to maintain remission, they managed to completely abandon the use of corticosteroids [47]. Infliximab also turned out to be the only drug at the moment, which made it possible to achieve not only clinical, but also endoscopic remission at BC [47]. The effect of infliximab develops quickly; after 2 weeks, the onset of clinical effect can be observed. The duration of its action is up to 30 weeks after a single infusion, however, after 8–12 weeks, the concentration of antibodies in serum decreases, therefore, in patients with the most stubborn form of the disease, repeated infusions are recommended every 8 weeks to maintain a clinical response [45]. At the Expert Council for the development of new standards for the use of infliximab in the EU and the USA in October 2005 (UC and CD: Treating to New Standarts Expert Round Table. 10.10.2005) it was proposed to include not only steroid refractory forms of BC and ulcerative forms Polititis (yak), but to recommend infliximab as a first -line drug in severe forms of these diseases. Currently, in Russia, infliximab is included in the list of Life preparations for the treatment of BC, included with the treatment standards of BC (MZ RF) and registered for the treatment of Yak. Inflocial therapy The issue of supporting BC therapy after reached remission remains not fully resolved. Sulfasalazine, oral mesalazine and prednisone in low doses are ineffective for maintaining remission. Budsonide at a dose of 6 mg leads to lengthening time before relapse, but does not allow maintaining remission throughout the year and does not prevent BC relapses after operations [31]. Antibiotics have not been studied in clinical research on this indication. Azatioprine, 6 - Merkaptopurin, are also effective as means of supporting therapy, but their use is limited by a large number of side effects. Thus, the data available at the moment do not allow us to recommend any drug for maintaining therapy, as a favorable ratio of effectiveness/safety. In this regard, some authors recommend only therapy to patients with mild and moderate BCs aimed at achieving remission with the subsequent renewal of its relapse [11]. The conclusion of this work pursued two goals: firstly, to demonstrate the insufficient validity of traditional approaches to the treatment of BC, primarily its lungs and moderate forms. Secondly, to show the possibilities of new drugs and their specific niche in the therapy of BC. Based on the above, the choice of treatment tactics with different forms of BC, taking into account the severity, localization and nature of complications, can be represented as shown in table 1.
Literature 1. Adler G. Crohn’s disease and ulcerative colitis. – Goetar – Med. – 2001. – 500 p. 2. Belousova E.A. Ulcerative colitis and Crohn's disease. – Triad. – 2002. – 128 p. 3. Morozova N.A., Belousova E.A. Systemic autoimmune manifestations of inflammatory bowel diseases (oral communication) 4. Belousova Pharmacotherapy and algorithm for the treatment of mild and moderate Crohn's disease from the perspective of evidence-based medicine. – Pharmateka. – 2004. – No. 13. – p. 8–18. 5. Anthonisen P, Barany F, Folkenborg O, et al. The clinical effect of salazosulphapyridine (Salazopyrin R) in Crohn's disease. Scand J Gastroenterol 1974; 9:549–54. 6. Van Hees PA, Van Lier HJ, Van Elteren PH, et al. Effect of sulphasalazine in patients with active Crohn's disease: a controlled double–blind study. Gut 1981; 22:404–9. 7. Summers RW, Switz DM, Sessions JT, et al. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology 1979; 77:847–69. 8. Malchow H, et al. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology 1984; 86:249–66. 