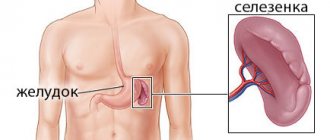
Sometimes after a heavy feast, we suddenly begin to experience spasmodic pain in the stomach, nausea and vomiting appear. The first thought that comes to mind is poisoning. Symptoms increase, become more frequent, and you have to call an ambulance. At the hospital, a not very pleasant thing turns out that the cause was intestinal obstruction. Most often this occurs in women.
Pathophysiology of ileus and intestinal obstruction
The intestines have a muscular wall that contracts in a coordinated manner, pushing food, fluids, and waste along its length . These coordinated movements are known as peristalsis. This allows intestinal contents to move from one segment of the intestine to another. To coordinate this process, nerves in the intestinal wall relax the muscles as new contents are delivered and contract the muscles to push the contents into the next segment.
Up to 50% of patients undergoing major abdominal surgery experience intestinal obstruction
These nerves communicate with each other in the intestinal wall and through reflexes in the spinal cord. Other nerves in the brain and spinal cord and hormones in the bloodstream can help speed up or slow down this process.
Hormones and chemicals
In addition to the chemical effect of anesthesia, which causes temporary paralysis of the muscles in the intestinal wall, other chemicals that occur naturally in the body can also have this effect. These natural chemicals play a variety of important roles in the body, but during a stress response, in this case caused by surgery, they interfere with the normal movement of the cup. Levels of various hormones may also increase or decrease during this time, further affecting peristalsis.
Worsening after surgery
It is known that for a certain period of time after surgery, disturbances in normal intestinal motility may occur:
- Stomach: 1-2 days
- Small intestine: several hours
- Large intestine: 3 to 5 days
However, some cases occurring after surgery and for other reasons may affect intestinal motility for a longer period of time.
Nerve problems
Any nerve damage or impairment commonly associated with anesthesia and surgery can affect normal bowel movements.
Any nerve damage or impairment typically associated with anesthesia and surgery can affect normal bowel movements. These effects are usually temporary and go away after a short time. Reflexes either within the intestinal wall, just behind it, or up to the spinal cord may be affected first. This damage and destruction of the nerve may be intentional or accidental during surgery. This can also occur with injuries to the abdomen and spinal cord.
The intestines can no longer perform their duties
With obstruction, paralysis of the intestines occurs at the stage of passage of food either in the small or large intestine. In this case, the intestines cannot help the stomach perform motor and secretory functions. The main job of the small intestine is to absorb important nutrients, vitamins, minerals and water from digested food through the mucous membrane into the blood. The large intestine thickens leftover food and removes it from the body.
If the digestion process is interfered with by an intestinal obstruction, this can have very serious consequences. Food accumulates in the stomach and rots. Due to putrefactive gases formed during the digestion process, the stomach can become bloated and swollen. As a result, the damaged intestine dies. This threatens with unpredictable consequences for the patient.
Ileus and intestinal obstruction causes
Abdominal surgery
The most common cause is intestinal obstruction after abdominal surgery. The entire digestive tract may be affected, but it is most noticeable in the stomach, small intestine, and large intestine. This is the reason why most patients experience constipation soon after surgery. However, it resolves spontaneously and usually does not require special treatment. Restoration of intestinal function occurs gradually from the ascending to the transverse and then the descending colon.
Other reasons
There are other causes of intestinal obstruction:
- Injury to the abdomen, spine or chest
- Medicines such as opioid analgesics, antacids, anti-clotting drugs such as warfarin, tricyclic antidepressants such as ammitriptyline, and antipsychotics.
- Head injury
- Neurosurgical procedures
- Septicemia (“blood poisoning”)
- Myocardial infarction (heart attack)
- Dehydration
- Other causes of electrolyte disturbances include low levels of potassium, magnesium, or sodium.
- Anemia
- Stones in the kidneys
- Gallstones
- Peritonitis
- Pneumonia
What diseases can it be associated with?
Ileus is usually an iatrogenic disorder, that is, it develops as a complication of surgery. However, there are diseases in the unfavorable course of which the development of ileus is likely and they must be taken into account when developing the corresponding symptoms:
- appendicitis,
- diverticulitis,
- perforation of duodenal ulcer,
- aortic aneurysm,
- compression fracture of the spine,
- fractures of the underlying ribs,
- hypokalemia,
- pneumonia of the lower lobes of the lungs,
- myocardial infarction.
