The birth of a healthy child is the natural desire of every pregnant woman. But, unfortunately, hopes for happy motherhood are not always justified. About 5% of newborns have various congenital diseases. Screening in the 1st and 2nd trimesters of pregnancy allows you to determine how high the risk of congenital pathology is in the unborn child.
Currently, there are quite effective methods for prenatal (prenatal) diagnosis of many fetal diseases that can be detected from the 11th week of pregnancy. Timely screening examinations make it possible to identify a wide range of fetal pathologies and see ultrasound signs of chromosomal abnormalities.
What is early prenatal screening and when is it performed?
Screening (from the English “sifting”) is a set of studies that allows us to identify groups of pregnant women who are at risk of having a child with chromosomal abnormalities and congenital defects. But early screening is only the initial, preliminary stage of the examination, after which women with an identified risk of congenital anomalies are recommended to have a more detailed diagnostic examination that will accurately confirm or exclude the presence of pathology.
This diagnostic complex is carried out for pregnant women at a period of 11–13 weeks + 6 days (when the coccygeal-parietal size of the fetus is from 45 to 84 mm). The most optimal time for screening is considered to be from 12 to 13 weeks, since such an examination evaluates both the signs of chromosomal pathology in the fetus and the anatomical structures of the unborn child, and at a period of 11 weeks - 11 weeks + 4 days it is very difficult to do this .
What does early prenatal screening include?
A comprehensive examination includes:
- Ultrasound of the fetus for the presence of markers (signs) of chromosomal pathology and congenital malformations;
- determination of the level of biochemical markers of chromosomal abnormalities in the blood of a pregnant woman: beta-hCG and PAPP-A. The study is carried out on special high-tech equipment Cobas e, Roshe, approved for calculating the risk of chromosomal syndromes in the Astraia (FMF) program;
- Doppler ultrasound of the uterine arteries
- cervicometry – measurement of the length of the cervix;
- collecting anamnesis (age, height, weight, number of pregnancies and births, smoking, diabetes, arterial hypertension, etc.);
- measuring blood pressure in both arms.
The data obtained: medical history, ultrasound and biochemical markers are placed in a specially developed Astraia program, which calculates the risk of having a child with congenital anomalies. The combination of these studies increases the efficiency of identifying fetuses with Down syndrome and other chromosomal diseases.
Medical Internet conferences
Introduction. One of the relevant aspects of modern pediatric urology is the diagnosis of defects of the urinary system at the antenatal stage; their share among all antenatally diagnosed defects is 26-28% [1]. The high prevalence and diversity of urinary organ defects, the severity of complications and often their fatal predetermination force us to consider the problem of their existence from the point of view of their prevention [2]. High-quality antenatal diagnostics can significantly increase the reliability of the prognosis, plan the timing, volume of treatment measures carried out at the stage of preclinical manifestations, which in its complex predetermines the outcome of the diagnosed pathological condition, prevents the development of life-threatening complications, and reduces the likelihood of having children with severe incurable defects [3 ].
Addressing pediatric urologists and surgeons, pediatricians, obstetricians, geneticists and ultrasound diagnostic specialists as the main audience for discussion on the problem of prenatal diagnosis (PD) of congenital defects (CD) of the urinary system (UCS), we propose to shift the focus when identifying this pathology to as early as possible gestational periods, that is, in the first trimester of gestation and discuss the prognostic significance of such a position.
On the one hand, today the priorities for solving the problems of the first trimester of gestation are still entirely assigned to ultrasound doctors, obstetricians and geneticists. On the other hand, currently, in accordance with existing legislation, a pediatric urologist must take part in prenatal consultation, namely, prenatal prognosis of diagnosed fetal pathology, and be responsible for decisions made. In the end, it is the pediatric urologist who is the doctor who subsequently treats these patients [4].