9. Das KM, Eastwood MA, McManus JP, et al. The relationship between metabolites and the response to treatment in inpatients. Gut 1973; 14: 631–41. 10. Taffet SL, Das KM. Sulfasalazine. Adverse effects and desensitization. Dig Dis Sci 1983; 28:833–42. 11. Sandborn WJ, Feagan BG Mild to Moderate Crohn's Disease – Defining the Basis for a New Treatment Algorithm. Aliment Pharmacol Ther 2003;18:263–77. 12. Tremaine WJ, Schroeder KW, Harrison JM, et al. A randomized, double–blind, placebo–controlled trial of the oral mesalamine (5–ASA) preparation, Asacol, in the treatment of symptomatic Crohn's colitis and ileocolitis. J Clin Gastroenterol 1994; 19: 278–82. 13. Gross V, Andus T, Fischbach W, et al. Comparison between high dose 5–aminosalicylic acid and 6–methylprednisolone in active Crohn's ileocolitis. A multicenter randomized double–blind study. German 5–ASA Study Group. Z Gastroenterol 1995; 33:581–4. 14. Scholmerich J, Jenss H, Hartmann F, the German 5–ASA Study Group. Oral 5–aminosalicylic acid versus 6–methylprednisolone in active Crohn's disease. Can J Gastroenterol 1990;4:446–51. 15. Prantera C, Cottone M, Pallone F, et al. Mesalamine in the treatment of mild to moderate active Crohn's ileitis: results of a randomized, multicenter trial. Gastroenterology 1999; 116:521–6. 16. Thomsen OO, Cortot A, Jewell D, et al. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide–Mesalamine Study Group. N Engl J Med 1998; 339:370–4. 17. Rijk MC, van Hogezand RA, van Lier HJ, et al. Sulphasalazine and prednisone compared with sulphasalazine for treating active Crohn disease. A double–blind, randomized, multicenter trial. Ann Intern Med 1991; 114:445–50. 18. Martin F, Sutherland L, Beck IT, et al. Oral 5–ASA versus prednisone in short term treatment of Crohn's disease: a multicentre controlled trial. Can J Gastroenterol 1990; 4:452–7. 19. Yang YX, Lichtenstein GR. Corticosteroids in Crohn's disease. Am J Gastroenterol 2002; 97:803–23. 20. Gross V, Andus T, Caesar I, et al. Oral pH–modified release budesonide versus 6–methylprednisolone in active Crohn's disease. German/Austrian Budesonide Study Group. Eur J Gastroenterol Hepatol 1996; 8:905–9. 21. Rutgeerts P, Lofberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med 1994; 331:842–5. 22. Prantera C, Zannoni F, Scribano ML, et al. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 1996; 91: 328–32. 23. Bar–Meir, S, Chower, Y, Lavy, A, et al. Budesonide versus prednisone in the treatment of active Crohn's disease. Gastroenterology 1998; 115:835. 24. Yang YX, Lichtenstein GR. Corticosteroids in Crohn's disease. Am J Gastroenterol 2002; 97:803–23. 25. Lyckegaard E, Hakansson K, Bengtsson B. Compassionate use of budesonide capsules (ENTOCORT EC) in patients with Crohn's disease. Gastroenterology 2002; 122:T1665. 26. Lofberg, R, Rutgeerts, P, Malchow, H, et al. Budesonide prolongs time to relapse in ileal and ileocecal Crohn's disease: A placebo controlled one year study. Gut 1996; 39:82. 27. Hellers, G, Cortot, A, Jewell, D, et al. Oral budesonide for prevention of postsurgical recurrence in Crohn's disease. Gastroenterology 1999; 116:294. 28. Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med 1994; 331:836–41. 29. Rutgeerts P, Lofberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med 1994; 331:842–5. 30. Kane SV, Schoenfeld P, Sandborn WJ, et al. Systematic review: The effectiveness of budesonide therapy for Crohn's disease. Aliment Pharmacol Ther 2002; 16:1509–17. 31. Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med 1994; 331:836–41. 32. Elson CO The effects of immunosuppressive agents on cytokines. //Aliment. Pharmacol. Ther.– 1996.– v.10.–suppl.2.–p.100–105. 