In the treatment of rectal fistulas, doctors at the proctology department of the Yusupov Hospital use the most modern techniques to guarantee a speedy and painless recovery for clinic patients who have undergone excision of a rectal fistula. The postoperative period after excision of a rectal fistula is supervised by the best specialists (proctologists, rehabilitation specialists) at the Yusupov Hospital, who provide round-the-clock medical support, attention and care to each patient.
What it is?
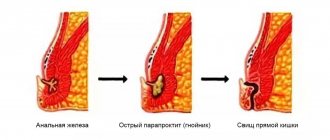
A rectal fistula is a tubular passage, the openings of which extend into the intestinal lumen and the skin of the buttocks.
Fistulas cause the patient a lot of discomfort:
- constant purulent discharge leaves marks on underwear;
- the patient suffers from frequent inflammation and suppuration;
- in severe cases, gross cicatricial changes occur in the anal area, severe pain;
- the functioning of the muscular apparatus of the rectum is disrupted;
- the risk of malignant tumors in the perianal area increases.
Benefits of rectal extirpation
- The technique allows you to remove foci of the malignant process to the maximum extent;
- In patients with stage III malignant tumors, the five-year survival rate can be increased;
- During the operation, blood loss is excluded;
- The use of modern equipment allows the operation to be carried out as carefully as possible;
- If it is possible to perform a sphincter-sparing operation, then the natural functioning of the intestines is preserved, and the stoma is formed only for the time necessary for healing.
Treatment
Complete relief from rectal fistula is guaranteed only by surgical intervention; conservative therapy in this case is absolutely ineffective.
The proctology department of the Yusupov Hospital uses laser welding of fistula tracts and their obliteration using special adhesives. Other progressive methods for eliminating fistulous tracts, which involve the absence of incisions in the rectal muscles, have been successfully used.
In addition, if necessary, surgical interventions are used to treat rectal fistula, during which the fistula tract is excised and primary sphincter repair is performed. Carrying out such operations is advisable in severe forms of pathology with a large number of branching fistulas and cavities along the fistula tracts. Radio wave surgery, electrical welding of tissues, laser coagulation, etc. can be used.
Thanks to an individual approach, complete recovery is achieved in the shortest possible time, minimal discomfort and preservation of patients’ ability to work.
results
Four patients were excluded from the study for various reasons: Hartmann's operation (2), a generalized process identified intraoperatively (1), acute cerebrovascular accident in the early postoperative period (1). As a result, the treatment results of 90 patients were analyzed. At the stage of surgical intervention, 7 out of 30 patients in the J-OR group underwent conversion to the IVC anastomosis and 1 - to the LHC anastomosis; only 22 (24.4%) had J-ORs formed. In the BV group, BV anastomoses were formed in 30 (33.3%) patients. The reasons for conversion were a narrow pelvis, obesity, and insufficient intestinal length. Patients who underwent anastomotic conversion were automatically transferred to the appropriate study groups. Accordingly, in the CVC group, 38 (42.2%) NPRs were performed with the formation of a CVC anastomosis.
The distribution of patients in groups by age and sex, body mass index (BMI), stage of the disease, tumor location and type of treatment is presented in Table. 1 .
Table 1. Characteristics of patients in groups
| Parameter | J-tank (n=22) | Side to end anastomosis (n=30) | End to end anastomosis (n=38) | p |
| Age (median) | 57 | 60 | 58 | 0,362 |
| Gender: male | 6 (27,3%)* | 18 (60,0%)* | 20 (52,6%) | <0,05* |
| BMI (median), kg/m2 | 24,9 | 26,5 | 27,2 | 0,101 |
| Distance from the anus, cm | 7,0 | 7,0 | 7,5 | 0,391 |
| T1 | 1 (4,5%) | 1 (3,3%) | 0 | >0,05 |
| T2 | 4 (18,2%) | 4 (13,3%) | 2 (5,2%) | >0,05 |
| T3 | 13 (59,1%) | 17 (56,7%) | 20 (52,6%) | >0,05 |
| T4 | 4 (18,2%) | 8 (26,7%) | 16 (42,1%) | >0,05 |
| N+ | 8(36,4%) | 8 (26,7%) | 13 (34,2%) | 0,742 |
| Neoadjuvant chemotherapy | 6 (19,3%) | 13 (43,3%) | 13 (34,3%) | |
| Prolonged chemoradiotherapy | 11 (50,0%) | 14 (46,7%) | 18 (47,3%) | >0,05 |
High ligation of the inferior mesenteric artery at its origin from the aorta was more often performed in the group where the creation of a J-OR was assumed. The groups were comparable in other characteristics (Table 2) .