The current European prenatal diagnostics is based on the ideology of early prenatal screening, formulated by the founder of the Fetal Medicine Foundation (FMF, London) Kypros Nicolaides. If previously it was believed that all complications of fetal development and the course of pregnancy arise or occur in the second half of pregnancy, and this predetermined an increase in the frequency of monitoring diagnostic procedures in order to identify pathology, then recent studies have proven that the formation of complications of fetal development occurs in periods of up to 12 weeks , the first screening period of 11-14 weeks of pregnancy is optimal for diagnosing most lethal and disabling congenital malformations and chromosomal abnormalities (CA). This thesis is declared in the form of the main brand of the Fetal Medicine Foundation: “to turn over the “diagnostic pyramid”, laying at its base high-quality early prenatal screening of the first trimester of gestation” [5].
Prenatal diagnosis of congenital and hereditary diseases (CHD) aims to identify congenital defects, chromosomal abnormalities, and monogenic diseases. According to the European registry, in newborns the frequency of congenital malformations is registered in the range of 2.5 - 3.0%, CA (mainly trisomies) account for a total of 2-3 cases per 1000 births, and monogenic diseases are registered in 0.5-1.4% born children. According to the WHO, the incidence of ICD among live and stillborn children does not exceed 4-6% [6, 7, 8].
The three-level system of prenatal diagnostics existing in Russia since December 1993, including ultrasound, biochemical screening of the second trimester, invasive methods (amniocentesis, cordocentesis) did not justify itself, and the summed up results were disappointing. Reform of the system of mass prenatal examination of pregnant women was based on the positive international experience of FMF and included in the national project “Health” since 2010 with transfer to the expert level of all subjects of the Russian Federation [6].
The algorithm of the Astraya program was chosen as the priority basis for PD, based on the recommendations of the FMF, including: 1) the decreed period for conducting research - 11-14 weeks; 2) a set of certified non-invasive diagnostic methods: ultrasound and biochemical screening and (determination of molecular serum markers: free beta unit of human choriogonic gonadotropin (hCG) and pregnancy-associated plasma protein A (PAPP-A); 3) combined calculation of the individual risk of chromosomal abnormalities with pregnant women whose risk was 1:100 or higher included in the risk group [6].
Along with this, in recent years significant progress has been made in the development of fundamental issues in antenatal urology. A diagnostic algorithm for prenatal ultrasound identification of variants of urinary system defects has been developed, which has brought us closer to making a prenatal diagnosis. Prognostic criteria for promising renal function and objective urodynamic criteria for “fatal defects”, etc. have been determined. [3, 9, 10]. However, it is important to note that all this applies to the second and, to a greater extent, the third trimesters of gestation, when the anatomical structures of the fetus, and at the same time the ultrasound markers of the pathology of the fetus, become most pronounced and accessible for visualization. However, in cases of diagnosis of prognostically unfavorable malformations, the time frame for terminating pregnancy is missed. It is these circumstances that forced us to support the main thesis of the Fetal Medicine Foundation and turn to a discussion of the issue of antenatal diagnosis of the pathology of MWS, shifting the focus to the first trimester of gestation.
For the first trimester of gestation, the manifest ultrasound marker of MBC defects is “megacystis syndrome”, which in the literary discussion has not an unambiguous interpretation, but accordingly conflicting tactical recommendations [11,12,13,14].
The detection rate of megacystis syndrome in the first trimester of pregnancy is 0.02% - 0.19% [13,14]. Most authors interpret fetal bladder enlargement syndrome as a result of low urinary tract obstruction, the causes of which may be urethral pathology in the form of atresia or fibrostenosis, dysgenesis of the cloaca with atresia of the rectum and urethra, which occurs in fetuses of both sexes. But the most commonly diagnosed syndrome is posterior urethral valve syndrome, which is characteristic of male fetuses [15].