33. Sanborn WJ Steroid–dependent Crohn's disease. // Scand. J. Gastroenterol..– 2000.– v. 14.– (Suppl. C).– 17C. 34. Sands BE Medical therapy of steroid–resistant Crohn's disease. // Scand. J. Gastroenterol.– 2000.– v. 14.– (Suppl. C).– 33C. 35. Steinhart H. Steroid resistant and steroid dependent Crohn's disease. // IBD, salicylates and other relevant therapies–Proceeding of the International IBD Symposium.– London.– 1999.– p.83–90 36. Lewis JD, Sanford–Schwartz J., Lichtenstein GR Azatioprine for maintenance of remission in Crohn's disease: benefits outweigh the risk of lymphoma // Gastroenterology. –2000.– v. 118.– p.1018–1024 37. Lemann M., Zenjari T., Cosnes J., Mesnard B. Methotrexate in Crohn's disease: long–term efficacy and toxicity.– Am. J. Gastroenterol. 2000.–v. 95.– p.1730–1734 38. Feagan BG, Fedorak RN, Irvine EJ, Wild G. et all. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease // N. Engl. J. Med.– 2000.– v.342.– p. 1627–1632 39. Belousova E.A., Morozova N.A., Nikitina N.V. Infliximab (Remicade) in the treatment of refractory forms of Crohn's disease.–Breast cancer.–2005.–vol.7.–No.1.–p.28–32 40. Sands B., Anderson F., Bernstein C. Infliximab Maintenance Therary for Fistulizung Crohn's Disease.– N.Eng.J.Med. 2004.–v.350.– p.876–885. 41. Lichtensteyn G., Yan S., Bala M. et all. Infliximab Maintenance Treatment Reduces Hospitalizations, Surgeries, and Procedures in Fistulizing Crohn's Disease. – Gastroenterology 2005.–v.128.–p.863–869 42. Present DH, Rutgeerts P., Targan S., Hanauer SB et all. Infliximab for the treatment of fistulas in patients with Crohn's disease.– N. Engl. J. Med. – 1999. – v. 340.– 1398–1405 43. Ricart E., Panaccione R., Loftus E., Tremain W. Successful management of Crohn's disease of the ileoanal pouch with infliximab // Gastroenterology.– 1999.– v. 117.– p. 429–432 44. Hanauer S., Feagan B., Lichtenstein G. et all. Maintenanse Infliximab for Crohn's Disease: the ACCENT I Randomised Trial.– Lancet 2002.–v.359.–p.1541–1549. 45. Rutgeerts P. A Critical Assessment of new Therapies in Inflammatory Bovel Diseases. // J. Gastroenterol. Hepatol. 2002.–V.17.–Suppl..–S177 QR. 46. Targan SR, Van Deventer SJH, et all. A short–term study of chimeric monoclonal antibody cA2 to tumor necrosis factor? for Crohn's disease.// N.Engl.J.Med. –1997.–v.337.–p.1029–1035 47. Rutgeerts P., D'Haens G., Targan S. et al. Efficacy and Safety of Retreatment with Anti–Tumor necrosis factor antibody (Infliximab) to maintain remission in Crohn’s disease // Gastroenterology, 1999.– V.117.– P.761–769.
Treatment
Basic treatments for UC include 4 groups of medications: 5-aminosalicylic acid (5-ASA) drugs, glucocorticosteroids (GCS), immunosuppressants and tumor necrosis factor α inhibitors. The first two groups are considered first-line drugs [1–3]. This article discusses only issues related to the treatment of UC with aminosalicylates.
5-ASA drugs (sulfasalazine and mesalazine) are used in the treatment of mild and moderate forms of UC to induce remission and as maintenance anti-relapse therapy in the remission stage [1–3]. The main mechanism of action of 5-ASA is the inhibition of the cyclooxygenase and lipoxygenase pathways of arachidonic acid metabolism, suppression of the synthesis of active inflammatory mediators, mainly leukotrienes, and especially leukotriene B4 [7]. In addition, 5-ASA suppresses the synthesis of pro-inflammatory cytokines: interleukin-1, -2 and tumor necrosis factor α, the production of antibodies by B lymphocytes and neutralizes free oxygen radicals [8]. It has also been shown that 5-ASA is capable of suppressing the nuclear transcription factor NF-κB and the activity of substances that stimulate it [9].
