Potent antibacterial drugs help fight serious diseases and advanced infections. Unfortunately, taking such medications often has a negative effect on all body systems. Antibiotics have many side effects, including:
- digestive disorders - constipation or diarrhea,
- bitterness or other unpleasant taste in the mouth,
- bloating and increased gas formation,
- nausea, abdominal pain, etc.
Negative reactions from the gastrointestinal tract are caused by the death of beneficial bacteria in the intestines. Strong antibiotics destroy not only infectious agents, but also beneficial microflora, so dysbiosis often develops after treatment. How to remove bitterness in the mouth after antibiotics and restore intestinal microflora? Find out what experts answer to these questions.
Content:
- Corona is always a risk
- Why does the liver suffer?
- Impact of COVID-19 on the liver
- Effect of Covid-19 therapy on the liver
- Hepatoprotective therapy
- Liver recovery after coronavirus infection
Many patients who have suffered from Covid-19 ask the doctor how to restore the liver after the disease. Liver pathologies indeed often develop due to a progressive viral infection. Their symptoms cannot be ignored, since it is not known whether the organ will be able to organize its work on its own.
It is much wiser to resort to methods that promote the renewal of liver cells and eliminate the local inflammatory process. In this article we will tell you how COVID-19 affects the body’s main “filter” and how to treat the liver after coronavirus.
Treatment of bitterness with folk remedies
Herbal medicine is appropriate in complex treatment under the supervision of a specialist. Chamomile infusion, flax seed jelly, and corn silk decoction will help get rid of such an unpleasant symptom. Therapy with freshly squeezed juices has proven itself well. For this purpose, potato is used, which activates the intestines and eliminates heartburn, carrot, beetroot, which is effective for diseases of the biliary tract, and cucumber. Juices have a general strengthening effect, help cleanse the organs of toxins and normalize digestion processes.
Corona is always a risk
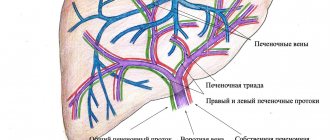
The liver is a vital organ. It performs a huge number of functions, takes part in immune processes, determines the body’s ability to resist viral agents and pathogenic flora. The smooth functioning of the liver structures determines whether antibodies that destroy Covid-19 will be produced and how far the inflammatory reactions will go.
Liver pathologies are rarely discussed after Corona, but this does not mean that they do not exist. The organ is sensitive to any disease. It filters toxins released during the life of pathogens. With severe intoxication, the load on it increases significantly.
You should think about liver restoration after Covid if:
- pain appeared in the right hypochondrium;
- Nausea occurs after eating fatty foods;
- there is an unpleasant bitter taste in the mouth;
- Laboratory tests show there is a problem.
The situation is especially bad for the patient if his skin has acquired a jaundiced tint and the whites of his eyes have become yellow.
Symptoms and diagnosis
The appearance of a bitter taste in the mouth when taking antibiotics may be accompanied by: bloating, heaviness in the stomach, nausea, belching and heartburn, putrid breath. In addition, patients experience pain in the right hypochondrium (in particular, during intense physical activity). If your mouth tastes bitter in the morning, the culprit may be overeating or the release of stomach acid into the esophagus. Discomfort after eating indicates a violation of bile outflow (for example, with cholelithiasis or dyskinesia).
Important! If bitterness in the mouth is a phenomenon that constantly bothers the patient, this is a reason to immediately seek help from a doctor. This phenomenon indicates serious problems with the liver or biliary tract.
Why does the liver suffer?
Approximately every second person undergoing treatment for COVID-19 in a hospital has elevated levels of liver enzymes. Doctors often identify both laboratory and clinical signs of dysfunction of the hepatobiliary system. "Crown" is capable of striking through three main mechanisms:
- Negative impact of the virus. The bile ducts and liver contain special type 2 angiotensin-converting enzymes. They are the main target of COVID-19.
- Excessive immune response. Sometimes a person’s immune system reacts too violently to a viral invasion. It happens that his response turns out to be more dangerous than the symptoms provoked by the disease itself. With an immunological failure, many internal organs begin to function incorrectly. Complications do not bypass the liver.
