Chronic pancreatitis (CP) is a common disease of the gastrointestinal tract. In Russia, the incidence of CP is 27-50 cases per 100 thousand population per year, and in 80% of cases the cause of its development is alcohol. Most often, CP affects men aged 25 to 50 years [1, 2]. In 20-40% of cases, chronic pancreatitis is complicated by pancreatic pseudocysts [3]. In 70-80% of cases, the cause of the development of pseudocysts is previous destructive pancreatitis [4]. In 15-40% of cases, pancreatic pseudocysts are complicated [3, 4]: 1) infection; 2) impaired patency of the extrahepatic bile ducts and duodenum against the background of their extraorgan compression; 3) breakthrough of contents into the free abdominal cavity; 4) thrombosis of the splenic or portal veins; 5) formation of a pseudoaneurysm of the splenic artery; 6) hemorrhage into the lumen of pseudocysts.
Currently, various types of surgical intervention are used to treat patients with pancreatic pseudocysts: laparotomic, laparoscopic (resection or drainage methods) and minimally invasive, in particular puncture-drainage method (PDM) with guidance from ultrasonography or computed tomography (CT) and endoscopic formation of cystogastro-/cystoduodenoanastomosis (CGA/CDA) under endosonography control [5, 6].
However, despite the variety of modern techniques, the type and extent of surgical intervention in the treatment of pancreatic pseudocysts complicated by infection and the presence of sequesters in the cyst cavity remains unresolved and controversial [7–9].
Etiology
PC can occur in 3 situations:
- PC can develop after an attack of acute pancreatitis in approximately 10% of cases [1,2]. Necrosis of peripancreatic tissues can reach the degree of liquefaction with subsequent organization and formation of a pseudocyst, which can communicate with the pancreatic duct. An alternative option is the occurrence of a pseudocyst as a result of massive necrosis of the parenchyma, which can lead to complete interruption of the pancreatic duct with massive leakage of pancreatic juice.
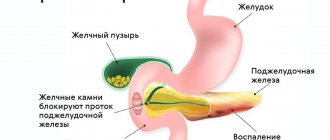
- Among patients with chronic pancreatitis, most often as a result of alcohol abuse, the formation of PC may be caused by exacerbation of pancreatitis or progression of pancreatic duct obstruction. Obstruction can develop either as a result of duct stricture or during the formation of an intraductal calculus from protein plugs. An increase in intraductal pressure can cause leakage of pancreatic juice with its accumulation in the prepancreatic tissues.
- Blunt or penetrating trauma can directly damage the pancreatic duct leading to the formation of pancreatic duct.
Clinic
Most PCs are asymptomatic, but they can have different clinical manifestations depending on their size and location.
- An enlarged pseudocyst can cause abdominal pain, obstruction of the duodenum, blood vessels or bile ducts. Fistulas with adjacent organs, the pleural cavity, or the pericardium may form.
- Spontaneous infection with abscess formation.
- Digestion of adjacent vessels can cause the formation of a pseudoaneurysm, which can cause a sharp increase in the size of the pancreas or bleeding from the gastrointestinal tract as a result of bleeding into the pancreatic duct [3].
- Pancreatic ascites and pleurisy can form when the pancreatic duct ruptures with the formation of a fistula with the abdominal or thoracic cavity or when the PC ruptures.
Alternative diagnoses
The first question is whether there is any possibility that the fluid collection is a cystic neoplasm or other “pseudo-pseudocyst”. Cystic neoplasm treated as PC can cause serious complications and can significantly complicate subsequent adequate surgical resection [5,6]. The following findings should raise concern that the encapsulated fluid collection is not PC:
- No history or symptoms of acute or chronic pancreatitis or trauma.
- No associated inflammatory changes on CT scan.
- The presence of internal partitions in the cyst cavity.
Although a high level of amylase in the PC contents as a result of its association with pancreatic flow usually indicates an inflammatory PC, a high level of doubt must remain because No single test can rule out cystic neoplasm. Many other non-malignant diseases can mimic PC, and as a result, extreme care is required to avoid diagnostic errors [2,8].