The first drug of the aminosalicylate group was sulfasalazine, synthesized in 1946. It is a 5-ASA linked by a nitrogen bond to sulfanilamide (sulfapyridine). The latter is an inert part of the molecule, prevents the absorption of the drug in the jejunum and actually serves as a carrier of 5-ASA to the colon. The bond between 5-ASA and sulfapyridine is cleaved in the ileum and colon under the influence of bacterial enzymes (azoreductases), and the released 5-ASA exerts its anti-inflammatory effect by blocking the synthesis of mediators in the colon mucosa. Only 20–30% free 5-ASA is absorbed from the colon, so its systemic effect is very small. The main part of the drug remains in the intestinal lumen and intestinal epithelium in a partially acetylated form. Thus, 5-ASA released from sulfasalazine has a mainly local effect.
Sulfasalazine is toxic and causes a wide range of side effects, including leukopenia with agranulocytosis, toxic-allergic skin lesions, renal dysfunction, pancreatitis, infertility in men, etc. These reactions occur among 15–20% of patients. The development of side effects is associated with the sulfonamide part of the drug, since sulfapyridine is almost completely absorbed from the colon and metabolized in the liver. The problem of toxicity, as is known, was solved by the creation of 5-ASA preparations without sulfapyridine in the molecule (mesalazine, olsalazine, balsalazide), which are not inferior to sulfasalazine in effectiveness, but without its side effects.
Until now, tablet preparations of mesalazine (Salofalk, Pentasa, Azakol, Mezakol) have been most widespread both abroad and in Russia, similar in mechanism of action and effectiveness, but differing in the nature of the enteric coating (L- or S-eudragit, acrylic or ethylcellulose ), respectively, the place and rate of 5-ASA release in the intestine [1, 10]. There is a clear correlation between the intraluminal concentration of 5-ASA and the clinical effectiveness of the drug, so the location of the lesion must be taken into account when prescribing aminosalicylate. The dissolution of L-eudragite enteric coating (Salofalk) and S-eudragite coating (Azacol) depends on the pH in the intestinal lumen, and it is destroyed at certain values in the terminal ileum and proximal colon, where the maximum therapeutic concentration of 5-ASA is achieved . Pentasa differs from these forms of mesalazine, having a microgranular structure and an ethylcellulose coating. The dissolution of the ethylcellulose coating is independent of intestinal pH and begins in the duodenum, providing a slow, gradual and uniform release of 5-ASA throughout the small intestine and proximal colon. All of these drugs are successfully used to treat common mild and moderate UC, creating an effective concentration of 5-ASA in the intestinal lumen. However, tablet preparations, unfortunately, are ineffective in the treatment of left-sided, especially distal, UC (proctitis), since the concentration of the active substance in the colon decreases in the caudal direction, as a result of which a minimal part of 5-ASA enters the descending and sigmoid colon, and there is practically none in the rectum. For the treatment of UC with damage to the rectum and left-sided colitis, there are dosage forms of mesalazine in the form of enemas, foams and suppositories. Often, to increase the effectiveness of therapy, a combination of oral mesalazine and rectal administration is used. Such treatment is quite expensive and technically inconvenient for patients, which reduces treatment adherence and therapeutic effect, increasing the risk of relapse.
Below are traditional treatment regimens for distal and widespread UC of mild and moderate severity. These schemes were developed by the Russian group for the study of IBD based on the European consensus [11].
Treatment of mild and moderate proctitis
1st line of therapy:
• suppositories with mesalazine 1–2 g/day. Onset of therapeutic response within 2 weeks. The duration of the induction course is 6–8 weeks.
In the absence of effect: 2nd line of therapy:
• a combination of mesalazine in suppositories 1 g and mesalazine in tablets 2–3 g or
• a combination of mesalazine in suppositories 1 g and rectal forms of GCS (suppositories with prednisolone 5-10 mg 1-2 times a day).
When clinical and endoscopic remission is achieved, maintenance therapy:
• mesalazine topically (suppositories) 1–2 g 3 times a week as monotherapy or in combination with mesalazine tablets 1.5–2.0 g – at least 2 years.