- Use of hepatotoxic drugs. In case of severe corona, the patient is prescribed antibiotics, antiviral, anti-inflammatory and drugs of other groups. Many of them turn out to be toxic to the organ.
Impact of COVID-19 on the liver
Describing the damage to the organ by the virus, doctors say that the bitter taste is a symptom of late damage to the parenchyma. The first dangerous sign is severe shortness of breath, which occurs even at rest. The second is an increase in body weight, despite maintaining the usual diet. Later appear:
- swelling of the legs;
- drying of the mucous membranes of the mouth;
- bitterness in the mouth;
- yellowing of the eye sclera.
All these symptoms indicate that there is a threat to the liver. When they occur, the patient must be examined to determine what exactly is happening to the organ and how the ailments can be eliminated.
It has been noticed that in Covid patients with chronic liver pathologies, the virus is more complicated. Infection provokes relapses of a chronic disease, making it more difficult for the body to overcome emerging difficulties.
“Liverworts” with a positive test for Covid-19 are more likely than other patients to encounter:
- arterial hypertension;
- atherosclerosis;
- diabetes mellitus;
- coronary heart disease;
- elevated cholesterol levels.
With the development of the listed diseases during COVID-19, the patient becomes more vulnerable and reacts extremely negatively to the viral attack and cannot cope with it. In no case should such patients self-medicate, as this will most likely lead to serious complications and will help all latent pathologies to manifest themselves.
Diagnostics
The most common causes of a bitter taste in the mouth are gastrointestinal diseases, so the patient should consult a gastroenterologist. First, complaints and medical history are collected, and the connection between bitterness and eating habits or time of day is clarified. Next, special laboratory and instrumental studies are used, the most informative of which are:
- Sonography
. The ultrasound method is indicated for studying the state of the digestive tract, identifying inflammatory and destructive changes, and neoplasms. A targeted ultrasound of the liver and gallbladder is performed. To detail the condition of the liver parenchyma, elastometry is used - a modern non-invasive method for determining the degree of fibrosis. - Duodenal sounding
. To prove the connection of the bitter taste with biliary diseases, 5 portions of bile are taken sequentially. The specialist evaluates the amount and rate of bile entering the intestines naturally and with pharmacological stimulation. Next, a bacteriological examination is performed. - Endoscopic examination
. To diagnose diseases of the esophagus and gastroduodenal zone, endoscopy is prescribed. During endoscopy, attention is paid to the integrity of the mucous membrane, the presence of areas of inflammation or atrophy. The condition of the major duodenal papilla and the initial parts of the duodenum is checked and a biopsy is performed. - Stool analysis
. Many diseases that are characterized by a bitter taste in the mouth cause specific changes in the stool. In case of violations of the bile secretion function, a large number of fatty inclusions are found in the stool; in case of damage to the pancreas, the stool contains undigested fibers and large carbohydrate molecules. - Laboratory diagnostics
. Women must be tested for hCG and sex hormones to exclude or confirm pregnancy. In a biochemical blood test for cholecystitis, the levels of bilirubin and the enzyme alkaline phosphatase are increased. If viral causes of hepatitis are suspected, serological testing of markers is required. - Additional methods
. An examination by a dentist is necessary to detect carious cavities, chronic periodontitis and other pathologies that cause a feeling of bitterness in the mouth. To diagnose lesions of the biliary system, cholangiopancreatography is performed. Patients with a complicated medical history should be examined by a neurologist.
Effect of Covid-19 therapy on the liver
The use of any drug does not go unnoticed. In addition to the toxic waste products of the virus, the organ is subjected to serious stress during treatment. Almost all medications prescribed to the patient exhibit pronounced hepatotoxic properties. Antibiotics that are prescribed to people with pneumonia are especially harmful in this regard.
They have a destructive effect on the functions of the gallbladder and cause serious damage to the health of the hepatobiliary system. If antibiotic therapy is prolonged, drug-induced hepatitis may develop, a disease in which serious inflammation is diagnosed. The filtration capacity of the liver deteriorates, and various digestive disorders occur.