Table 2. Characteristics of surgical interventions
| Parameter | J-tank (n=22) | Side to end anastomosis (n=30) | End to end anastomosis (n=38) | p |
| Minimally invasive total mesorectumectomy | 17 (77,3%) | 26 (86,6%) | 26 (68,5%) | 0,210 |
| Operation time, min | 185 | 230 | 210 | 0,144 |
| Volume of blood loss, ml | 75 | 50 | 125 | 0,201 |
| Ileostomy | 11 (50,0%) | 22 (73,4%) | 21 (53,8%) | |
| Transversostomy | 11 (50,0%) | 8(26,6%) | 16 (46,1%) | 0,422 |
| High dressing a. mesenterica inf. | 20 (91,0%) | 20 (66,6%) | 22 (57,9%) | 0,074 |
| Mobilization of the splenic flexure | 20 (91,0%) | 7 (23,3%) | 9 (23,7%) | <0,001 |
| Length of hospital stay after surgery, bed days | 8 | 8 | 8 | 0,401 |
There were no significant differences in the frequency of postoperative complications in the groups, although a tendency towards a higher number was observed in the group of patients with anastomosis of the CVK (J-OR - 13.6%, BVK - 16.7%, CVK - 34.2%, p = 0.705 ). At the same time, the frequency of anastomotic suture failure was noted in 1 (4.5%) observation in the J-OR group, in 1 (3.3%) observation in the group with BV anastomosis and in 3 (7.9%) observations in the group with anastomosis KVK.
The initial indicators of anorectal manometry immediately before planning surgical treatment in patients of all study groups did not have significant differences, as well as the number of points on the Wexner scale (0 points in the J-OR group and 1 point each in the groups with anastomoses of the BVC and CVC (p>0 ,05).From the moment of surgical treatment to the stage of reconstructive surgery/closure of the preventive intestinal stoma, a persistent tendency towards a decrease in the tone of the external sphincter was observed, however, already in the interval from 6 to 12 months after the closure of the preventive stoma, positive dynamics were observed (Fig. 1) .Comparative general and intergroup analysis of the average contraction pressure in the anal canal revealed that their values before the closure of the stoma and up to 12 months after the closure of the stoma were greater than before the operation.The highest indicators of the average contraction pressure in all groups were noted before the closure of the preventive stoma and 3 months after this (Fig. 2) .
Rice. 1. Indicators of average resting pressure in the anal canal, mm Hg.
Rice. 2. Indicators of average pressure in the anal canal during contraction, mm Hg.
Before reconstructive surgery and 3 months after it, a decrease in maximum pressure in the anal canal at rest was observed in each study group (Fig. 3) . A comparative intergroup assessment of digital indicators of maximum pressure in the anal canal during volitional contraction revealed a similar tendency towards a decrease in values in the interval from the moment of rectal failure to closure of the preventive stoma. However, in the next 6-12 months there was a tendency towards a statistically significant improvement in these indicators (p<0.0001) (Fig. 4) .
Rice. 3. Maximum pressure in the anal canal at rest, mm Hg.
Rice. 4. Maximum pressure in the anal canal during contraction, mm Hg.
In the time interval after NPR of the rectum up to 3 months after closure of the preventive intestinal stoma, a statistically significant decrease in the duration of contraction of the anal sphincter was observed in all groups. And already from 3 to 12 months after closure of the stoma, there was an improvement in muscle endurance, which means an increase in the duration of voluntary contraction of the external sphincter (Fig. 5) .
Rice. 5. Duration of contraction of the anal sphincter (endurance), pp.
The threshold value of the sensitivity of the reconstructed rectum in the groups of patients with anastomoses of the CVC and BVC did not have statistically significant differences (p>0.05) during the period of 3-12 months of observation. The highest threshold of neorectal sensitivity was observed in the group with J-OR (p<0.0001) (Fig. 6) . The largest volume of the first urge to defecate was recorded in the group of patients with J-OP without a consistent tendency to change throughout the year (Fig. 7) .