Ileus and intestinal obstruction types
The terms ileus can be used in conjunction with various conditions that interfere with intestinal motility and various causes. It can be broadly classified as follows:
Postoperative intestinal obstruction
This is the most common type of intestinal obstruction and mainly occurs during abdominal surgery. Ileus can also occur, but to a lesser extent, with other operations on the brain, spinal cord, and some nerves.
Paralytic ileus
This can also happen after surgery and for most of the reasons mentioned above. The muscles of the intestinal wall are partially or completely paralyzed, and peristalsis cannot proceed normally.
Gallstone obstruction
This type of intestinal obstruction occurs due to gallstones. This is a mechanical intestinal obstruction associated with one or more large gallstones. This is not the result of hypomobility due to insufficient activity of the intestinal muscles.
Meconium ileus
Meconium is the first stool of a newborn, containing contents that enter the uterus. It usually goes away within 24 hours after birth. However, sometimes it becomes very sticky and causes intestinal obstruction. This is called meconium ileus.
Other animals
Ileus is a cause of colic in horses due to functional intestinal obstruction. This is most commonly seen in horses after surgery, especially after surgery for colic.[10] Horses suffering from intestinal obstruction are at risk of gastric rupture due to rapid progression of reflux and require intensive medical treatment with frequent nasogastric intubation.[10] Ileus may increase adhesion formation because intestinal segments have longer contact, and intestinal distension causes serous damage and ischemia. It is typically treated with aggressive fluid support, prokinetics, and anti-inflammatory agents.[10]
Symptoms of ileus and intestinal obstruction
Symptoms of intestinal obstruction are nonspecific. Many other types of abdominal and gastrointestinal diseases present with the same symptoms and must be excluded as possible causes.
Nausea and vomiting are symptoms of ileus
These symptoms include:
- Mild abdominal pain (no cramps)
- Bloating is a feeling of fullness in the abdomen.
- Bloating (bloating)
- Nausea
- Vomit
- Bowel sounds are reduced or absent
- Constipation (sometimes) with little or no gas (gas)
Types of disease
Depending on the anatomical and functional features of development, the following types of ileus are distinguished:
- dynamic intestinal obstruction (divided into spastic and paralytic);
- mechanical intestinal obstruction (divided into strangulation, obstruction and mixed);
- vascular intestinal obstruction.
There is also a classification of intestinal obstruction according to the level of localization. Thus, high and low small intestinal obstruction and large intestinal obstruction are distinguished.
Ileus diagnosis
The symptoms themselves may not indicate an intestinal obstruction, especially when the patient is still passing stool and gas. However, medical history is important, and intestinal obstruction should be suspected after abdominal surgery. Therefore, further diagnostic studies are important.
Tests
Blood tests can help determine the cause of an intestinal obstruction if it is due to dehydration or infection. However, it cannot definitively diagnose intestinal obstruction. Therefore, imaging studies such as x-rays are necessary.
Abdominal x-ray
A simple x-ray of the abdomen can reveal swelling of the small and large intestines. Radiocontrast dye injected into the small intestine through a nasal tube may provide better detection. This is called an enteroclyster. The dye can be seen on an x-ray and should reach the cecum of the colon within 4 hours.
We recommend reading: Improve bowel function at home.
Treatment
If the presence of ileus is suspected, the patient is hospitalized. Carrying out a pre-medical examination, using laxatives, enemas, administering painkillers or gastric lavage is strictly prohibited, as this can worsen your health.
If the absence of peritonitis is confirmed, decompression of the gastrointestinal tract is performed using aspiration of the contents of the stomach and intestines through a thin nasogastric tube with a siphon enema.
When the patient experiences severe pain, antispasmodics are administered:
- Atropine;
- Platyfillin;
- Drotaverine.
In case of intestinal paresis, organ motility is restored by taking Neostigmine. To restore the water-salt balance, saline solutions are administered.
If the patient's condition does not stabilize, this indicates the presence of mechanical obstruction requiring surgical procedures. Surgery is necessary to relieve the obstruction with resection of the dead portion of the intestine (small or large). During the operation, it may be necessary to untwist the intestinal loops and dissect adhesions.
For tumors, hemicolectomy with temporary colostomy is used. For malignant neoplasms, a bypass anastomosis can be performed, and if the pathology is complicated by peritonitis, a transversostomy is performed.
After all procedures, antibacterial, detoxification treatment is prescribed with correction of the balance of electrolytes and proteins, stimulation of intestinal motility.
During the rehabilitation period, the patient adheres to a special diet: products must contain fiber and provide a laxative effect. Avoid eating foods that increase gas formation or cause constipation.