Stages of formation
Based on their location, false cysts are divided into formations of the head, tail or body. In addition, they can be postoperative, post-traumatic and pancreatic.
There are several stages of formation of neoplasms:
- Initial. It takes about one and a half months and is characterized by the formation of a cyst cavity.
- Second. The stage can last about three months. In this case, new loose tissue develops in the formed area.
- Third. Characterized by the completion of the formation of a fibrous capsule.
- The last one. The capsule becomes denser. The process of its formation is completed.
Pancreatic pseudocysts: what are they and how can they be located? False cysts can be single or present in large numbers in the organ area. A pathology is considered acute if it remains in the organ for three months, subacute - about six months and chronic - more than this period.
Possible presence of pseudoaneurysm
The next question is whether a pseudoaneurysm is present, a complication that occurs in approximately 10% of patients with PC [9–11]. Severe or even fatal bleeding occurs after endoscopic drainage unless the patient is suspected of having an existing pseudoaneurysm. Unless arterial embolization has been performed first, pseudoaneurysm is an absolute contraindication to endoscopic intervention. Three clinical signs may indicate the presence of a pseudoaneurysm:
- Unexplained gastrointestinal bleeding.
- Unexpected increase in PC size.
- Unexplained drop in hematocrit.
We believe that carefully performed bolus dynamic CT with early arterial phase imaging should be routine testing in all patients being considered candidates for endoscopic drainage to detect pseudoaneurysms. Doppler scanning of the abdomen can be useful but has lower sensitivity. Angiography is the definitive diagnostic test and is increasingly used to embolize pseudoaneurysms with radiocontrast coils or foam [12]. Among the first 57 patients referred to our institution for endoscopic treatment of pseudocysts, we were able to diagnose 5 pseudoaneurysms before drainage was performed. These patients were treated with a multidisciplinary approach, including embolization or resection [12]. More recently, we cautiously performed endoscopic drainage after precise angiographic embolization in patients who were not good candidates for surgical resection [13].
Diagnostics
- In order to obtain an accurate clinical picture, the doctor will usually conduct a thorough interview with the patient. The history of his illness can also provide important information. To complete the diagnosis, the following procedures are performed:
- X-ray;
- ultrasound examination of the organ (ultrasound);
- tomography using computer technology;
- endoscopic studies.
After the necessary data has been collected, the doctor can determine the type and stage of the tumor in order to prescribe adequate treatment.
The role of conservative treatment
Traditional training in surgery is based on the classic study that PCs present for more than 6 weeks rarely resolve and have complications in 50% of cases during follow-up [14]. After week 13, no further resolution was observed and the complication rate increased sharply. Surgery was recommended after a 6-week observation period to ensure that spontaneous resolution had not occurred and to allow time for the PC walls to mature enough to allow a direct cystoenterostomy to be performed by suturing. This approach is widely accepted among surgeons and is often cited [15–18]. Two other reviews, however, recommend a more conservative watchful waiting approach in the patient with established absence of cystic neoplasm, pseudoaniurysm, or more than minimal symptoms. A retrospective review of 68 patients with PC treated conservatively found that serious complications occurred in 9% of cases, most of which occurred within the first 8 weeks after diagnosis [19]. Complications included the formation of pseudoaneuryses in 3 patients, perforation into the free abdominal cavity in 2 patients, and spontaneous abscess formation in 1 patient. In addition, 1/3 of patients underwent elective surgery due to pain associated with cyst enlargement. However, 43 patients (63%) experienced either spontaneous resolution or no symptoms or complications at a mean follow-up of 51 months. Similar observations were noted in another study of 75 patients [20]. Surgery was performed only for severe abdominal pain, complications, or progressive enlargement of the cyst. 52% of patients underwent surgery for the above indications; the remaining patients were managed conservatively. Among the patients in the latter group, 60% experienced complete resolution of the cyst within up to 1 year, and only one experienced complications associated with PC. Other patients in this group had no symptoms, and the PC either persisted or gradually decreased in size. It is impossible to predict on the basis of etiology or CT which patients will experience complete resolution of PC, but in general PCs in patients in the conservative treatment group were smaller in size than in patients requiring surgical treatment. A detailed description of the anatomy of the pancreatic duct, which could help predict the development of the disease, was not provided in any of these studies [21].