Rice. 6. Threshold of rectal sensitivity, cm3.
Rice. 7. First urge to defecate, cm3.
Indicators of the volume that causes a constant urge to defecate in patients against the background of inflation of the balloon cuff in the lumen of the reconstructed intestine in J-OR have a statistically significant superiority of these structures in the ability to accumulate and retain intestinal contents compared to other types of anastomoses in a period of 3-12 months, respectively. (p<0.0001: J-OR vs. BVK, J-OR vs. BVK) (Fig .
Rice. 8. Constant urge to defecate, cm3.
The highest value of the maximum tolerated volume of the reconstructed rectum was also observed in patients with J-OP at all time intervals from 3 to 12 months after closure of the preventive intestinal stoma (Fig. 9) .
Rice. 9. Maximum tolerated volume, cm3.
Before treatment, the Wexner score did not exceed 2 (0-2) points, which indicated normal values in the majority of patients who retained all components of stool and gases (Table 3) .
Table 3. Wexner scale score, points
| Assessment deadline | J-tank* (n=22) | Side to end anastomosis** (n=30) | End to end anastomosis*** (n=38) | p |
| Before surgery | 0 (0—4) | 1 (0—4) | 1 (0—4) | >0,05 |
| 3 months after surgery | 5 (4—8) | 6 (4—10) | 10 (5—12) | *0,011*** *0,044** **0,015*** |
| 6 months after surgery | 3 (3—6) | 5 (3—6) | 6 (3—9) | *0,01*** *0,02** **0,042*** |
| 12 months after surgery | 0 (0—2) | 1 (0—3) | 1 (0—4) | >0,05 |
At 3, 6 and 12 months after closure of the preventive intestinal stoma, the best scores on the Wexner scale were observed in patients in the J-OR group.
Patients with LVC anastomosis had better results compared to patients with LVC anastomosis at 3 and 6 months, but by 12 months the indicators of all groups were equal (Fig. 10) .
Rice. 10. Wexner scale score, points.
After 12 months after closure of the preventive intestinal stoma, the frequency of stool in patients with J-OP was significantly less frequent compared to patients with anastomosis of the BVC and CVC - 1.3, 1.6 and 1.8 times per day, respectively (Table 4) .
Table 4. Average stool frequency at 3, 6 and 12 months after closure of a preventive intestinal stoma
| Number of bowel movements per day after surgery | J-tank* (n=22) | Side to end anastomosis** (n=30) | End to end anastomosis*** (n=38) | p |
| After 3 months | 2,4 | 4,2 | 5,2 | *<0,0001*** **<0,0001*** *<0,0001** |
| After 6 months | 2,1 | 3,2 | 3,5 | <0,0001 **0,135*** <0,0001 |
| After 12 months | 1,3 | 1,6 | 1,8 | *<0,003*** **0,16*** *0,05** |
However, along with a decrease in stool frequency, in the J-OR group there was a problem with emptying the reservoir structure, the presence of fragmented multi-stage stool (in one visit to the restroom, requiring a long stay in the toilet), complaints of constipation, requiring the use of laxatives and the use of microenemas. In an intergroup pairwise comparison at 3 and 6 months after stoma closure, microenemas were used significantly more often in the group with J-OR (in 9 patients after 3 months and 13 patients after 6 months) than in the group with CVC anastomosis (7 and 8 observations, respectively ) and the group with LVC anastomosis (7 and 10 observations, respectively) (J-OR vs LVC anastomosis: p=0.002 and p=0.007; LVC anastomosis vs LVC anastomosis: p=0.823 and p=0.021; LVC anastomosis vs J-OR: p=0.005 and p=0.013).
Rehabilitation after surgery
The duration of rehabilitation and the course of the recovery period depend on which surgical technique was used to remove the rectal fistula.
After laser coagulation
Laser coagulation is the most modern method of treating the disease. During this procedure, the lumen is cleared of pus and epithelium using special instruments, after which a laser fiber is inserted into it. Thanks to laser radiation, the fistula tract is sealed and all microorganisms in it are destroyed.
For laser coagulation, regional anesthesia is used, so there is no pain. Recovery after excision of a rectal fistula involves the use of rectal suppositories, which accelerate regenerative processes. As a rule, the patient is discharged from the hospital 7-10 days after surgery.