Drug-induced hepatitis after coronavirus is a consequence of the fact that antibacterial components destroy hepatocytes. Then the liver tissue becomes inflamed, necrosis occurs, leading to severe liver failure or even cirrhosis. The condition is characterized by:
- disturbance of local blood circulation;
- blockage of the hepatic ducts by blood clots;
- violation of the production and excretion of bile;
- intestinal dysbiosis;
- allergic manifestations;
- irritable bowel syndrome;
- hemolytic problems.
Antibiotics not only destroy hepatocytes, but also change the functions of the hematopoietic system and contribute to the appearance of anemia. Many patients develop cholecystitis, a chronic inflammation of the gallbladder. If it is not diagnosed and treated promptly, stones may form. Then the person will be indicated for surgery to remove the gallbladder.
Prevention
To avoid discomfort associated with antibiotic therapy, it is recommended:
- take medications in the dosage prescribed by the doctor (at regular intervals);
- take the tablets with plenty of water;
- adjust your diet (at least for the duration of treatment), give up fatty, fried and other “heavy” foods, so as not to further overload the liver, gall bladder and stomach;
- Upon completion of the course, you should definitely take probiotics (drugs that “introduce” beneficial bacteria into the intestines).
As you can see, the appearance of bitterness in the mouth is a common side effect of antibacterial therapy for various diseases. The cause of the problem lies in the increased functional load of such medications on the liver and gall bladder and, as a consequence, general intoxication of the body. In addition, such treatment often leads to exacerbation of diseases of the gastrointestinal tract, which, in turn, also provokes periodic or constant discomfort. It is necessary to combat bitterness in a comprehensive manner, based on the reason for its occurrence.
Hepatoprotective therapy
Considering all the risks to the liver, patients with severe Corona are necessarily prescribed hepatoprotectors. These are medications that increase the organ’s resistance to pathological influences and toxins, promote its speedy recovery, and enhance its neutralizing functions by increasing the activity of the liver system.
Hepatoprotectors can be of chemical, synthetic and natural origin. Depending on the composition, they can dilute and remove bile, exhibit antioxidant activity, and relieve inflammation.
Liver recovery after coronavirus infection
The organ is unique. It has the ability to self-heal. Even after a serious defeat, he can normalize his work if a person helps him with this. Basic requirements for the speedy restoration of liver structures:
- Eat a balanced diet. Refractory fats, spices, herbs, alcohol, offal, ice cream, and carbonated drinks are prohibited. A person who is recovering should eat porridge, vegetables, fruits, steamed and boiled meat, cottage cheese, vegetable soups, savory cookies, and a protein omelet. It is also important to drink plenty of clean water.
- Take hepatoprotectors prescribed by your doctor. It is unacceptable to use medications purchased at a pharmacy without medical advice or recommended by friends. This approach to treatment is ineffective and dangerous. The pharmaceutical market offers a wide range of geratoprotective medications. They all have their own side effects, dosages and indications. Only an experienced doctor can determine whether a particular patient needs a particular drug composition.
- Move more. If you no longer need to stay in bed, you should take walks in the fresh air more often. Activity contributes to the stable functioning of hepatobiliary structures.
- Don't overeat. This is the sin of many people who are forced to stay in a hospital for a long time. In order for all internal organs to function normally, it is important to eat four to five times a day in small portions.
- Follow doctor's orders. This is the main condition for a speedy recovery. If the doctor told you to take laboratory tests and undergo ultrasound diagnostics, then that’s what you need to do. By ignoring the doctor's recommendations, a person makes things worse for himself.
If you are sick with coronavirus, be sure to take care of your liver health. Don't ignore the symptoms of inflammation. Then, after discharge from the hospital, you will not have to take hepatoprotectors for a long time.
Clarithromycin
The simultaneous use of clarithromycin and the following drugs is contraindicated due to the possibility of serious side effects.