Treatment of left-sided and total mild UC
• 1st attack or relapse: mesalazine in tablets orally 3 g/day (or sulfasalazine 4 g/day) in combination with mesalazine in enemas 2–4 g/day (depending on endoscopic activity). The onset of therapeutic response is up to 2 weeks. The duration of the induction course is 6–8 weeks.
If there is no effect, add rectal forms of GCS (microenemas with a suspension of hydrocortisone 125–250 mg/day).
When clinical and endoscopic remission is achieved, maintenance therapy:
• mesalazine in tablets 1.5 g/day orally + mesalazine in enemas 2 g 2 times a week;
• it is acceptable to prescribe sulfasalazine 3 g instead of mesalazine.
Treatment of left-sided and total UC of moderate severity
First attack or relapse: mesalazine tablets 4–5 g/day in combination with mesalazine enemas 2–4 g/day (depending on endoscopic activity).
When remission is achieved, maintenance therapy:
• mesalazine in tablets 1.5 g/day orally + mesalazine in enemas 2 g 2 times a week;
• it is acceptable to prescribe sulfasalazine 3 g/day instead of mesalazine.
Treatment of mild to moderate ulcerative colitis
Speech by Professor Shifrin O.S. within the framework of the II International Internet Congress of Internal Medicine Specialists.
Professor Shifrin O.S.: – Good afternoon, dear colleagues. I will give a talk today on the topic of inflammatory bowel disease.
(00:13) Screensaver: 5-aminosalicylic acid preparations in the treatment of nonspecific ulcerative colitis.
Professor Shifrin O.S.: – And it will be devoted to the treatment of ulcerative colitis: how to optimally use 5-aminosalicylic acid preparations in the treatment of this severe, diagnostically and therapeutically difficult disease. What goals does a clinician pursue when taking on the complex task of supervising a patient with ulcerative colitis? First of all, he must achieve clinical remission, but this is not enough. He should try to achieve clinical remission without the use of steroid drugs. Maintenance of clinical remission should be long-term. A very important key to the success of long-term maintenance of clinical remission is the achievement of endoscopic remission, and in the future we will strive to achieve histological remission. As is known, a relatively small number of drugs are used in the treatment of inflammatory bowel diseases. There is a tendency that a number of drugs that were previously used in the treatment only of Crohn's disease are now successfully used for ulcerative colitis. This applies to azathioprine, anticytokine drugs, and some antibiotics that are also used for toxic forms of ulcerative colitis.
There are two approaches to the treatment of patients with ulcerative colitis and Crohn's disease: either we prescribe drugs sequentially from the least strong effect and, accordingly, from the weakest side effects, to stronger drugs that give a greater number of side effects. This approach seems to be the most logical. Another approach is when, already in the early stages, and this is especially true for Crohn’s disease, anti-cytokine drugs are prescribed. These are drugs that do not change the natural course of the disease, but at the same time threaten the occurrence of many severe side effects. It should be remembered that 5-aminosalicylic acid drugs in the first line of treatment for ulcerative colitis have the highest degree of evidence of the effectiveness of their action. Already by 2000, based on a large number of controlled randomized studies, it was shown that these drugs in their effectiveness were supported by the highest level of evidence - 1A.
The first drug of 5-aminosalicylic acid, which began to be used for ulcerative colitis, was “Sulfasalazine”, consisting of two parts: 5-aminosalicylic acid combined with sulfapyridine, which is used as a carrier of 5-aminosalicylic acid under the action of azoreductases produced by colon bacteria , the drug molecules disintegrate into their original parts and exhibit a pharmacological effect. I will dwell a little later on the numerous side effects of this drug. And the prescription of Sulfasalazine now in the 21st century should be treated with great caution.
So, let us remember the recommendations of the European Society for the Study of Colitis and Crohn's Disease in terms of the use of 5-aminosalicylic acid preparations for ulcerative colitis. In the case of a widespread form of colitis, with total colitis, but with moderate activity, at least 2 grams of mesalazine per day is indicated as the basic therapy for exacerbations. This is supported by a high level of evidence of 1A. And what is very important, for total colitis you should also use local topical preparations of mesalazine - this dramatically increases the effectiveness of treatment.