After excision with obturation surgery
During this surgical intervention, the fistula tract is removed along its entire length. The operation is considered low-traumatic, yet quite effective even in the most difficult cases. To carry out the intervention, spinal or general anesthesia is used, so there is no pain at all.
After the operation, the patient remains in the hospital for four to five days; after discharge, he must observe a “protective regime” for 2-3 weeks.
This surgical intervention is used to treat patients with “superficially” located fistula tracts. Spinal anesthesia is used to perform the operation. The postoperative period after excision of a rectal fistula is almost painless. The hospitalization period ranges from two to three days.
After excision with primary sphincterplasty
Previously, such traditional surgical intervention was used in cases where the characteristics of the fistula tracts prevented the use of laser and other new techniques.
During the operation, the fistula tract is eliminated along its entire length, then layer-by-layer suturing of the wound is performed from the skin and rectum.
This surgical procedure is performed under spinal or general anesthesia, making it absolutely painless. For pain relief during the postoperative period, patients are prescribed non-narcotic analgesics.
The length of hospitalization ranges from three to seven days.
Rectal cancer. Postoperative prevention of disorders
Surgical treatment of rectal cancer at the beginning of the development of this problem was associated with ensuring the safety of surgical interventions and combating intraoperative complications, then with an increase in the radicality of operations and expanding the area of lymph node dissection. For the first time, radical surgery for rectal cancer—abdominal-perineal extirpation—was performed by W. Miles. The essence of the operation was the case removal of the rectum itself. In domestic surgery, priority in the development and implementation of anterior (transperitoneal) rectal resection in clinical practice belongs to B.A. Petrov, who first performed this MTR in 1937.
Dear patients! Please pay attention to the following site materials:
|
New opportunities for increasing the proportion of SSO appeared with the introduction into surgical practice of the latest models of circular staplers in combination with a clamp for applying a purse-string suture and monofilament threads on straight bendable needles. All this simplified the performance of rectal resections that were extremely low from the anocutaneous line. To this were added special studies on the features of the intraintestinal spread of the cancer field in the distal direction from the visible edge of the tumor. It turned out that it is enough to retreat 1.5 - 2 cm from the lower border of the palpable part of the cancer tumor so that the resection line falls along the healthy intestine, unaffected by tumor cells. Not only low, but also ultra-low rectal resections began to be performed. There have been reports from highly qualified and specialized medical and scientific institutions about transsphincteric resections of PC.
Prevention of postoperative colorectal disorders in rectal cancer
In recent years, in order to prevent postoperative colorectal disorders, operations to form a colonic reservoir during rectal resections have become widespread. The starting points of this proposal are obvious - to create, instead of the storage and evacuation functions lost after resection of the rectum, a semblance of them, using a reservoir from the colon. There is quite a lot of work in this regard. In this case, many years of experience in the formation of small intestinal reservoirs during total proctocolectomy were used. Over time, it became obvious that purely mechanically replacing the lost functions of the rectum could not be solved so easily, and an assessment of the results of operations with the formation of colonic reservoirs, reports of changes in the surgical technique and new proposals followed. The term "neorectum" began to be used less and less.
Initially, they tried to create a reservoir that was quite voluminous, 7.5-10 cm. As the functional results of PC resections with the creation of colonic reservoirs were studied, it became obvious that the best data were revealed with smaller reservoirs - 5 cm. Thus, Jin-Ichi Hida et all. (1996) conducted randomized studies with the formation of reservoirs of 10 and 5 cm (length). The first group (10 cm) - 20 people, the second group (5 cm) - also 20 people. The results were assessed after 1 year. The evacuation function with a 5 cm reservoir was significantly higher than with the “neorectum” 10 cm. Sphincter function was the same in both groups. Assessing function across a number of parameters, the authors strongly favor the creation of a 5 cm colonic pouch. They write: “The 5 cm J-reservoir provides adequate pouch function without compromising evacuation.” After 3 years, the authors returned to this issue and confidently came to the conclusion that the observed difficulties with the evacuation of intestinal contents after operations are definitely related to the size of the reservoir - the larger it is, the more often the difficulties are observed.