In addition, an enlarged bladder may accompany MVD defects, the pathogenesis of which is not associated with the presence of anatomical obstruction of the lower urinary tract, and the obstruction in such cases is functional in nature. Currently, two syndromes are known: megacystis-megaureter-microcolon syndrome of intestinal hypoperistalsis and prune-belle syndrome, which are among them. The cause of functional obstruction of the detrusor is due to a sharp decrease, or rather, the absence of its contractility, which is morphologically manifested by degeneration and sparseness of smooth muscles, proliferation of fibrous tissue, and the presence of massive hyaline deposits. Mortality in these syndromes ranges from 20 to 50% due to pulmonary failure in the neonatal period or renal failure in early childhood [15,15,16,17,18].
Most authors interpret the diagnosis of fetal megacystis as a prognostically unfavorable sign, threatened by perinatal losses due to decompensated defects of the urinary system associated with dysplasia of the renal parenchyma. The most unfavorable option is the formation of cystic dysplasia of the renal parenchyma (mainly type IV, according to Potter) [7,8,11,14,20].
On the other hand, N. Sebire and Sahid S. report independent spontaneous regression of fetal megacystis syndrome and the birth of healthy children [21]. This apparently occurs due to the fact that the formation of smooth muscle muscles and innervation of the bladder does not end by the 13th week of gestation and continues in the following days, providing the basis for self-resolution of the problem in subsequent weeks of intrauterine development of the fetus [22].
Considering that megacystis syndrome is combined with chromosomal pathology in 25-40% [23], the results of genetic studies and karyotyping of fetal material play a significant role in deciding whether to prolong or terminate pregnancy. Most authors agree that posterior urethral valves, as well as prune-belly syndrome, are not a genetically inherited pathology, but this does not exclude the possibility of chromosomal damage. AWLiao states that when the size of the bladder increases from 7 to 15 mm in fetuses of 10-14 weeks of gestation, in 25% of cases the presence of trisomy on chromosomes 13 and 18 is determined [23].
The variety of literature data and the ambiguity of tactical recommendations compels us to discuss this problem in detail and purposefully among pediatric urologists and antenatal diagnostic doctors, recognizing the complexity and responsibility when making decisions both in cases of prolonging the course of pregnancy and in cases of its termination.
The purpose of this work is to analyze perinatal outcomes of pregnancies and develop an algorithm for tactical decisions in identifying fetal megacystis syndrome in the first trimester of gestation.
Materials and methods. We analyzed the course of 11 pregnancies in the first trimester with an antenatal diagnosis of fetal megacystis syndrome, which were included in the perinatal observation group of a geneticist at the Regional Perinatal Center and a pediatric urologist.
The longitudinal size of the bladder exceeded the conventional standard of 7 mm and varied from 8 to 31 mm (Fig. 1).
Rice. 1. Pregnancy 13 weeks. Fetal megacystis syndrome.
It is noteworthy that in 85% of cases the age of women ranged from 31 to 41 years. Of the 11 observed pregnant women, only 6 women underwent early prenatal screening under the Astraya program, 5 women were not examined. Based on the results of biochemical screening, hCG levels were normal in all cases; PAPPA levels deviated from normal values in 2 of 6.
Results and its discussion. Ultrasound markers of congenital malformation and CA were detected in the form of an increase in the nuchal space to 2.6 and 3.0 mm in 2 fetuses; the presence of a concomitant malformation – omphalocele – in 1 fetus; In 1 case, umbilical cord cysts were diagnosed. In none of the examined fetuses did the calculated individual risk of chromosomal pathology exceed the permissible values (that is, in all cases it was more than 1:100) (Table 1).
At the time of diagnosis of this condition, only 2 cases had unilateral or bilateral pyelectasis (Fig. 2).
Rice. 2. Pregnancy 12 weeks with the presence of dilation of the pelvis of both kidneys.
During dynamic observation for 2 weeks, against the background of an increase in the size of the bladder, bilateral pyelectasis was detected in 5 cases (Fig. 3).
Rice. 3. Pregnancy 15 weeks. Dilatation of the upper urinary tract in a fetus with megacystis syndrome.
The result of the study was an analysis of pregnancy outcomes.