For left-sided colitis with moderate activity, a combination of oral mesalazine and topical drugs is also indicated. At the same time, the effectiveness of treatment significantly increases with a level of evidence of 1B. For proctitis with mild moderate activity, it is possible to initially prescribe suppositories with mesalazine with a high level of evidence. But if there is no effect, this local therapy should be supported by the administration of oral forms of the drug. Maintenance therapy with 5-aminosalicylic acid drugs is recommended for all patients in whom we managed to achieve clinical and endoscopic remission after using 5-aminosalicylic acid drugs as an exacerbation therapy.
Oral therapy is the first line of maintenance therapy and should be used for total colitis, necessarily with the use of oral forms. For proctitis or left-sided colitis, it is possible to use only local forms of mesalazine to maintain remission (not to induce remission). If it is not possible to successfully maintain remission with local forms only, the latter should be combined with tablets. And we must not forget the minimum doses that are necessary to effectively maintain remission. They must be at least 1 gram. At the same time, topical mesalazine preparations, in particular suppositories, should be prescribed at least three times a week for distal colitis. Of course, recommendations are recommendations. And a clinician who has experience in treating such patients can, of course, independently decide many questions about prescribing the drug. The ECCO guidelines do not require scrupulous adherence to them. First of all, the real clinical picture decides.
Thus, according to the experience of our clinic, it is very important to consider the clinical features of the onset of the disease. They also, to some extent, predispose to maintaining remission in the future with one drug or another. Thus, in the case of a purely diarrheal variant of the onset (not diarrheal-hematochezian) of ulcerative colitis, 5-aminosalicylic acid preparations also turned out to be most indicated. 5-aminosalicylic acid preparations are also important in preventing the development of colorectal cancer; unfortunately, a frequent complication of ulcerative colitis is a decisive line for the prevention of colorectal cancer. Screening and genetic studies have not yet been fully developed. Therefore, special importance is attached to the long-term administration of 5-aminosalicylic acid drugs in order to prevent the development of colorectal cancer in our patients.
The first 5-aminosalicylic acid drug was Sulfasalazine. It showed an effect, but it also showed a high incidence of various side effects when using it. According to our data, 40% of patients with ulcerative colitis who took Sulfasalazine experienced some side effects. After Sulfasalazine, the so-called diazo compounds of 5-aminosalicylic acid were developed: Olsalazine, in which two molecules of 5-aminosalicylic acid are connected by a diazo bond, and Balsalazide, in which 5-aminosalicylic acid is connected to a 4-amino-benzoyl-alanine residue. But, unfortunately, these drugs showed approximately the same frequency of side effects as Sulfasalazine.
Later, releasing systems appeared; in the beginning, they were predominantly pH-dependent. That is, the release of mesalazine contained in the tablet depended primarily on the pH of the intestinal contents. The disadvantage of this group of drugs is that, since inflammation of the intestinal wall changes the normal intestinal pH, these drugs often do not work. The situation changed after the development of mesalazine preparations, namely mesalazine microgranules coated with an ethylcellulose coating, the release of which depends on the time the substance remains in the intestine.
The release of the drug "Pentasa", which represents this form, begins already in the duodenum. That is, this drug is good for treating not only ulcerative colitis, but is very suitable for treating Crohn's disease. The drug is evenly distributed in the small and large intestines. What is very important is that it does not linger in the stomach, which avoids a sharp increase in concentration in the blood. Thanks to its gradual release, the drug reaches the distal parts of the intestine. And the rate of drug release remains constant over a wide range of pH of the intestinal contents. That is, in this regard, the drug is very advantageous. The distribution of the drug has been repeatedly studied using various methods, in particular scintigraphy. And here it is shown that mesalazine is released in both the small and large intestines. And the area of application of this drug is both Crohn’s disease and ulcerative colitis. Moreover, various forms are used for these diseases. When studying the distribution of the drug, we noted that the distribution of Pentas was very uniform and applied both to the initial parts of the small intestine, starting with the duodenum, and to the distal parts of the colon.
