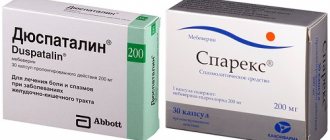
Duspatalin relieves pain caused by spasms of the intestinal muscles. Helps speed up the treatment of irritable bowel syndrome, and also significantly reduces the risk of relapse. Having a pronounced antispasmodic effect, Duspatalin does not cause hypotension of the smooth muscles of the gastrointestinal tract.
Duspatalin: what kind of medicine is it?
Duspatalin is an antispasmodic agent, the effect of which is mainly directed on the smooth muscles of the intestine. At the same time, there will be no effect on the normal functioning of the digestive organ, so the drug does not cause relaxation of the gastrointestinal tract tissues. In addition, Duspatalin has an anesthetic effect on the gastrointestinal tract.
The drug is taken for pain associated with intestinal dysfunction. Duspatalin acts symptomatically. That is, it does not treat the disease that caused the intestinal spasm, but only relieves the symptom - relaxes the smooth muscles where it is necessary, without affecting healthy areas of the intestine. This pain is especially typical during sudden attacks of diarrhea, when intestinal spasms and cramps interfere with a normal lifestyle. At the same time, Duspatalin can stop frequent and loose stools, reducing chaotic intestinal activity, which accelerates the process of movement of feces along its entire length.
What is the difference between Duspatalin and No-shpa
The main differences between Duspatalin and No-shpa are:
- The mechanism of action of the drugs differs in that the active substance Duspatalin blocks channels from the penetration of sodium ions into cells, and drotaverine No-shpa changes the content of cyclic nucleotides that regulate the concentration of calcium inside cells.
- Duspatalin acts primarily on the intestinal muscles, relaxes the digestive tract, and No-shpa affects all smooth muscles.
- Duspatalin lasts longer.
- No-shpu is not prescribed to children under 6 years of age, Duspatalin is not prescribed to children under 16 years of age.
- Taking No-shpa does not affect the reaction speed when driving a car. During treatment with Duspatalin, especially at the initial stage, it is better to refrain from driving vehicles and work that requires concentration.
- Duspatalin tablets are 3-3.2 times more expensive than No-shpa tablets.
The norm for Duspatalin for adults is 3 tablets per day: one 30 minutes before meals. Taking No-shpa tablets does not depend on food intake. The maximum daily dose for adults is 240 mg (6 tablets), for children 6-12 years old - 80 mg, over 12 - 160 mg. The dose is divided into 3 doses.
The norm for Duspatalin for adults is 3 tablets per day.
Is it possible to take Duspatalin for pancreatitis?
Pancreatitis is a disease of the pancreas in which the process of releasing enzymes into the duodenum, intended for the breakdown of fats and carbohydrates, is disrupted. Without coming into contact with food, and remaining in the body or ducts of the pancreas, enzymes begin to destroy the organ that produces them. Which leads to severe pain, in addition to the dangerous consequences of the release of decay products through the bloodstream into other organs.
The cause of impaired enzyme release may be dysfunction of the sphincter responsible for their release into the duodenum. Duspatalin, by relaxing the muscles of the tight sphincter, can facilitate the process of emptying the pancreas, which will improve the functioning of the gastrointestinal tract. In addition, the drug has an analgesic effect, which is very helpful for acute pain in the left hypochondrium.
Material and methods
This work was planned as a prospective open non-comparative study and was carried out at the clinical sites of the Department of Propaedeutics of Internal Medicine and Gastroenterology of the Moscow State Medical University. The study included persons of both sexes aged from 18 to 70 years. At the first stage, patients were selected with complaints of episodes of recurrent spastic pain in the right hypochondrium and/or epigastric region. All patients underwent standard laboratory tests (general blood and urine tests, biochemical blood test), ultrasound of the abdominal organs and esophagogastroduodenoscopy.