Literature
- Cameron, JL. Acute pancreatitis. In: Surgery of the Alimentary Tract 2nd edition, Shackelford, RT, Zuideme, GD (Eds), WB Saunders, Philadelphia 1983. p.31.
- O'Malley, V.P., Cannon, J.P., Postie, R.G. Pancreatic pseudocysts: Cause, therapy, and results. Am J Surg 1985; 150:680.
- Risti, B, Marincek, B, Jost, R, et al. Hemosuccus pancreaticus as a source of obscure upper gastrointestinal bleeding: Three cases and literature review. Am J Gastroenterol 1995; 90:1878.
- Runyon, B.A. Amylase levels in ascitic fluid. J Clin Gastroenterol 1987; 9:172.
- Sperti, C, Cappellazzo, F, Pasquali, C, et al. Cystic neoplasms of the pancreas: Problems in differential diagnosis. Am Surg 1993; 59:740.
- Warshaw, A.L., Rutledge, P.L. Cystic tumors mistaken for pancreatic pseudocysts. Ann Surg 1987; 205:393.
- Lewanodtrowski, KB, Southern, JF, Pines, MR, et al. Cystic fluid analysis in the differential diagnosis of pancreatic cysts. Ann Surg 1993; 217:41.
- Levin, M.F., Vellet, A.D., Bach, D.B., et al. Peripancreatic fluid collections: Vascular structures masquerading as pseudocysts. Can Assoc Radiol J 1992; 43:267.
- el Hamel, A, Parc, R, Adda, G, et al. Pseudoaneurysms in chronic pancreatitis. Br J Surg 1991; 78:1059.
- Kiviluoto, T, Schroder, T, Kivilaakso, E, et al. Acute hemorrhage associated with pancreatic pseudocyst and chronic pseudocyst and chronic pancreatitis. Ann Chir Gynaecol 1984; 73:214.
- Pitkaranta, P, Haapiainen, R, Kivisaari, L, et al. Diagnostic evaluation and aggressive surgical approach in bleeding pseudoaneurysms associated with pancreatic pseudocysts. Scand J Gastroenterol 1991; 26:58.
- Marshall, G.T., Douglas, D.A., Hanson, B.L., et al. Multidisciplinary approach to pseudoaneurysms complicating pancreatic pseudocysts. Arch Surg 1996; 131:278.
- Elton, E, Howell, DA, Amberson, SM, Dykes, TA. Combined angiographic and endoscopic management of bleeding pancreatic pseudoaneurysms. Gastrointest Endosc 1997; 46:544.
- Bradley, E.L. III, Clements, J.L. Jr., Gonzales, A.C. The natural history of pancreatic pseudocysts: A unified concept of management. Am J Surg 1979; 137:135.
- Martin, EW Jr, Catalano, P, Cooperman, M, et al. Surgical decision-making in the treatment of pancreatic pseudocysts. Am J Surg 1979; 138:821.
- Sankaran, S, Walt, AJ. The natural and unnatural history of pancreatic pseudocysts. Br J Surg 1975; 62:37.
- Shatney, CH, Lillehei, RC. The timing of surgical treatment of pancreatic pseudocysts. Surg Gynecol Obstet 1981; 152:809.
- Wilheim, K. Pancreatitis: Pancreatic pseudocysts and their complications surgical management. Gastroenterology 1977; 73:601.
- Vitas, G. J., Sarr, M. G. Selected management of pancreatic pseudocysts: Operative versus expectant management. Surgery 1992; 111:123.
- Yeo, C. J., Bastidas, J. A., Lynch-Nyhan, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet 1990; 170:411.