Ileus and intestinal obstruction treatment
In most cases, postoperative ileus resolves spontaneously. This means that it goes away on its own, often without treatment. Therefore, most clinicians may initially take a watchful waiting approach with supportive measures in place to prevent complications.
In most cases, postoperative ileus resolves spontaneously
Some of the recommended measures include:
- Intravenous (IV) fluids
- Enteral nutrition through a nasogastric tube.
- You should stop taking medications that may cause intestinal obstruction, but only under medical supervision.
- NSAIDs (non-steroidal anti-inflammatory drugs) can be used to replace opioid analgesics for post-operative pain.
- Chewing gum can stimulate gastrointestinal motility.
- Walking after surgery (ambulance) may be helpful, but this has not been clinically proven.
Treatment for intestinal obstruction depends on the cause. Most of the above measures are relevant for postoperative intestinal obstruction. Other causative conditions need to be treated accordingly.
Post Views: 487
Recommendations
- thefreedictionary.com>Ileus Quoting:
- Dorland's Medical Dictionary for Healthcare Consumers. 2007
American Heritage Medical Dictionary Copyright 2007
- Mosby's Medical Dictionary, 8th edition. 2009
- Saunders' Complete Veterinary Dictionary, 3rd ed. 2007
- Merriam-Webster Medical Dictionary. Retrieved November 9, 2010
- Townsend CM, Beauchamp RD, Evers BM, Mattox KL (2004). "Biological foundations of modern surgical practice." Sabiston Textbook of Surgery
(17th ed.). Elsevier Saunders. - Livingston EH, Passaro EP (January 1990). "Postoperative intestinal obstruction." Dig the ground.
Dis. The science .
35
(1): 121–32. Doi:10.1007/bf01537233. PMID 2403907. - Feldman M., Friedman L.S., Brandt L.J., Sleizenger M.H. (2006). "Intestinal Obstruction and Ileus". Diseases of the Gastrointestinal Tract and Liver by Sleizenger and Fordtran
(8th ed.). Elsevier Saunders. - "Intestinal obstruction Ileus." www.baermed.ch
. Retrieved 2019-10-05. - Kitabchi A.E., Umpierres G.E., Murphy M.B. and others (January 2001). "Management of hyperglycemic crises in patients with diabetes." Diabetes care
.
24
(1): 131–53. Doi:10.2337/diacare.24.1.131. PMID 11194218. - McClave S.A., Martindale R.G., Vanek V.V. et al. (2009). “Recommendations for the provision and evaluation of nutritional maintenance therapy for critically ill adult patients: Society of Critical Care Medicine (SCCM) and American Society of Parenteral and Enteral Nutrition (ASPEN).” JPEN J Parenter Enteral Nutr
.
33
(3):277–316. Doi:10.1177/0148607109335234. PMID 19398613. - Fitzgerald JE, Ahmed I (December 2009). "Systematic review and meta-analysis of chewing gum therapy in reducing postoperative paralytic ileus after gastrointestinal surgery." World J Surg
.
33
(12):2557–66. doi:10.1007/s00268-009-0104-5. PMID 19763686. - ^ a b c
Larson, Erica.
"Equine Postoperative Ileus Insights." www.thehorse.com
. Horse. Retrieved July 4, 2014.
Meconium ileus. Clinical and genetic characteristics and outcomes of meconium ileus in CF.
03Sep 2022 by Eustace 1 Comment
Source: https://www.krasotaimedicina.ru/diseases/children/meconium-ileus
Adhesive disease: prevention and treatment. St. Petersburg, 2013 Filenko B.P., Zemlyanoy V.P., Borsak I.I., Ivanov A.S. The thoughts presented in the monograph are of interest, and the presented results of experimental and clinical research, illustrated by a large number of practical cases, convince of the feasibility of the proposed measures for the prevention and treatment of adhesive disease in the abdominal cavity.
ADHESION-DISEASE-PREVENTION-AND-TREATMENT
Meconium ileus is one of the clinical forms of cystic fibrosis, characterized by obstruction of the ileum against the background of its obstruction by compacted meconium masses. It is manifested by the absence of passage of original feces, vomiting with an admixture of bile and feces, bloating, dilation of the veins of the anterior abdominal wall, intoxication syndrome and exicosis. Diagnosis of cystic fibrosis involves visualization of intestinal obstruction using radiography and laboratory confirmation of the pathology. Treatment involves softening and evacuating the meconium through colonic lavage or surgery.