The need to form a colonic reservoir arises with low rectal resections. True, the concept of “low resection” is defined differently and every centimeter matters here. Usually we are talking about cancer of the mid-ampullary rectum with the determination of the lower limit of resection. T.S. Odaryuk et al. (2000) write that this is on average 1.5 cm from the anorectal line. The authors determined the need for a reservoir due to the development of “Low anterior resection syndrome” after direct anal anastomoses.
Jin-ichi Hida et all (1998) come to the conclusion that when resection of the rectum above 4 cm from the dentate line, a direct coloanal anastomosis should be performed, and below 4 cm, a J-reservoir should be formed.
To assess colorectal function in patients after rectal resections with the formation of a colonic reservoir, a survey method (questionnaire) and objective studies of anorectal function at different times after surgery are used.
Along with the unconditionally positive assessment of rectal resection for cancer with the formation of a colonic reservoir, a number of authors draw attention to the disadvantages of such an operation. G.I. Vorobiev et al. rightly note. (2000) that “to this day, instability and unpredictability of functional results remain, especially in the long term after this intervention (operation with reservoirs).” Thus, in 15-20% of patients it is not possible to achieve the required continuity.
In connection with the identified negative aspects of J-reservoirs, the literature began to pay attention to the peculiarities of the formation of the colonic reservoir itself. G.A. Pokrovsky et al. (1998) instead of a J-shaped one, an E-shaped reservoir was created, but the number of such operations is small - only five operated patients. GAMauer et all (1999) express the opinion that the short colonic reservoir is more physiological and is closer to the “neorectum”. In an experiment on pigs, they formed several types of reservoirs, and the most acceptable was a simple design with transverse coloplasty, which creates something like a rectal ampulla. They anastomosed it to the rest of the anal canal. R. Ruppert & D. Staimmer (1999), knowing these experimental studies on pigs and seeing the shortcomings of J-reservoirs, used coloplasty in 31 patients. Functional results were noticeably better.
An interesting variant of SSO was proposed by V.V. Yanova et al. with low PC resections, when the tumor is located no higher than 3 cm from the dentate line of the anal canal. Since 1989, these authors have been making two longitudinal incisions in the lowered intestine and then stitching them crosswise, which creates an S-shaped bend and increases the volume of the intestine. The function of the finkter apparatus was considered good and satisfactory in 60.5% of patients: 5-year survival - 69%. The authors' experience is the treatment of 700 patients with RCC.
The search for the best options for the formation of colonic reservoirs and the assessment of coloanal function in such cases continues. Improving coloplastic operations in this regard will probably take a decisive place.
Tendency to lymphotropism in rectal cancer
Due to the fact that rectal cancer, according to research, has a tendency to lymphotropism, lymphadenectomy in the surgical treatment of rectal cancer is given great importance. Lymphogenic metastasis of rectal cancer is one of the likely causes of locoregional relapse after surgery. Therefore, along with removal of the primary tumor, the goal of surgical treatment is radical removal of areas of regional tumor metastasis.
Back in 1908, W. Miles identified three main routes of lymphatic metastasis in rectal cancer - ascending, descending and lateral, which served as the beginning of the development of the principles of lymph node dissection for neoplastic formations of the colon.
- Ascending metastasis covers the pararectal, upper rectal and lower mesenteric lymph nodes.
- Lateral metastasis affects the middle rectal, obturator, internal iliac and common iliac nodes.
- Descending metastasis - inguinal lymph nodes.
Perineural invasion
In the presence of tumor invasion into the nervous tissue, metastases in the lymph nodes are detected in 81%, and without it - in 37%.
Primary rectal cancer is characterized by the fact that the tumor remains localized for a long time and grows relatively slowly compared to other tumors of the digestive tract. Localized and slow growth is also characteristic of metastases to the lymph nodes. Based on this biological feature of rectal cancer, it has been suggested that by increasing the volume of lymphadenectomy, a decrease in the number of relapses can be achieved.
Extended lymphadenectomy was first described by Kuru in 1942. However, study results did not show a statistically significant difference in 5-year survival between the groups of patients who underwent standard and extended lymphadenectomy. Interest in extended lymphadenectomy reappeared in the 1980s when Heald et al. They suggested performing a total mesorectumectomy, i.e. removal of a set of tissues and organs located within the fascial membrane of the rectum, including adirectal tissue, blood and lymphatic vessels. Using this technique, these authors were able to reduce the rate of local recurrence to 5%, which, however, other researchers could not reproduce, but interest in extended lymphadenectomy arose again.