The study did not include patients with organic pathology of the gastroduodenal region (cholelithiasis, chronic cholecystitis, chronic pancreatitis, gastric and duodenal ulcers, etc.). Patients who underwent cholecystectomy in the absence of signs of choledocholithiasis were not excluded from the protocol. Other functional gastrointestinal pathologies (functional dyspepsia and irritable bowel syndrome) were excluded based on a detailed analysis of the clinical picture, localization of pain, and its relationship with food intake and defecation. The protocol did not include patients with signs of functional biliary disorder CO types I and II (increased levels of alanine and aspartate aminotransferase, bilirubin or alkaline phosphatase more than 2 norms and an expansion of the diameter of the common bile duct more than 8 mm), since in such cases the high the likelihood of its structural changes. Other criteria for non-inclusion were known hypersensitivity to mebeverine, recent use of any antispasmodic drugs and analgesics, severe concomitant pathology (cardiovascular, pulmonary, renal or hepatic failure, vascular pathology of the brain, blood diseases, decompensated diabetes mellitus, cancer, etc.) , alcoholism, drug addiction, substance abuse, mental illness, taking antidepressants, pregnancy and lactation, lack of informed consent.
As a result, at the second stage, 69 patients (49 women and 20 men) with a diagnosis of “functional disorder of the biliary tract” were included in the study, divided into two groups. The 1st group included 31 patients with preserved GB (22 women and 9 men, average age - 33.5 years), and the 2nd group included 38 patients who had previously undergone cholecystectomy (28 women and 10 men, average age - 52 ,4 years).
To clarify the nature of the violation of the motor-tonic function of the biliary tract, patients in both groups underwent dynamic hepatobilis scintigraphy. The study was carried out using the Siemens Orbiter gamma camera. Bromezide 99 mTc was used as a radiopharmaceutical. The examination was performed on an empty stomach. The radiopharmaceutical solution was administered intravenously at the rate of 1.7 mBq per 1 kg of patient body weight. Recording was carried out in 1 frame per minute mode for 1.5 hours. At the 30th minute, to stimulate bile secretion and reduce the gallbladder, patients were given a choleretic breakfast (2 raw egg yolks).
Based on the results of the study, the following main functional parameters were assessed: the duration of the latent time (from the introduction of a choleretic breakfast to the beginning of the contraction of the gallbladder), the time of maximum accumulation and half-life of the radiopharmaceutical from the gallbladder, the time of maximum accumulation and half-life of the radiopharmaceutical from the common bile duct, the time and volume of entry of the radiopharmaceutical into the duodenum [7 ].
The study protocol included two visits to the doctor. During the first visit, which was carried out after hepatobiliscintigraphy, all patients were prescribed mebeverine at a dose of 200 mg 2 times a day 20 minutes before meals. The duration of therapy was 28 days. During the second visit (on the 29th–30th day from the start of therapy), a clinical assessment of the treatment results was carried out.
The effectiveness and safety of therapy were assessed using questionnaires filled out by patients during the first and second visits. The examined patients recorded the presence or absence of recurrent pain during the week before the first and second visits. Pain intensity at baseline and at the end of the study was determined using a 100-mm visual analogue scale (VAS), with 0 being designated as “no pain” and 100 as “the worst pain imaginable.” In addition, patients noted the overall effectiveness of treatment as “very good” or “good”, “satisfactory”, “unsatisfactory”. Patients of group 1, after completing the course of drug treatment, to assess the motor function of the gallbladder, underwent dynamic transabdominal ultrasound before and after taking cholekinetics according to the standard method.
Statistical analysis was carried out using the Biostat program; χ-square and Student's t-tests were used.
Is it possible to take Duspatalin for gastritis?
Gastritis is an inflammation of the gastric mucosa, which can be caused by various reasons. If the disease is of bacterial or fungal etiology, therapy is prescribed with narrowly targeted antibiotics or antifungal agents that affect the type of pathogenic microorganisms. Duspatalin can be used as an antispasmodic if the disease is accompanied by pain that occurs due to increased tone of the stomach muscles. Taking Duspatalin will not affect the treatment of diagnosed gastritis.
Which is better: Duspatalin or Trimedat
The active ingredient of Trimedat is trimebutine, one of the auxiliary components of which is lactose monohydrate. In this regard, the drug Trimedat becomes dangerous for patients with lactase deficiency.