- Howell, DA, Lehman, GA, Baron, TH, et al. Pancreatic ductal anatomy in patients undergoing endoscopic pseudocyst drainage: Implications of follow-up data (abstract). Gastrointest Endosc 1996; 43:467.
- D'Edogo, A, Schein, M. Pancreatic pseudocysts: A proposed classification and its management implications. Br J Surg 1991; 78:981.
- Grimm, H, Binmoeller, KF, Soehendra, N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc 1992; 38:170.
- Kohler, H, Schafmeyer, A, Ludtke, FE, et al. Surgical treatment of pancreatic pseudocysts. Br J Surg 1987; 74:813.
- Lohr-Happe, A, Peiper, M, Lankisch, PG. Natural course of operated pseudocysts in chronic pancreatitis. Gut 1994; 35:1479.
- Segal, I, Epstein, B, Lawson, HH, et al. The syndromes of pancreatic pseudocysts and fluid collections. Gastrointest Radiol 1984; 9:115.
- Frey, C. F. Pancreatic pseudocyst—operative strategy. Ann Surg 1978; 188:652.
- Adams, D.B., Anderson, M.C. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocysts. Ann Surg 1992; 215:571.
- Szentes, MJ, Traverso, LW, Kozarek, RA, et al. Invasive treatment of pancreatic fluid collections with surgical and non-surgical methods. Am J Surg 1991; 161:600.
- van Sonnenberg, E, Wittich, GR, Casola, G. Complicated pancreatic inflammatory disease: Diagnostic and therapeutic role of interventional radiology. Radiology 1985; 155:355.
- Fritscher-Ravens, A, Izbicki, JR, Sriram, PV, et al. Endosonography-guided, fine-needle aspiration cytology extending the indication for organ-preserving pancreatic surgery. Am J Gastroenterol 2000; 95:2255.
- Chak, A. Endosonographic-guided therapy of pancreatic pseudocysts. Gastrointest Endosc 2000; 52:S23.
- Hariri, M, Slivka, A. Carr-Locke, DL, et al. Pseudocyst drainage predisposes to infection when pancreatic necrosis is unrecognized. Am J Gastroenterol 1994; 89:1781.
- Baron, T.H., Thaggard, W.G., Morgan, D.E., et al. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755.
- Venu, R.P., Brown, R.D., Marrero, J.A., et al. Endoscopic transpapillary drainage of pancreatic abscess: Technique and results. Gastrointest Endosc 2000; 51:391.
| back |
Drainage options
In the past, when drainage became necessary due to complications or intractable symptoms associated with PC, surgical drainage remained the only treatment option. Currently, there are two more treatment options that have achieved increasing popularity: percutaneous and endoscopic drainage. The remaining controversy is which of these methods should be offered to the patient as the initial type of therapy. Currently, there are no randomized comparative studies of these two methods and doctors use the one they are better at. The disadvantage of percutaneous drainage is the long stay of the catheter and the possible formation of an external fistula.
Internal surgical drainage.
Most surgeons, if possible, use the internal drainage technique, the technique of which depends on the location of the pseudocyst:
- Cysto-gastro or duodenostomy when a cyst is fused with the stomach or duodenum.
- Cystojejunostomy can be used for other anatomical variants.
- The PCs of the tail of the pancreas can be removed during pancreatic resection; in these conditions, papilosphincterotomy is often necessary.
The reported complication rate of internal drainage is approximately 15% with a mortality rate of less than 5%. The postoperative recurrence rate is about 10% [22–26]. When there is obstruction of the main pancreatic duct below the level of the anastomosis, some surgeons prefer PC resection rather than internal drainage in an attempt to minimize recurrence rates.
External surgical drainage
may be necessary if it is impossible to create an internal anastomosis. External pancreatic fistulas are a common outcome of this approach [27].
Percutaneous drainage with a catheter.
Percutaneous catheter drainage is as effective as surgical drainage in draining and closing both sterile and infected cysts [28–30].