Decision-making tactics were motivated by the degree of damage to the urinary system, that is, the degree of dilation of the bladder, the presence of retention of the upper urinary tract.
Considering the high percentage of concomitant genetic pathology in the presence of megacystis syndrome in fetuses, karyotyping of fetal material (chorionic villi) was performed in 5 cases. Moreover, in 1 case, trisomy of the 18th pair of chromosomes with karyotype 46, XX+47.18 was detected, and the pregnancy was terminated; in other cases, no karyotype pathology was detected.
In 5 cases, after the initial visualization of an enlarged bladder ≥14≤30 mm, dynamic observation was continued for 2 weeks, which made it possible to establish an increase in the enlargement of the bladder, the appearance of dilatations of the upper urinary tract in 4 fetuses, and justified the termination of pregnancies.
In 4 observations, the initial significant increase in the bladder to 29-30 mm made it possible to associate this with severe urethral obstruction and terminate pregnancies without additional studies.
In one case, a frozen pregnancy was noted. Overall, 10 of the 11 pregnancies were terminated.
Only in one case, with an initial increase in the size of the bladder to 8 mm, dynamic observation made it possible to observe positive dynamics and normalization of the size of the bladder, and as a result, prolongation of the course of pregnancy. However, it is not yet possible to assess its outcome, since the birth has not yet occurred.
The analysis of diagnostic measures and pregnancy outcomes allowed us to develop a diagnostic algorithm that involves mandatory karyotyping of the fetal material in the presence of bladder sizes within the range of ≥7≤15 mm. If a karyotype abnormality is confirmed, it is advisable to terminate the pregnancy, otherwise, dynamic observation until convincing markers for the prognosis of the diagnosed condition appear.
An initial significant enlargement of the bladder ≥ 20-30 mm clearly indicates severe obstruction of the lower urinary tract and does not require dynamic monitoring; it is advisable to terminate the pregnancy.
The presence of dilation of the upper urinary tract does not always complement megacystis syndrome in the early stages of gestation (11-13 weeks) and is most clearly defined in the second and third trimesters of gestation. However, in all cases its presence is a factor aggravating the prognosis.
Thus, summing up the analysis of literature data and the results of our own observations, several conclusions are obvious.
Conclusions.
1. The set of measures included in early prenatal screening of 11-14 weeks of gestation (molecular genetic and ultrasound markers of congenital malformation and CA) does not allow determining the likelihood of a fetus having congenital malformation of the urinary system. Identification of a risk group for fetuses at risk due to the presence of congenital pathology of VUS occurs on the basis of the diagnosis of an increased longitudinal size of the bladder ≥ 7 mm, which is interpreted as “fetal megacystis” syndrome and requires careful diagnostic measures to predict the outcome.
2. Fetal megacystis syndrome is considered as a manifestation of severe disturbances in the urodynamics of the lower urinary tract of anatomical or functional origin, underlying the development of obstructive disorders of the upper urinary tract and dysplastic development of the renal parenchyma, including its cystic dysplasia, which predetermines decompensation of renal functions and an unfavorable outcome.
3. A set of diagnostic procedures that clarify the prognosis when identifying a group of fetuses with megacystis syndrome includes karyotyping of fetal material (chorionic villi) in the absence of dilatation of the upper urinary tract and bladder size ≤ 20 mm and termination of pregnancy in cases of detected chromosomal pathology.
4. The presence of bladder enlargement ≥ 20 mm isolated or in the presence of dilatations of the upper urinary tract is an indication for termination of pregnancy.
5. Prolongation of pregnancy is recommended in all cases of moderate bladder dilation within ≥7≤15mm in the absence of dilation of the upper urinary tract, positive dynamics of observation for 2-3 weeks, restoration of bladder size.
6. Carrying out diagnostic measures and choosing tactical decisions should be carried out with the participation of a pediatric urologist, including the ante- and postnatal stages of dynamic observation and necessary treatment.