Takahashi T. et al. proposed to distinguish several options for lymphadenectomy: limited, standard and extended. However, such a division is not generally accepted.
- Limited lymphadenectomy corresponds to total mesorectumectomy.
- Standard lymphadenectomy is limited + high ligation of the inferior mesenteric artery at the aorta and removal of lymph nodes along the iliac vessels.
- Extended lymphadenectomy - standard + lymph node dissection in the obturator space.
According to Japanese surgeons, limited lymphadenectomy is indicated for the early stages of rectal cancer, standard - for advanced cancer of the upper parts of the rectum, extended - for advanced cancer of the lower parts of the rectum.
Data on the effect of extended lymphadenectomy on 5-year survival of patients with Dukes stage B and C cancer are very contradictory. Further multicenter studies are needed.
Disadvantages of lymphadenectomy for rectal cancer
The main disadvantage of lymphadenectomy is the disturbance of urinary and sexual functions, which occur in 30% and 80 - 100% of cases, respectively. Such disorders can be associated with various reasons, but undoubtedly they occur when the autonomic nerves of the pelvis are crossed. While clarifying the functional results of SSO performed for RPC, we also paid attention to the frequency of genitourinary dysfunction in these patients. This was the reason for conducting our own research to clarify the structural features and location of the autonomic nerves of the pelvis.
It would seem obvious that in order to obtain better functional results after low resection of the pelvis, care must be taken to preserve the autonomic innervation of the pelvic organs, but there are very few special studies in this regard. Yasutomi Masayuki et all are probably right. (1995), who write that in previous years all attention was focused on increasing the radicalization of operations. To this we must add that there are no, and we were specifically interested in, specific methods for identifying nervous structures (coloring, luminescence, etc.).
The problem is the preservation of innervation during resection of the pelvis, and a number of surgeons call for careful treatment of the nervous structures of the pelvis. In this regard, it is necessary to note a certain persistence and consistency of research by Japanese surgeons.
Preservation of the autonomic nerves of the pelvis during operations in patients with PC is realistic in the initial forms of cancer at Dukes stages A and B. The question of the possibility and feasibility of preserving the hypogastric nerves in more common forms of tumor lesions of the PC, without neglecting oncological interests, is ultimately unclear and controversial.
Original research by T.Kono et all. (1998), who conducted a study in rats on transplantation of pelvic nerves after their preliminary excision. After 6 weeks, they already recorded rhythmic contractions of the bladder in all experimental subjects. The authors make a bold conclusion that pelvic nerve transplantation successfully restores bladder function and therefore such an operation can be recommended in practice.
Yasutomi M. et al. during low resections of PC in 185 patients with early forms of cancer, they always tried to preserve bundles of autonomic nerves. Urinary dysfunction after surgery decreased to 15% (previously - 33%), sexual potency decreased to 21% (previously - 81%), 5-year survival rate - 81%.
Sugihara Kenichi et al., preserving nerve bundles on both sides during rectal resections, noted the ability to voluntarily urinate in 93.5%, and sexual potency in 70.4%. In early rectal cancer, the authors note, the nerves should be preserved completely, and if tumor metastases are detected in the pelvis, one should strive to preserve the nerves on at least one side. The 5-year survival rate for Dukes stages A was 96.4%, B was 84.0%, and C was 67.3%. Ishikura S. et al. (1999) operated on 50 patients with low PKC, preserving the pelvic nerve bundles. The average 3-year survival rate was 88%. At stages I-II - 97%, at stage III - 73%. The authors emphasize that preservation of the nerve bundles was not the cause of local recurrence of the cancerous tumor. Matsumoto A. et al. (1995) draw attention to the fact that dissection of the hypogastric nerve bundle on one side does not impair bladder function. Sugihara K. et al. (1996) during the operation of 214 patients with low RPC, the pelvic nerves were preserved. 70.4% of men had preserved sexual function. The 5-year survival rate after such operations was: for stage A - 96.4%, B - 84%, C - 67.3%.