It is necessary to maintain patency of the catheter by careful irrigation. The catheter is left in place until the discharge level drops to 5-10 ml. in a day. In one study of 52 patients, the average drainage time was 42 days [28]. If such a decrease in the level of discharge does not occur, then the administration of octreotide (50-200 mg subcutaneously, every 8 hours) may be useful. A control CT scan should be performed when the amount of discharge decreases to ensure that the catheter has not dislodged from the PC cavity. The main complication of this procedure is catheter infection, which occurred in half of the patients in one study [28]. It is unknown whether obstruction of the main pancreatic duct should deter percutaneous drainage. Endoscopic approach.
Multiple reports confirm the high success rate of endoscopic cysto-gastro (ECG) and cysto-duodenostomy (ECD). ECD is the procedure of choice due to its higher safety, easier achievement of a perpendicular approach to the cyst during drainage, and greater involvement of the duodenum than the stomach in most cases of PC. The resolution level of PC during endoscopic treatment varies from 65 to 89%. The main complications of endoscopic drainage are bleeding (which, due to its severity, requires surgical treatment in up to 5% of cases), retroperitoneal perforation, infection and failure to achieve resolution of PC. There is virtually no mortality associated with this procedure, with a recurrence rate of 6-18%. The incidence of perforation or bleeding can be minimized by detecting the PC prior to endoscopic puncture. We prefer detection of PC by endoscopic puncture, although the increasing popularity of endoscopic ultrasound may make this technique a viable alternative.
The role of endoscopic ultrasound
The popularity of endoscopic ultrasound in the diagnosis of pancreatic pseudocysts is currently growing due to the fact that this technique allows one to recognize the complex structure of the walls and contents of the pancreas. In combination with aspiration biopsy, it can help in the differential diagnosis of PC and cystic neoplasm [31]. The presence of well-differentiated septa, echogenic mucin, and masses indicates a cystic neoplasm requiring resection rather than drainage. As mentioned above, endoscopic ultrasound can help in choosing the site of pseudocyst puncture - to exclude the presence of large veins or arteries in the drainage area [32]. Thus, it is theoretically possible that this technique may have an advantage by reducing the risk of bleeding and perforation, although this has not been demonstrated in controlled studies.
Symptoms of the disease
The formations themselves are most often found during ultrasound examination. However, their occurrence is usually accompanied by certain symptoms. The following signs of pseudocyst development are characteristic:
- pain in the abdominal area (occurs in more than 80% of cases), usually aching and localized near the hypochondrium;
- nausea and vomiting;
- jaundice (due to compression of the bile ducts);
- weight loss (rare);
- increase in body temperature (if false cysts become infected).
Patients often experience worsening sleep and appetite, high levels of fatigue, drowsiness, and weakness. In advanced stages, bleeding, rupture of cysts, and heart rhythm disturbances are possible.
Presence of pancreatic necrosis
We believe that the most important question on which the decision to use endoscopic, surgical or radiological drainage depends is whether there are signs of underlying pancreatic necrosis associated with PC, as determined on CT with additional contrast. The presence of dense inclusions, dendrite, and the presence of non-vascularized areas of the pancreas parenchyma may indicate that a significant amount of dead tissue may be present. The decision to use a transmural approach depends on how organized the necrosis appears. Infectious complications are common when endoscopic and radiological drainage is used in these settings [33]. Although most complications arising from endoscopic drainage can be treated endoscopically by an experienced specialist, failure to recognize necrosis, resulting in inadequate drainage/washing of the necrotic lesion, can lead to serious infectious complications, including death. Thus, the presence of pancreatic necrosis should be a significant reason to doubt the performance of endoscopic drainage, although it does not exclude its attempts. Surgical drainage allows probing of the PC to extract the necrotic dendrite and achieve complete evacuation of the contents before anastomosis. The endoscopic approach with transmural puncture allows for nasogastric lavage, orifice dilatation with the insertion of multiple stents, and may be an alternative to surgery in carefully selected patients in specialized centers. The problems that can arise are illustrated in a report of 11 patients who underwent endoscopic drainage for this type of cyst (defined as “organized pancreatic necrosis”) [34]. Using aggressive endoscopic techniques, success was achieved in 9 patients. Multiple procedures were required with a complication rate of 50%, although most were treated endoscopically.