Cisapride, pimozide, terfenadine and astemizole
When clarithromycin was taken together with cisapride, pimozide, terfenadine, astemizole, an increase in the concentration of the latter in the blood plasma was reported, which can lead to an increase in the QT interval and the appearance of cardiac arrhythmias, including ventricular tachycardia, ventricular fibrillation and torsade de pointes (see section "Contraindications").
Ergot alkaloids
When clarithromycin is used together with ergotamine or dihydroergotamine, the following effects associated with acute poisoning with drugs of the ergotamine group are possible: vascular spasm, ischemia of the limbs and other tissues, including the central nervous system. The simultaneous use of clarithromycin and ergot alkaloids is contraindicated (see section "Contraindications").
HMG-CoA reductase inhibitors (statins)
Co-administration of clarithromycin with lovastatin or simvastatin is contraindicated (see section "Contraindications") due to the fact that these statins are largely metabolized by the CYP3A4 isoenzyme, and combined use with clarithromycin increases their serum concentrations, which leads to an increased risk of developing myopathy, including Rhabdomyolysis Cases of rhabdomyolysis have been reported in patients taking clarithromycin concomitantly with these drugs. If clarithromycin is necessary, lovastatin or simvastatin should be discontinued during therapy.
Clarithromycin should be used with caution in combination therapy with statins. It is recommended to use statins that do not depend on CYP3A metabolism (for example, fluvastatin). If coadministration is necessary, it is recommended to take the lowest dose of statin. The development of signs and symptoms of myopathy should be monitored.
Effect of other drugs on clarithromycin
Drugs that are CYP3A inducers (for example, rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's wort) may induce the metabolism of clarithromycin. This may result in subtherapeutic concentrations of clarithromycin, resulting in reduced effectiveness. In addition, it is necessary to monitor the concentration of the CYP3A inducer in the blood plasma, which may increase due to inhibition of the CYP3A isoenzyme by clarithromycin. When rifabutin and clarithromycin were used together, an increase in plasma concentrations of rifabutin and a decrease in serum concentrations of clarithromycin were observed with an increased risk of developing uveitis.
The following drugs have a proven or suspected effect on clarithromycin plasma concentrations; if used concomitantly with clarithromycin, dosage adjustments or switching to alternative treatment may be required.
Efavirenz, nevirapine, rifampicin, rifabutin and rifapentine
Strong inducers of the cytochrome P450 system, such as efavirenz, nevirapine, rifampicin, rifabutin and rifapentine, can accelerate the metabolism of clarithromycin and, thus, reduce the plasma concentration of clarithromycin and weaken the therapeutic effect, and at the same time increase the concentration of the 14-OH-clarithromycin metabolite, also being microbiologically active. Since the microbiological activity of clarithromycin and 14-OH-clarithromycin differs against different bacteria, the therapeutic effect may be reduced when clarithromycin is used together with enzyme inducers.
Etravirine
The concentration of clarithromycin decreases with the use of etravirine, but the concentration of the active metabolite 14-OH-clarithromycin increases. Because 14-OP-clarithromycin has low activity against Mycobacterium avium complex (MAC) infections, overall activity against these pathogens may be affected, and alternative treatments should be considered for the treatment of MAC.
Fluconazole
Coadministration of fluconazole 200 mg daily and clarithromycin 500 mg twice daily in 21 healthy volunteers resulted in an increase in mean clarithromycin minimum steady-state concentration (Cmin) and AUC by 33% and 18%, respectively. However, co-administration did not significantly affect the average steady-state concentration of the active metabolite 14-OH-clarithromycin. No dose adjustment of clarithromycin is required when taking fluconazole concomitantly.
Ritonavir
Coadministration of ritonavir 200 mg every eight hours and clarithromycin 500 mg every 12 hours resulted in a marked suppression of the metabolism of clarithromycin. When co-administered with ritonavir, clarithromycin Cmax increased by 31%, Cmin increased by 182% and AUC increased by 77%. Complete suppression of the formation of 14-OH-clarithromycin was noted. Due to the wide therapeutic range of clarithromycin, dose reduction is not required in patients with normal renal function. In patients with renal failure, it is advisable to consider the following dose adjustment options: with creatinine clearance of 30-60 ml/min, the dose of clarithromycin should be reduced by 50%; if creatinine clearance is less than 30 ml/min, the dose of clarithromycin should be reduced by 75%. Ritonavir should not be co-administered with clarithromycin in doses exceeding 1 g/day.