What is the Astraia program
Astraia is a professional program that calculates the likelihood of chromosomal abnormalities in a fetus. The program was developed by the Fetal Medicine Foundation (FMF) in London and was successfully tested on a huge amount of clinical material in many countries around the world. It is constantly being improved under the leadership of a leading specialist in the field of prenatal diagnostics, Professor Kypros Nicolaides, in accordance with the latest world advances in the field of fetal medicine.
The specialist conducting early prenatal screening must have an international FMF certificate, which gives the right to perform this diagnosis and work with the Astraia program. The certificate is confirmed annually after a statistical audit of the work done during the year and passing the certification exam. This ensures high diagnostic accuracy of the obtained risks.
Conducting early prenatal screening using this program is regulated by Order of the Ministry of Health of the Russian Federation dated November 1, 2012 No. 572n “On approval of the procedure for providing medical care in the field of obstetrics and gynecology (except for the use of assisted reproductive technologies).”
Early prenatal screening allows you to calculate the following risks:
- Down syndrome (trisomy 21) in the fetus;
- Edward syndrome (trisomy 18) in the fetus;
- Patau syndrome (trisomy 13) in the fetus;
- the risk of developing early (before 34 weeks) and late (after 37 weeks) preeclampsia (preeclampsia) in the pregnant woman herself.
What is assessed during ultrasound in the 1st trimester
Coccygeal-parietal size (CPS) of the fetus
This indicator accurately determines the gestational age (pregnancy), especially if a woman does not remember the 1st day of her last menstruation, or if her menstrual cycle is not regular. In conclusion, the gestational age is set according to the fetal calf temperature, and not according to the date of the last menstruation.
Correct measurement of fetal CTE
Markers of chromosomal pathology:
- nuchal translucency thickness (TN) is the main sign of chromosomal pathology in the fetus. A pathological value is considered to be an increase in TVP greater than the 95th percentile for each gestational age. Each increase in TVP increases the risk of a chromosomal abnormality in the fetus.
TVP is normal TVP is pathological
It is important to understand that an increase in TVP is a sign (marker), but not an accurate diagnosis of chromosomal abnormalities in the fetus. Only invasive diagnostics followed by genetic analysis can determine the presence of Down syndrome and other diseases in an unborn child.
- nasal bone. In fetuses with Down syndrome, the nasal bone may be absent or reduced in size (hypoplastic). Very rarely, this can occur in completely healthy children. An accurate diagnosis can only be established using genetic analysis.
Normal nasal bone Absent nasal bone
- blood flow in the ductus venosus is a small vessel in the fetal liver. With reverse (retrograde) blood flow in this vessel, it can be assumed that the fetus has a chromosomal syndrome or a congenital heart defect.
Normal blood flow in the ductus venosus
But it is important to correctly obtain this blood flow and evaluate it. This requires certain skills and qualifications of a doctor, which are confirmed by annual FMF certification.
- blood flow through the tricuspid valve into the fetal heart. Here, retrograde (reverse) blood flow also indicates a chromosomal pathology, or can manifest itself in congenital heart defects.
Anatomical structures of the fetus and exclusion of major congenital defects
Fetal hand Fetal brain in the form of a “butterfly” is normal
Cervical length
Walls of the uterus and appendages (ovaries)
Blood flow in the uterine arteries
Ultrasound can be performed either transabdominally or transvaginally.
What is screening in the second trimester of pregnancy?
According to order No. 572n dated November 1, 2012, the second screening consists of an ultrasound examination of the fetus at 18-21 weeks of pregnancy. At this age, blood is no longer tested for biochemical markers. The fetus has a mass of about 300-500 grams and a length of 20-25 cm, and ultrasound allows you to analyze in detail all the anatomical structures of the fetus and identify most developmental defects. At the same time, the amount of amniotic fluid, the location and structure of the placenta, the length of the cervix, etc. are assessed.
After an ultrasound scan at these stages of pregnancy, most questions of prenatal diagnosis are considered closed.