General information Meconium ileus is a pathological condition in pediatrics and neonatology that occurs against the background of cystic fibrosis and is manifested by obstruction of the distal parts of the small intestine with compacted original feces. The incidence is about 1:2000, affecting 10-20% of children with cystic fibrosis. This pathology is the cause of more than 30% of all intestinal obstructions in newborns. The clinical picture of meconium ileus was first described in 1905 by Landsteiner. Despite the possibility of complete elimination of intestinal obstruction in most patients (approximately 85% of cases), complete cure cannot be achieved, since cystic fibrosis is an incurable genetically determined pathology. Mortality in children under one year of age is more than 50%. The cause of death is most often chronic respiratory diseases.
Causes of meconium ileus Meconium ileus is one of the clinical forms of the autosomal recessive disease - cystic fibrosis. This pathology is caused by a mutation in the long arm gene of chromosome VII, which encodes the protein CFTR, a cystic fibrosis transmembrane conductance regulator. Meconium ileus itself develops against the background of the absence of the pancreatic enzyme, trypsin. A deficiency of this hydrolase causes excessive viscosity of the physiological secretion of the gastrointestinal tract, which is why the secretion “sticks” to the mucous membranes.
As a result, against the background of the accumulation of a large number of meconium masses in the distal part of the small intestine (ileum - ileum) even in the womb, obstruction occurs. In addition, other pathological changes in this part of the digestive tract are observed: pronounced atrophy of the mucous membrane, expansion of the lumen of the excretory ducts of the mucous glands, and enlargement of the lymphatic slits. The intestinal loops located distal to the site of obstruction are completely or almost completely deprived of original feces and decrease in size. This phenomenon is called microcolon.
Symptoms of meconium ileus The clinical picture of meconium ileus develops in the first 24-48 hours after birth. Normally, a newborn’s first-born stool passes on the first, and rarely on the second, day. With this form of cystic fibrosis, stool does not pass. After about 1-2 days, the child’s general condition worsens - he becomes restless, sleep and appetite are disturbed, and unreasonable crying occurs. On the 2nd day (sometimes from the moment of birth), regurgitation appears, frequent vomiting of gastric contents, often mixed with bile or feces. After some time, pallor and decreased skin turgor, dilation of the veins of the anterior abdominal wall and bloating occur. The child gradually becomes apathetic and inactive. Intoxication and dehydration syndromes are associated: increased body temperature, severe thirst, dry skin, decreased blood pressure, increased RR and heart rate, etc. Intercurrent diseases are often associated - pneumonia, bronchitis and others.
In approximately 50% of cases, meconium ileus is the cause of atresia, perforation, or incomplete rotation of the intestine. This is due to the inversion of stretched and weakened loops, the formation of a pseudovalve and impaired blood supply to the walls of the digestive canal. With prolonged ischemia, intestinal infarction occurs, followed by closure of the lumen (atresia) or perforation of the intestine, the development of meconium peritonitis or pseudocyst.
Diagnosis of meconium ileus Diagnosis of meconium ileus consists of collecting anamnestic data, physical, laboratory and instrumental examination of the child. When collecting anamnesis, the pediatrician pays attention to the presence or absence of meconium discharge and the dynamics of the development of the disease. Cases of cystic fibrosis in parents or other relatives must be established.
An objective examination of a child with meconium ileus reveals mixed shortness of breath, tachycardia, tachypnea and increased percussion sound over the abdominal cavity. Peristaltic sounds are not detected by auscultation. By palpation it is often possible to detect dilated loops of the proximal parts of the small intestine that have a dough-like consistency. A rectal examination can confirm increased tone of the anal sphincter and microcolons.
To confirm intestinal obstruction, contrast-enhanced radiography is used. This study indicates multiple distensions of the small intestinal loops, and occasionally the presence of a horizontal fluid level. The diagnostic criterion for meconium ileus is the symptom of “soap bubbles” in the right iliac region, caused by the mixing of meconium masses with air bubbles. In the presence of peritonitis, areas of calcification are found throughout the abdominal cavity - from the space under the domes of the diaphragm to the pelvic cavity.
Carrying out irrigography for a child can reveal a decrease in the size of the distal parts of the intestine - microcolons. Ultrasound diagnostics is widely used, which can indicate pathological changes in the intestine even in the antenatal period. Laboratory tests are aimed at confirming the diagnosis of cystic fibrosis. These include a BM test using the first meconium sample collected, a pilocarpine sweat test for chloride concentration, and DNA testing. Differential diagnosis of meconium ileus includes Hirschsprung's disease, intestinal atresia, meconium plug syndrome and functional spasm syndrome of the descending colon.