Definition of pathology
A pseudocyst is a paralogous area of the pancreas. Organs acquired during the development of diseases are called false cysts. They differ from ordinary ones in the absence of internal epithelium.
The formation of such an education can be considered a serious and dangerous process, since it is often accompanied by the following:
- suppuration;
- malingization;
- the emergence of new formations that require surgical intervention;
- perforation.
Treatment of false cysts is usually prescribed taking into account the individual characteristics of the patient.
Presence of pancreatic abscess
A localized collection of pus within or adjacent to the pancreas has traditionally been described as an infected pseudocyst, a condition requiring prompt incision and drainage. Recently, endoscopic drainage has been used in a group of patients at high surgical risk due to the presence of systemic complications of pancreatitis [35]. Important factors include adequate drainage, the need to relieve outflow obstruction, and diligent patient education and monitoring. We prefer the transmural approach to abscess drainage because... it allows for further drainage of the cystenterostomy channel, insertion of a nasogastric irrigation catheter and multiple stents to prevent complications associated with insufficient catheter function and residual contents.
Recommended approach
Currently, we recommend active tactics in patients with pancreatitis arising as a complication of chronic or acute pancreatitis with the presence of symptoms and duration of pancreatitis of at least 4 weeks. We perform ERCP when we believe that the patient is a candidate for attempting endoscopic drainage. During endoscopy, it is necessary to exclude portal hypertension and obstructive gastric emptying disorders. RCCP is performed to detect signs of compression of the biliary tree, especially if liver parameters are elevated. Pancreatography is necessary in all patients to identify underlying pancreatic duct obstruction. Unexpected pancreatic duct strictures and calculi are often detected, and a stricture caused by a malignant tumor may even occur. Because Endoscopic drainage can be performed either by transmural puncture or transpapillary stent placement; a pancreatogram is very important for choosing between these two options. Endoscopic ultrasound may be useful in the differential diagnosis of cystic lesions of the pancreas and drainage of the pancreas, although it is not routinely used. Patients with large, persistent or enlarging pancreatic ducts often exhibit severe pancreatic duct damage, which determines the need and type of treatment used [10]. In our experience, pancreatic duct obstructions and complete strictures are common in this group of patients and do not resolve after resolution of the pseudocysts. In contrast, leaks from peripheral branches close after endoscopic treatment leading to resolution of the cyst.
We recommend:
- The role of intraluminal endoscopic interventions in the complex treatment of chronic pancreatitis and its complications
- Diagnostic RCP. Execution technique
- Diagnosis and treatment of pancreatic pseudocysts
- Endoscopy for primary sclerosing cholangitis: clinical recommendations of the European Society
- Recommendations for surgical treatment of chronic pancreatitis 2016
- Stenting and ERCP under ultrasound
- Draft national clinical guidelines “Obstructive jaundice”
Articles on the topic - see the selection of articles at the bottom of the page
Prevention, prognosis, possible complications
People suffering from pancreatic diseases especially need preventive measures against pseudocysts. One of the most important of them is the correct and timely treatment of diseases. In addition, both such patients and healthy people must comply with the following rules:
- to refuse from bad habits;
- undergo medical examinations in a timely manner;
- establish a healthy lifestyle and a balanced diet;
- avoid traumatic situations;
- provide the body with proper rest.
With proper treatment, complete relief from the pathology is possible. However, in 30% of cases, according to statistics, relapses are possible. In addition, if surgery is required, the risk of death is almost 50%.
Thus, escalating the problem to severe forms is very harmful and extremely dangerous. A complete lack of treatment threatens the appearance of suppuration and abscesses, the transition of pseudocysts to a malignant formation, the development of pleurisy and ascites. If the false cyst bursts, it can even lead to coma and even death.