Let me tell you about a case from our clinical practice. A 35-year-old patient came to our clinic with complaints: loose stools mixed with mucus, sometimes blood, up to 5-7 times a day, flatulence, weakness, weight loss of almost 7 kg in a year. Ill for two years. The young woman was quickly diagnosed with ulcerative colitis; unfortunately, this is not always the case. As with Crohn's disease, we have big problems in the country with the early detection of these diseases. A patient with Crohn's disease was recently admitted to our clinic but had not been diagnosed during his 24 years of follow-up with a typical clinical presentation. That is, of course, we must study the clinical picture of these diseases more deeply.
So, the patient developed hematochezia, diarrhea, and began to lose weight. The correct diagnosis was made - ulcerative colitis. The doctors mechanically followed the path of increasing the dose of Sulfasalazine. At first she took 2 grams, then she began to take 3 grams and, finally, 4 - there was no effect. Fortunately, this patient had no side effects. But the clinical effect of prescribing Sulfasalazine was not achieved. In our clinic, a diagnosis of “nonspecific ulcerative colitis, total form, Truelove I activity” was made, and it was confirmed. We widely use radiation methods to confirm the diagnosis, in particular computed tomography. The computer colonography method allows you to clarify the lesion, at what stage the intestine is affected, the severity of the lesion, the thickness of the intestinal wall, evaluate the fiber adjacent to the intestine, the presence of lymph nodes, and so on.
We prescribed Pentasa to the patient - 3 g/day. As is known, this drug can be used once a day - this increases patient compliance. Naturally, it is easier for a person to take medications once than three times a day. And, accordingly, patients will be more willing to follow the doctor’s recommendations. According to the perfect ECCO recommendations, therapy with an oral drug - Pentasy tablets - had to be supplemented with a new form of Pentasy, which is now proposed, and which we already have at our disposal - suppositories, Pentasy suppositories, which contain 1 gram of mesalazine. Pentasy suppositories can be used once, and not twice a day, as was previously proposed for their use. And with the use of local and oral therapy, we quickly managed to achieve an improvement in the clinical picture.
But I have already said that our goal is to achieve not only clinical remission, but also endoscopic remission. To assess the dynamics of the condition in this regard, we use the Mayo clinical-endoscopic index, which combines both clinical indicators and endoscopic indicators. After treatment with Pentasa tablets and suppositories, the patient’s stool frequency normalized, rectal bleeding and hematochezia disappeared, and the endoscopic picture completely normalized.
What we have as a result: disease activity is 0 points, we have achieved clinical and endoscopic remission. Should we stop therapy? No. And according to our experience and the ECCO recommendations, the recommendations of the American Gastroenterological Association, maintenance therapy should be continued, but the dose of the drug can be reduced. We gradually reduced the Pentasa tablets from 3 grams of the drug per day to 1 gram. We suggested that the patient use Pentasy suppositories three times a week. What can be concluded? 5-aminosalicylic acid preparations remain the first line of treatment for ulcerative colitis. Fortunately, new and increasingly effective drugs containing mesalazine are becoming available to doctors.
(17:25) Screensaver.
Professor Shifrin O.S.: – Several questions were received: “What is a terrible side effect from the use of mesalazine?”
I must say that this is a fairly safe drug. If we compare it with drugs from other groups, this is perhaps the safest drug. It can give cephalalgia, it can give abdominalgia, it can give stool upset. Sometimes it causes such pancreatotoxic reactions, but this is not common. And I repeat, this drug is the safest among all other drugs used in the treatment of inflammatory bowel diseases.
Question: “Should I take probiotics when treating ulcerative colitis?”
This is a controversial issue that is being debated. The Escherichia coli 1917 strain is believed to have some effect and is even included in the recommendations. But nevertheless, the issue of using other strains of microorganisms is being discussed. In particular, with Crohn's disease - Saccharomyces boulardii and so on. This question requires further development, but is very interesting in itself.