Treatment of meconium ileus Treatment tactics for meconium ileus depend on the severity of the child’s condition, the presence or absence of complications. In uncomplicated cases of the disease, intestinal lavage is performed with a hyperosmolar solution (cystografin). The high osmotic pressure of the solution allows fluid to be “pulled” into the intestinal lumen from surrounding tissues, thereby liquefying thick meconium masses and facilitating their passage. Washing is carried out no more than 2-3 times.
If the child’s general condition is severe, complications develop, or there is no effect from intestinal lavage with a hypertonic solution, surgical intervention is performed by midline laparotomy. After opening the abdominal cavity, to simplify the manipulation of compacted meconium, cystografin is injected into the lumen of the ileum with a syringe. Next, using an elastic catheter inserted into the appendix, the softened secretion is evacuated through the anus. If this manipulation is ineffective, opening the intestinal lumen and mechanical removal of meconium masses with subsequent restoration of the integrity of the digestive tract is indicated. In some cases, a proximal stoma is formed, bringing the intestine to the anterior abdominal wall. If necessary, carry out symptomatic treatment.
Prognosis and prevention of meconium ileus The prognosis for the recovery of children with meconium ileus is unfavorable. Without treatment, death occurs in the first 3-7 days of a child's life. With effective elimination of intestinal obstruction, the survival rate is about 85%. Further prognosis depends on the course of cystic fibrosis and associated intercurrent diseases, the adequacy and effectiveness of therapy. About 50% of children die at an early age from severe infectious diseases.
Specific prevention of meconium ileus and cystic fibrosis has not been developed. Nonspecific preventive measures include medical and genetic counseling of married couples, pregnancy planning, regular visits to antenatal clinics, undergoing all necessary studies during gestation, and examination of patients with suspected cystic fibrosis.
Research Article. Clinical and genetic characteristics and outcomes of meconium ileus in cystic fibrosis E.I. Kondratyeva, V.D. Sherman, E.L. Amelina, A.Yu.Voronkova, S.A. Krasovsky, N.Yu. Kashirskaya, N.V. Petrova, A.V. Chernyak, N.I. Kapranov, V.S. Nikonova, L.A. Shabalova 1. Federal State Budgetary Institution "Medical Genetic Research FMBA of Russia", Moscow, Russia
The purpose of the study is to study the prevalence of meconium ileus in the Russian population of patients with cystic fibrosis, its clinical and genetic characteristics and outcomes based on an analysis of data from the 2014 register of the Russian Federation. We studied the characteristics of the disease in 142 patients with cystic fibrosis who had meconium ileus at birth from the 2014 register of the Russian Federation, which included data from 2131 patients. Thus, the proportion of patients with cystic fibrosis who suffered meconium ileus at birth was 6.6%. In the group of children in the first year of life, meconium ileus was diagnosed in 22.1% of patients, which reflects its real prevalence. In the group of children aged 1 to 7 years, 10.7% of patients had a history of ileus, 5.6% of patients aged 7 to 18 years, and 1.5% of patients over 18 years of age. The age of diagnosis of cystic fibrosis in patients with ileus was 5 times less compared to the group of patients without ileus: 0.76±2.01 years versus 3.72±6.16 years, (p<0.0001). The level of sweat chlorides in the group with meconium ileus was significantly higher, and the body mass index was lower than in the group without ileus. Electrolyte disturbances, aspergillosis, and liver cirrhosis were more common in patients who had meconium ileus. The homozygous state for the F508del mutation (class II) and the “severe” class I mutation CFTRdele2 were more often recorded in the group with meconium ileus. “Mild” mutations were more common in patients without meconium ileus. Survival and age at death were lower in patients with a history of meconium ileus. All newborns with meconium ileus should be evaluated for cystic fibrosis.
Meconium ileus is diagnosed in 15–20% of newborns with cystic fibrosis. If in European countries patients with meconium ileus are subject to mandatory examination for cystic fibrosis, then in the Russian Federation this approach has not received proper distribution, despite methodological recommendations. With the introduction of mass screening of newborns since 2006 and the creation of the national register of the Russian Federation, it became possible to assess the prevalence of meconium ileus at birth and its outcomes. It was initially thought that chloride channel dysfunction in cystic fibrosis primarily affected pancreatic function; It is now known that they also influence bowel function, determining the development of meconium ileus. This condition in some cases is diagnosed by ultrasound examination of the fetus in utero and manifests itself in the first days after birth, requiring emergency therapeutic or surgical interventions. Research into the effect of meconium ileus at birth on the course of cystic fibrosis continues to attract interest. The purpose of the study was to study the prevalence of meconium ileus in the Russian population of patients with cystic fibrosis, its clinical and genetic characteristics and outcomes based on an analysis of data from the 2014 Russian Federation register.