Effect of clarithromycin on other drugs Antiarrhythmic drugs (quinidine and disopyramide)
Ventricular tachycardia of the “pirouette” type may occur with the combined use of clarithromycin and quinidine or disopyramide. When clarithromycin is coadministered with these drugs, the electrocardiogram should be regularly monitored for prolongation of the QT interval, and serum concentrations of these drugs should also be monitored. During post-marketing use, cases of hypoglycemia have been reported during co-administration of clarithromycin and disopyramide. It is necessary to monitor the concentration of glucose in the blood while using clarithromycin and disopyramide.
Oral hypoglycemic agents and insulin
When clarithromycin is used together with oral hypoglycemic agents (for example, sulfonylureas) and/or insulin, severe hypoglycemia may occur. During concomitant use of clarithromycin and certain drugs that lower glucose concentrations, such as nateglinide, pioglitazone, repaglinide and rosiglitazone, inhibition of the CYP3A isoenzyme by clarithromycin may occur, which may result in hypoglycemia. Careful monitoring of glucose concentrations is recommended.
Interactions due to CYP 3 A
Co-administration of clarithromycin, which is known to inhibit the CYP3A isoenzyme, and drugs primarily metabolized by the CYP3A isoenzyme, may be associated with a mutual increase in their concentrations, which may increase or prolong both therapeutic and side effects. Clarithromycin should be used with caution in patients receiving drugs that are substrates of the CYP3A isoenzyme, especially if these drugs have a narrow therapeutic index (for example, carbamazepine) and/or are extensively metabolized by this enzyme. If necessary, the dose of the drug taken together with clarithromycin should be adjusted. Also, whenever possible, serum concentrations of drugs primarily metabolized by CYP3A should be monitored.
The following drugs/classes are metabolized by the same CYP3A isoenzyme as clarithromycin, for example, alprazolam, carbamazepine, cilostazol, cyclosporine, disopyramide, methylprednisolone, midazolam, omeprazole, indirect anticoagulants (eg, warfarin), quinidine, rifabutin, sildenafil, tacrolimus, triazolam and vinblastine. Also, agonists of the CYP3A isoenzyme include the following drugs that are contraindicated for combined use with clarithromycin: astemizole, cisapride, pimozide, terfenadine, lovastatin, simvastatin and ergot alkaloids (see section “Contraindications”). Drugs that interact in this manner through other isoenzymes within the cytochrome P450 system include phenytoin, theophylline, and valproic acid. Co-administration of clarithromycin, which is known to inhibit the CYP3A4 isoenzyme, and quetiapine, which is a CYP3A4 substrate, may lead to increased exposure of the antipsychotic quetiapine and possible toxic effects.
There have been post-marketing reports of somnolence, orthostatic hypotension, altered states of consciousness, neuroleptic malignant syndrome, and QT prolongation when these drugs are used together.
Caution should be used when quetiapine is used in combination with CYP3A4 inhibitors such as clarithromycin. The dose of quetiapine may need to be reduced.
Indirect anticoagulants
When taking warfarin and clarithromycin together, bleeding and a marked increase in the international normalized ratio (INR) and prothrombin time are possible. In case of combined use with warfarin or other indirect anticoagulants, it is necessary to monitor the INR and prothrombin time.
Omeprazole
Clarithromycin (500 mg every 8 hours) was studied in healthy adult volunteers in combination with omeprazole (40 mg daily). When clarithromycin and omeprazole were used together, steady-state plasma concentrations of omeprazole were increased (Cmax, AUCo-24 and T1/2 increased by 30%, 89% and 34%, respectively). The mean 24-hour gastric pH was 5.2 when omeprazole was taken alone and 5.7 when omeprazole was taken with clarithromycin.