Duspatalin does not differ from Trimedat in its mechanism of action. If the patient has acute intolerance to the components of one of the drugs, it can be replaced with another. The difference in composition can become a selection criterion for allergy sufferers, due to the fact that the compositions of the drugs are different, but the mechanism of action is the same.
results
According to dynamic hepatobiliscintigraphy, in group 1, 71.0% of patients had SO spasm, and 29.0% had a hyperkinetic functional disorder of the gastrointestinal tract. In 58% of patients, hypomotor dyskinesia of the gallbladder was recorded, which was always combined with SO spasm. In group 2, SO spasm was present in 89.5% of cases. In 10.5% of patients, no motor function disorders were detected, however, taking into account the presence of a typical clinical picture of biliary pain, these patients were not excluded from the protocol.
Initially, as noted earlier, 100% of patients in both groups had periodic (at least once a week) recurrent spasmodic pain in the right hypochondrium and/or epigastric region. Analysis of the dynamics of pain syndrome showed that after a 4-week course of monotherapy with mebeverine in group 1, pain continued to bother only 48.4% of patients (p < 0.0001; Fig. 1). At the same time, in 25.8% of the examined patients there was a noticeable decrease in the frequency and intensity of abdominal pain syndrome. Only in 22.6% of cases was it not possible to achieve any significant positive dynamics in the clinical picture. A significant positive effect was also noted when assessing pain intensity using VAS. The initial pain intensity in group 1 was 4.4 ± 1.1 cm (hereinafter the standard deviation is indicated), while after 4 weeks this indicator decreased to 1.5 ± 1.9 cm (p < 0.0001 , 95% confidence interval – 2.1–3.6; Fig. 2). The general self-assessment of therapy by patients in this group showed that 71.0% of them considered its effectiveness to be very good or good (Fig. 3). According to dynamic ultrasound, after treatment, hypokinetic type disorders of the motor function of the gallbladder continued to be detected in only 13% of the examined patients.
In the 2nd group of patients, significant positive dynamics in pain syndrome were also recorded, but this was observed in a smaller number of patients. By the end of treatment, 52.3% of patients complained of pain (p < 0.0001; Fig. 1), and in 26.3% of cases a decrease in the intensity and frequency of pain episodes was noted. In 29.0% of patients, no significant changes in the clinical symptoms of functional disorders of the gastrointestinal tract were detected. As in group 1, in group 2, significant positive dynamics were also achieved in terms of pain intensity. Before the start of therapy in group 2, it was 5.1 ± 1.3 cm, while by the end of the 4th week of taking mebeverine it was already 1.8 ± 2.1 cm (p < 0.0001, 95% confidence interval – 2.5–4.1; Fig. 2). In the second group, 60.5% of patients noted the effectiveness of treatment as “very good” or “good” (Fig. 3).
Mebeverine was well tolerated. No treatment-related side effects were reported in any case.
Which is better: Duspatalin or Drotaverine
Drotaverine, like Duspatalin, is an antispasmodic that affects smooth muscles. On the shelves, Drotaverine is often hidden under the trade name No-shpa. This drug is prescribed for certain types of pain caused by cholecystitis, cholangitis, pyelitis and cystitis. But, unlike Duspatalin, Drotaverine dilates blood vessels. Its spectrum of action is not limited to the smooth muscles of the gastrointestinal tract.
No-spa is prescribed to pregnant women with a threat of premature birth, if there is increased uterine tone. In addition, I use the drug during labor if delivery is prevented by spasm of the uterine pharynx.
A contraindication for taking Drotaverine is lactase deficiency, so in such patients it is better to relieve spasms of the intestines and other gastrointestinal organs with products that do not contain lactose among the auxiliary elements.
Contraindications for Duspatalin include, in addition to childhood and intolerance to the components, pregnancy. The same cannot be said about Drotaverine: it is prescribed to pregnant women, however, despite the stated safety, expectant mothers must coordinate the use of any medications with their local obstetrician-gynecologist.
Reviews from doctors
Chistyakov A.S., gastroenterologist, 7 years of experience, Moscow
I prescribe Dustapalin for irritable bowel syndrome, Crohn's disease, colitis and pain of other origins in the abdominal cavity. Patients tolerate it well, there were no complaints. The drug will not cure the disease, especially a chronic one, but it will prevent it from developing and relieve pain.
Novikova L.T., pediatrician, 10 years of experience, Tyumen
I prescribe No-shpa for colic, spastic constipation and as an adjuvant to reduce high fever. The drug is effective and safe even for children. But parents should remember not to overdose.
[morkovin_vg video=”mc3W-5AMuYY;GRwG85lg7A4"]