Characteristics of children and research methods The study included data from children and adults entered into the national register in 2014. In total, the 2014 register included data from 2131 patients: 1847 patients from 30 regions with cystic fibrosis centers and 284 patients from 44 regions of Russia in which centers cystic fibrosis are missing or information from them is partially presented. The 2014 register included 141 patients with a history of meconium ileus. The study was retrospective in nature. The age of the patients ranged from 1 month to 65 years. The average age in 2014 was 12.8±9.7 years, the median age was 10.2 years. The register included 1509 children under 18 years of age (up to 1 year - 78, from 1 year to 7 years - 708, from 7 to 18 years - 723) and 622 adults (from 18 to 25 years - 338, from 25 to 32 years - 216 , over 32 years old – 68). The following data were assessed: age at diagnosis, sweat chloride levels during a sweat test. A sweat test carried out using the classical Gibson–Cook method was considered positive when the readings were > 60 mmol/l, borderline – 40–60 mmol/l, negative – when < 40 mmol/l; indicators when the test was carried out using the express method (devices "Nanodact" and "Marodact") were: > 80, 60–80 and < 60 mmol/l, respectively. Clinical data are presented in accordance with the requirements of the European Cystic Fibrosis Society Patient Registry.
The microbial inflammatory process in the bronchopulmonary system was studied based on an analysis of the frequency of occurrence of Staphylococcus aureus, Pseudomonas aeruginosa, Burkholderia cepacia and non-tuberculous mycobacteria. Pulmonary function analysis was carried out in a group of children aged 6 to 18 years (n=817), taking into account their ability to conduct the specified study (spirometry). The state of lung function was analyzed according to forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1). The studies were conducted in accordance with the criteria of the European Cystic Fibrosis Registry. In all age groups, the microbial landscape of the respiratory tract and complications of cystic fibrosis (cystic fibrosis-dependent diabetes mellitus, cirrhosis of the liver, nasal polyps, hemoptysis and pulmonary hemorrhages, episodes of pneumothorax) were determined. The nutritional status of patients with cystic fibrosis was assessed using body mass index (BMI) according to Quetelet (weight (kg) / height (m)2). In the genetic study of CFTR gene mutations, the multiplex amplification method was used to identify insertion/deletion mutations; to register point mutations, the allele-specific ligation method followed by amplification was used. A number of patients had their nucleotide sequence determined using direct automatic sequencing on an Applied Biosystems device according to the manufacturer’s protocol. Statistical data processing was carried out using the Statistica v.10 application package (StatSoft, Inc., USA). Depending on the type of distribution, the measures of central tendency and dispersion were the mean (M) ± standard deviation (SD) or median (Me) (interquartile range). When comparing means or medians, the Student t test or Mann–Whitney test was used. Survival analysis was performed using the Kaplan–Meier curve. The log-rank test was used to assess differences in survival between groups. Differences were considered significant at p<0.05.
Results Clinical, laboratory and genetic analysis was carried out in children and adults who had meconium ileus at birth, according to the Russian Federation register for 2014. The number of patients with meconium ileus in the Russian Federation register in 2014 was 141 (6.6% of the total number of patients cystic fibrosis in the registry), including 117 (5.49%) who received surgical treatment, 24 (1.13%) were not operated on. There was no meconium ileus in 1971 (92.5%) patients; Accurate data were missing in 19 (0.89%) patients. In the 2014 register, in patients with cystic fibrosis in the first year of life, 17 (22.1%) of 60 children were diagnosed with meconium ileus. Among 628 children aged 1 to 7 years, meconium ileus was present in 75 (10.7%), among 680 patients aged from 7 to 18 years - in 40 (5.6%), among 603 patients over 18 years - only in 9 (1.5%). Analysis of the results of clinical and laboratory studies was carried out in stages: in the general group of patients (children and adults), in the group of children, and then in different age groups. The age of diagnosis in patients with cystic fibrosis (children and adults) with meconium ileus was 5 times less compared to the group of patients without meconium ileus and 0.76±2.01 years versus 3.72±6.16 years, p<0. 