Sildenafil, tadalafil and vardenafil
Each of these phosphodiesterase-5 inhibitors is metabolized at least in part by CYP3A. However, CYP3A may be inhibited in the presence of clarithromycin. Concomitant use of clarithromycin with sildenafil, tadalafil or vardenafil may result in increased phosphodiesterase inhibitory effects. When using these drugs together with clarithromycin, consider reducing the dose of sildenafil, tadalafil and vardenafil.
Theophylline, carbamazepine
When clarithromycin and theophylline or carbamazepine are used together, the concentration of these drugs in the systemic circulation may increase.
Tolterodine
The primary metabolism of tolterodine occurs through the 2D6 isoform of cytochrome P450 (CYP2D6). However, in part of the population lacking the CYP2D6 isoenzyme, metabolism occurs through CYP3A. In this population, inhibition of CYP3A results in significantly higher serum concentrations of tolterodine. In populations that are poor metabolizers via CYP2D6, a reduced dose of tolterodine may be required in the presence of CYP3A inhibitors such as clarithromycin.
Benzodiazepines (eg, alprazolam, midazolam, triazolam)
When midazolam was co-administered with clarithromycin tablets (500 mg twice daily), midazolam AUC increased by 2.7 times after intravenous midazolam and 7 times after oral administration. Concomitant oral administration of midazolam and clarithromycin should be avoided. Concomitant use of clarithromycin with oral midazolam is contraindicated. If intravenous midazolam is used concomitantly with clarithromycin, the patient's condition should be carefully monitored for possible dose adjustment. The same precautions should be applied to other benzodiazepines that are metabolized by CYP3A, including triazolam and alprazolam. For benzodiazepines whose elimination is not dependent on CYP3A (temazepam, nitrazepam, lorazepam), a clinically significant interaction with clarithromycin is unlikely.
When clarithromycin and triazolam are used together, central nervous system (CNS) effects such as drowsiness and confusion are possible. Therefore, if coadministration occurs, it is recommended to monitor for symptoms of CNS impairment.
Interactions with other drugs
Aminoglycosides
When taking clarithromycin concomitantly with other ototoxic drugs, especially aminoglycosides, caution should be exercised and the functions of the vestibular and auditory systems should be monitored both during and after therapy.
Colchicine
Colchicine is a substrate of both CYP3A and the P-glycoprotein (Pgp) transporter protein. Clarithromycin and other macrolides are known to be inhibitors of CYP3A and Pgp. When clarithromycin and colchicine are taken together, inhibition of Pgp and/or CYP3A may result in increased effects of colchicine. The development of clinical symptoms of colchicine poisoning should be monitored. There have been post-marketing reports of cases of colchicine poisoning when taken concomitantly with clarithromycin, most often in elderly patients. Some of the reported cases occurred in patients suffering from kidney failure. Some cases were reported to be fatal. The simultaneous use of clarithromycin and colchicine is contraindicated (see section "Contraindications").
Digoxin
Digoxin is suspected to be a Pgp substrate. Clarithromycin is known to inhibit Pgp. When clarithromycin and digoxin are co-administered, inhibition of Pgp by clarithromycin may result in increased effects of digoxin. Coadministration of digoxin and clarithromycin may also result in increased serum concentrations of digoxin. Some patients have experienced clinical symptoms of digoxin toxicity, including potentially fatal arrhythmias. When clarithromycin and digoxin are used together, serum digoxin concentrations should be carefully monitored.
Zidovudine
Concomitant use of clarithromycin tablets and oral zidovudine in adult HIV-infected patients may result in decreased steady-state zidovudine concentrations.
Because clarithromycin interferes with the oral absorption of zidovudine, the interaction can be largely avoided by taking clarithromycin and zidovudine 4 hours apart.
This interaction was not observed in HIV-infected children taking clarithromycin pediatric suspension with zidovudine or dideoxyinosine. Since clarithromycin may interfere with the absorption of zidovudine when administered concomitantly orally in adult patients, such an interaction is unlikely to occur when clarithromycin is used intravenously.