The work of surgeons from the Netherlands is interesting. Surgeons in this country drew attention to the fact that after operations for rectal cancer, a significant number of genitourinary disorders occur, which, of course, affects the quality of life of these patients. In this regard, they invited Japanese surgeons and with their help operated on 47 patients with low-grade cancer of the pelvis, in whom the pelvic nerves were preserved. None of the patients developed urinary disorders. 19 out of 30 men were sexually active. According to their observations, sexual impotence is associated with damage to the hypogastric plexus. To preserve ejaculation, it is necessary to preserve the superior hypogastric plexus. The authors conclude that pelvic nerve-preserving operations should become the surgical standard for the surgical treatment of early forms of rectal cancer.
Domestic authors also report on the possibility of preserving pelvic nerves during rectal resections: G.I. Vorobyov et al., P.V. Tsarkov et al. A.I. Temnikov and Yu.P. Dugin.
We conducted our own study to study some features and options for the location of the superior hypogastric plexus, hypogastric nerves and their branches, with the aim of possibly preserving them during operations for low-grade PC cancer.
The material for the study was 12 embalmed and 5 unfixed corpses of people who died from causes unrelated to pathology of the pelvic organs. The research methodology consisted of general and special anthropometry, dissection, sketching, recording, photography, and video recording.
The superior hypogastric plexus is formed by the fusion of the autonomic nerves located in front of the lower part of the abdominal aorta and, as a rule, its width corresponds to the transverse dimensions of the aorta in the area of its bifurcation. As a result of the studies, mainly two variants of the structure of the superior hypogastric plexus were identified - multi-branched and few-branched forms. The multi-branched form is represented by a nerve plexus in the form of a wide “plate”, having a triangular or trapezoidal shape with the base facing down, formed by a large number of nerve fibers forming a dense network below the aortic bifurcation. In this case, the plexus is located posterior to the retrorectal tissue and rectal vessels. Behind the superior hypogastric plexus is the parietal layer of the pelvic fascia and the left common iliac vein. The lower edge of the plexus was clearly defined when isolated with a tupper and was located below the promontory of the sacrum (along the midsagittal line) by 0.4 - 2.4 cm. The right and left hypogastric nerves depart from the lower edge of the plexus, being a continuation of it. They are located on the posterolateral surface of the pelvis and are directed downward, along and medial to the ureters and internal iliac artery. At the intersection of the ureter with the uterine artery or spermatic duct, these nerves are located so that the uterine artery (spermiferous duct) is in front and the ureter is posterior to the nerves. In this zone, the nerves split primarily into three groups of nerve fibers, forming the inferior hypogastric bilateral plexus. These groups of nerve fibers are directed accordingly: to the bladder, to the uterus (seminal vesicles, prostate), to the rectum. The nerve fibers approach the intestinal wall as part of the lateral ligament of the rectum together with the middle rectal artery and vein, located randomly relative to each other.
The low-branched form of the nerve structure was characterized by the presence of individual, few, relatively thick fibers with single anastomoses between them. The plexus fibers are also located posterior to the retrorectal tissue and superior rectal vessels and are not associated with these formations.
Taking into account the peculiarities of the topographic and anatomical location of the autonomic nerves of the pelvis, we used a nerve-saving operating technique when performing operations on the rectum for cancer of its various localizations.
The indication for surgery in all patients was adenocarcinoma. Most of the neoplasms corresponded to Dukes B stage with a high and moderate degree of tumor differentiation and its localization in the middle and lower ampullary sections. The predominant type of operation was low anterior hardware resection and abdominoperineal extirpation of the rectum. The required stages of the operation in all patients were: high ligation of the inferior mesenteric artery and vein directly at the aorta, excision of mesorectal tissue, determination of the shape of the superior hypogastric plexus, isolation, holding and mobilization of the right and left hypogastric nerves.