0001 (Table 1). The level of sweat chlorides during a sweat test in the group with meconium ileus was significantly higher than in the group without it (see Table 1). BMI in the general group was significantly higher in patients without meconium ileus. At the same time, lung function (FEV1) was better with meconium ileus. In the group of patients without meconium ileus, 122 mutations in the CFTR gene were registered, and in the group with meconium ileus - only 25 mutations. In the group of patients with meconium ileus, the following mutations were determined: F508del - in 59% of cases, CFTRdele2.3 - in 9.4%, G542X - in 2.7%, W1282X - in 2.2%, 394delTT - in 1.8% , N1303K – 1.3%, 3821delT – 1.3%, 2143delT – 0.9%, S1196X – 0.9%. Moreover, 16 mutations (2184insA, 1677delTA, R553X, 3849+10kbC>T, E92K, L1335P, L138ins, R1158X, 712-1G->T, R1066C, S466X-R1070Q, Dup ex 6b-10, 1898+2T->C , 583delC, W496X, R851X) occurred less frequently than in 0.45% of cases. No differences were found in the frequency of the F508del allele between the group with and without meconium ileus. However, homozygotes for the F508del mutation were more often registered in childhood with meconium ileus: in 44 (39.3%) of 112 patients versus 352 (29.2%) of 1203 without meconium ileus, p = 0.034. A similar situation was in the general group of patients (see Table 1). Noteworthy is the high frequency of CFTRdele2, 3 (the so-called “Slavic” mutation, which is classified as “severe”) in sick children with meconium ileus - 10% versus 5.69% in patients without meconium ileus. The most common mutations in patients with meconium ileus were G542X (2.50% versus 1.08%) and 3821delT (1.25% versus 0.37%). All children with meconium ileus had chronic insufficiency of the exocrine function of the pancreas and were characterized by a lower frequency of “mild” mutations, which belong to classes IV, V mutations of the cystic fibrosis gene and are characterized by normal or slightly impaired pancreatic function. In these patients, “mild” mutations were recorded with a frequency of 4.44% versus 23.85% in the group of patients without meconium ileus (p=0.00192). Mutations 2184insA, 1677delTA, R553X, 3849+10kbC>T, E92K were encountered once and with a frequency less than 0.42% in patients with meconium ileus. At the same time, these mutations in patients without meconium ileus were observed with a frequency of 1.02 to 2.8%. In childhood, in the general group of children, differences were recorded in relation to the average age of patients and the age of diagnosis. Sweat test scores were higher in children with meconium ileus (Table 2, as in the general group of patients, see Table 1).
The clinical and microbiological status of patients in different age groups was assessed to clarify differences that may appear with age. The frequency of diagnosis by neonatal screening in groups under 7 years of age did not differ and was about 35%. Children born in 2014 and after 7 years of neonatal screening (from 2007 to 2013, group from 1 to 7 years of age) did not differ in the main indicators, except for the age of diagnosis (0.19±0.29 years versus 0.52±0.85 years; p=0.0011). In other groups, the differences increased. In the group from 7 to 18 years (during this period neonatal screening for cystic fibrosis was not carried out), differences were noted in the age of diagnosis (1.47 ± 3.06 years versus 2.89 ± 3.42 years; p = 0.0101), in body weight (28.8±9.8 kg vs. 33.7±12.9 kg; p=0.0203) and body length (134.4±17.8 cm vs. 140.9±17.5 cm; p=0.0269).
Reliable information has been obtained that patients with meconium ileus are prone to the formation of liver cirrhosis, allergic bronchopulmonary aspergillosis, and electrolyte disturbances (pseudo-Bartter syndrome). Electrolyte disturbances were observed in 11 (7.97%) of 138 patients with meconium ileus versus 66 (3.54%) of 1916 without meconium ileus; p=0.0091. Allergic bronchopulmonary aspergillosis was present in 3 (2.16%) patients out of 139 with meconium ileus and in 26 (1.38%) out of 1887 patients without meconium ileus. In the group of adults with a history of meconium ileus, allergic bronchopulmonary aspergillosis was diagnosed in 1 (11.1%) versus 3 (0.99%) of 302 adults without meconium ileus. Moreover, in the general group of children (from birth to 18 years), it was diagnosed in 14 (1.05%) out of 1332. With regard to microbial agents of the respiratory tract, no differences were registered in the observation groups. Liver cirrhosis in the general group of patients with ileus (children and adults) was diagnosed in 7 (5%) patients in the group of children without meconium ileus - in 42 (3.15%) out of 994, and in the group of children with meconium ileus - in 4 (3.05%) out of 131.