Phenytoin and valproic acid
There is evidence of interactions between CYP3A inhibitors (including clarithromycin) and drugs that are not metabolized by CYP3A (phenytoin and valproic acid). For these drugs, when used together with clarithromycin, it is recommended to determine their serum concentrations, as there are reports of their increase.
Bidirectional drug interactions
Atazanavir
Clarithromycin and atazanavir are both substrates and inhibitors of the CYP3A isoenzyme. There is evidence of a bidirectional interaction between these drugs. Coadministration of clarithromycin (500 mg twice daily) and atazanavir (400 mg once daily) may result in a twofold increase in clarithromycin exposure and a 70% decrease in 14-OH-clarithromycin exposure, with a 28% increase in atazanavir AUC. Due to the wide therapeutic range of clarithromycin, dose reduction is not required in patients with normal renal function. In patients with moderate renal failure (creatinine clearance 30 - 60 ml/min), the dose of clarithromycin should be reduced by 50%. In patients with creatinine clearance less than 30 ml/min, the dose of clarithromycin should be reduced by 75% using the appropriate clarithromycin dosage form. Clarithromycin in doses exceeding 1000 mg per day should not be used in conjunction with protease inhibitors.
Blockers of "slow" calcium channels
When using clarithromycin simultaneously with blockers of “slow” calcium channels that are metabolized by the CYP3A4 isoenzyme (for example, verapamil, amlodipine, diltiazem), caution should be exercised as there is a risk of arterial hypotension. Plasma concentrations of clarithromycin, as well as slow calcium channel blockers, may increase with simultaneous use. Arterial hypotension, bradyarrhythmia and lactic acidosis are possible when taking clarithromycin and verapamil simultaneously.
Itraconazole
Clarithromycin and itraconazole are substrates and inhibitors of the CYP3A isoenzyme, which determines the bidirectional interaction of the drugs. Clarithromycin may increase plasma concentrations of itraconazole, while itraconazole may increase plasma concentrations of clarithromycin. Patients taking itraconazole and clarithromycin concomitantly should be closely monitored for symptoms of increased or prolonged pharmacological effects of these drugs.
Saquinavir
Clarithromycin and saquinavir are CYP3A substrates and inhibitors, resulting in a bidirectional drug interaction. Coadministration of clarithromycin (500 mg twice daily) and saquinavir (soft gelatin capsules, 1200 mg three times daily) in 12 healthy volunteers increased the AUC and Cmax of saquinavir by 177% and 187%, respectively, compared with saquinavir. separately. The AUC and Cmax values of clarithromycin were approximately 40% higher than with clarithromycin monotherapy. When these two drugs are used together for a limited time at the doses/dosage forms indicated above, no dose adjustment is required. Results from drug interaction studies using saquinavir soft gelatin capsules may not be consistent with the effects observed with saquinavir hard gelatin capsules. The results of drug interaction studies with saquinavir monotherapy may not be consistent with the effects observed with saquinavir hard gelatin capsules. The results of drug interaction studies with saquinavir monotherapy may not be consistent with the effects observed with saquinavir/ritonavir therapy. When taking saquinavir with ritonavir, consider the potential effect of ritonavir on clarithromycin.
Literature:
- Complete reference book for infectious disease specialists / N.I. Zryachkin et al. - M.: Eksmo, 2004 (JSC Mozhaisk Printing Plant). – 990 s.
- RMJ. 2022, No. 9: Clinical recommendations and algorithms for practicing physicians. — 2022. — 80 p.
- COVID-19 and liver damage/ Ilchenko Lyudmila Yurievna, Nikitin I.G., Fedorov I.G./ 2022 / Journal “Archives of Internal Medicine”
The text was checked by medical experts: Head of the socio-psychological service of the Alkoklinik MC, psychiatrist-narcologist L.A. Serova.
CAN'T FIND THE ANSWER?
Consult a specialist
Or call: +7 (495) 798-30-80
Call! We work around the clock!