We hope that this information will help you better understand the importance and necessity of screening in the first and second trimesters of pregnancy. In our clinic you have a unique opportunity to undergo a high-quality examination and obtain the most objective data about the condition of your fetus.
Sign up for prenatal screening at the Family Doctor clinic by phone in Moscow, through the online registration form or at the reception.
Fetal development anomalies: classification
The following diseases belong to this category:
Neural tube developmental abnormalities
. The most common pathology of the fetal central nervous system is anencephaly - the congenital absence of the cranial vault and cerebral hemispheres. It is diagnosed by ultrasound at 11-12 weeks. Hydrocephalus is detected at the 18th week of pregnancy - by the expansion of the anterior and posterior horns of the lateral ventricles. Pathological reduction of the fetal head (microcephaly) occurs, as a rule, against the background of other related diseases, and very rarely - in its pure form. In such cases, the doctor performs an ultrasound scan several weeks apart (to obtain the most reliable picture). A rounded protrusion in the area of the bones of the cranial vault (encephalomeningocele) is most often diagnosed on the back of the head, and such a pathology also requires a repeat ultrasound.
Deviations in the development of the spine
observed mainly in the lumbar and cervical region. You can see the spine on an ultrasound from the 15th week of pregnancy; from about the 18th week, defects of its structure are diagnosed (if any). The worst thing is cystic hygroma, which affects the lymphatic system. An ultrasound with this diagnosis shows a cyst in the cervical spine. If this disease is associated with other pathologies of the lymphatic system, the fetus dies. It is difficult to detect myelomeningocele (a formation consisting of fluid and elements of the spinal cord) and spina bifida - these pathologies require the use of expert-class equipment.
Among the pathologies of development of the gastrointestinal tract
Duodenal atresia is most often detected. This disease is often accompanied by polyhydramnios, damage to the kidneys, heart, and nervous system. It is very difficult to detect small intestinal atresia using ultrasound; most often it is detected in mid-pregnancy or late pregnancy. Colonic atresia cannot be diagnosed by ultrasound. Of the structural disorders of the anterior abdominal wall, omphalocele is detected in most cases.
Some of the abnormalities of the urinary system
lead to very dire consequences, so if the pathology is detected in the early stages, the doctor may advise termination of pregnancy. If the disease is detected late, pregnancy management tactics are changed. Ultrasound can detect pathological bilateral obstruction, multicystic kidney; diagnosing agenesis (absence) of an organ can be difficult. Determining hydronephrosis will require repeat ultrasound.
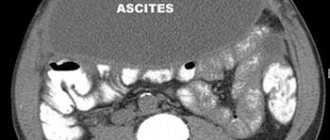
Ascites
– free fluid – on ultrasound it is seen as an anechoic zone that surrounds the fetal organs. Only after careful study of the examination results is a final diagnosis made. Dropsy is indicated by thickening of the skin or the presence of fluid in at least two natural cavities. This disease is caused by:
- congenital heart defects;
- Rhesus conflict;
- obstruction of lymphatic and blood vessels;
- arrhythmias.
To accurately diagnose dropsy, the patient is prescribed an additional expert ultrasound.
Polyhydramnios can also be detected using ultrasound. The development of this anomaly is facilitated by:
- hydrops fetalis;
- diabetes mellitus (in mother);
- disturbances in the formation of the gastrointestinal tract;
- neural tube pathologies;
- multiple pregnancy;
- skeletal dysplasia of the chest;
- structural defects of the anterior abdominal wall.
Low hydramnios is caused by:
- bilateral renal anomaly;
- intrauterine growth retardation;
- post-term pregnancy;
- damage to the amniotic membranes;
- intrauterine fetal death.
The ultrasound procedure is absolutely safe for the life and health of the mother and child, there are no restrictions on the number of sessions, so do not neglect the doctor’s recommendations and undergo the prescribed examination in a timely manner.
All information is for informational purposes only. If you have any health problems, you need to consult a specialist.