In the group of adult patients with meconium ileus (n=9), 3 (33.3%) patients had cirrhosis with portal hypertension. In the group of adults without meconium ileus (n=570), it occurred in 29 (5.09%) patients with portal hypertension (p=0.0002, Mann–Whitney test; data are presented for patients who had complete information about cirrhosis and meconium ileus). Thus, differences appeared only in adults. In the group of patients with meconium ileus in 2014, 4 (2.84%) children died (average age 1.44±1.59 years); 137 patients (mean age 6.54±5.66 years) are alive (data as of December 31, 2014). In the group without meconium ileus, 34 (1.73%) patients died (mean age 16.83±9.69 years), 1937 patients are alive (mean age 13.09±9.69 years). Using survival analysis and log-rank test, significant differences were identified in the groups: the survival rate of patients with meconium ileus was significantly lower compared to patients without meconium ileus (p = 0.0462).
Discussion The prevalence and health status of patients with meconium ileus in cystic fibrosis continues to be debated by researchers. According to the Federal Register (2014), the incidence of meconium ileus in patients with cystic fibrosis is 6.6%. The prevalence of meconium ileus in children under 1 year of age was 22.1%, and according to medical history in the group of adults – 1.5%. According to other registries (USA, Italy, Germany), the prevalence varies from 13 to 21%.
The prevalence of meconium ileus in the first year of life reflects the real situation. The study showed the positive role of neonatal screening in the diagnosis of meconium ileus in the country and maintaining the health status of patients with cystic fibrosis in the first years of life, which is confirmed by other studies. With age, the number of patients who have had meconium ileus in the neonatal period decreases, which can be associated with the lack of neonatal screening before 2006, late diagnosis of the disease, and the death of children from electrolyte disturbances and other complications of cystic fibrosis. This is indicated by the age of death of patients with meconium ileus - the first three years of life. At older ages, the cause of death may include cirrhosis of the liver. According to the literature, children who have had meconium ileus have low body weight and length. According to the study, BMI in patients with a history of meconium ileus was lower in the general group of children and adults. At the age of up to 7 years, no differences in height and weight were observed, but in the age group from 7 to 18 years, these indicators were lower in children with a history of ileus. The cause of death in adult patients was probably liver cirrhosis or a more aggressive course of the disease, characteristic of “severe” genotypes.
Similar information about the condition of the liver in children with meconium ileus was presented in the study. The data obtained on the high frequency of registration of patients with meconium ileus homozygous for the F508del mutation and the low frequency of “mild” mutations are consistent with the results of previous studies. We found a high frequency of CFTR del 2, 3 and G542X mutations, which is confirmed in the literature.
Conclusion Thus, patients who suffered meconium ileus at birth continue to remain a difficult category of patients, despite the developed and successful therapy for cystic fibrosis with early diagnosis of the disease through neonatal screening and increased awareness of doctors. The identified features dictate the need to continue to inform doctors about the high risk of cystic fibrosis with meconium ileus, the need for more careful monitoring of electrolyte balance in this category of patients, measures to prevent a decrease in nutritional status, early diagnosis of liver cirrhosis and allergic bronchopulmonary aspergillosis. Further in-depth and extended prolonged observations of this category of patients are required.
0
Author of the publication
offline for 2 months
Eustace
15
Comments: 7Publications: 399Registration: 01-07-2019
Related posts:
- The book Breath of Salt: A Deadly Genetic...
- Nutrition system for cystic fibrosis
- For nasal polyps
- In the treatment of laryngitis
- Antibacterial therapy when detected in sputum...
- Antibacterial therapy for MRSA infections
- Measures to prevent cross infection in CF
- The effectiveness of long-term antibacterial…
Basic information
Paralytic ileus (ICD-10 assigns code K56.0 to this disease) is not an independent pathology. As a rule, this syndrome develops against the background of other diseases. Therefore, it is more fair to consider intestinal obstruction a symptom of a particular illness. If we talk about how critical this condition is, then more than 3% of people actually experienced quite serious complications that could lead to death.
However, as a rule, a person quickly determines that certain negative changes are occurring in his body and turns to a surgeon. By and large, paralytic ileus is a syndrome that is caused by a huge number of reasons. There are also different forms of this pathology.
To get rid of the disease, you need to promptly consult a specialist and undergo a course of treatment.
Features of nutrition after surgery
If the patient has undergone surgery, he must remain in bed for several days afterward. For the first 12 hours, the patient should not drink or eat anything. During this period of time, he receives food through a special tube.
After the doctor allows solid food, the patient must adhere to the diet. He is forbidden to overeat and drink more than 2 liters of liquid per day. You should not eat foods that increase gas formation.
You need to give up sweets, legumes, cabbage, and carbonated water. It is prohibited to consume alcohol, fatty, spicy, fried foods. All dishes must be warm. The diet is based on juices, decoctions, jellies, slimy porridges and weakly concentrated meat and chicken broth.