After preliminary ligation of the lower mesenteric vessels near the aorta and intersection of the sigmoid colon, we considered it necessary to find out the structural features of the superior hypogastric plexus, and then proceeded to isolate the right and left hypogastric nerves, before the stage of mobilization of the rectum. The reference point for identifying the superior hypogastric plexus is the pelvic promontory, where it is easy to visualize its lower edge. The adipose tissue was moved over the surface of the nerve with a tupper, it was taken onto a “holder” and, preferentially, sharply isolated from the adirectal tissue, moving the “holder” down to the lateral ligament of the rectum. Using this technique, the nerve on the other side was isolated. After this, the hypogastric nerves became mobile and shifted laterally by 5-6 cm. This circumstance is very important, as it makes it possible to excise the mesorectal tissue in the required volume, without compromising the oncological principles of the operation. In most patients, the hypogastric nerves are not distinguished in any way, but merge with the surrounding tissues and appear in the form of a thin and narrow “strip”. It is in such patients that the hypogastric nerves can be easily damaged during mobilization of the rectum or excision of mesorectal tissue. Only in three patients the hypogastric nerves were well contoured, had fairly thick trunks and were easily visualized.
During further mobilization of the rectum, we tried to isolate and visualize its lateral ligaments (which we cross with clamps), so that only those branches of the lower bilateral plexus that are directed to the rectal wall would be included in the instrument, along with the middle rectal vessels. The branches going to other pelvic organs were preserved with the surrounding tissue removed. In cases where the cancerous tumor spread to the surrounding tissues and involved the hypogastric nerve on one side, we neglected it, observing oncological principles of surgery and tried to preserve the integrity of the hypogastric nerve on the other side. We operated on three patients this way.
In cases where the tumor spreads to surrounding tissues involving the autonomic nerves of the pelvis on both sides, it should be removed en bloc along with the nerves.
Urinary function was assessed by asking patients about the nature of the urge, frequency of urination and volume of urine excreted, after removal of the urinary catheter, which was inserted for 2 - 4 days. 94% of patients had free, voluntary urination immediately after removal of the Foley catheter. Patients did not note any differences in the nature of the urge, frequency of urination and volume of urine compared to the preoperative period. All sexually active men had preserved sexual function.
- We presented the current state of the problem associated with functional results after operations on the rectum with cancerous lesions.
- We are not inclined to give categorical recommendations regarding the choice of one single treatment method to reduce the incidence of functional disorders after rectal surgery.
- Knowledge of the literature on this problem is a guarantee for operating surgeons to correctly select the appropriate tactics in each specific case. However, there is no doubt that compliance with oncological principles of surgery must be kept in mind above all. Further, one should always strive to perform SSO, even if this requires intersphincteric resection of the PC. Undoubtedly, surgeons operating on PC need to master the technique of forming colonic reservoirs and coloplasty.
- Taking care of the pelvic nerves is another concern during rectal surgery. This is real, as evidenced by both literature data and our targeted research and the results of surgical interventions.
FAQ
Do I need special preparation for rectal extirpation?
Before surgery, radiotherapy may be prescribed to reduce the size of the tumor and prevent recurrence in the future. It is also possible to prescribe antibacterial drugs. A few days before surgery, foods high in fiber are excluded from the diet. On the eve of the intervention, the intestines should be cleansed using a cleansing enema and laxatives. The day before the procedure, only liquid food is allowed. On the day of surgery, a cleansing enema is performed, the last meal is at least 8 hours before the intervention.
How is abdominoperineal extirpation of the rectum performed?
During the operation, two stages are distinguished: intra-abdominal and perineal; in our Center, the intervention is performed by two teams of surgeons.
At the abdominal stage, a laparotomy is performed, during which the sigmoid colon is cut off 12-15 cm above the upper border of the tumor, its descending segment is slightly sutured to reduce the lumen and brought into the wound, then sutured to the anterior wall of the peritoneum - a colostomy is formed, intended for the removal of feces . Then the rectum is mobilized: the arteries are ligated, the fixing ligaments are cut, after which the wound is sutured.
- At the perineal stage, a circular incision is made in the tissue around the anus, the tissue surrounding the rectum is excised, after which the descending part of the sigmoid colon is removed, and the perineum is sutured at the site of the anus. If cancer cells damage neighboring organs, additional manipulations are required, which affects the duration of the operation, but on average it takes 2-3 hours.
Are there possible complications during rectal extirpation and what are they?
Complications include, first of all, colostomy failure as a result of weakening or rupture of the suture between the skin and the intestine, which causes peritonitis; Treatment is only surgical. If nerves are damaged during surgery, sexual dysfunction, urinary incontinence, and discomfort in the urethral area are possible. Due to shortening of the large intestine, indigestion, bloating, and unpleasant odor of stool may occur; in this case, correction is carried out with the help of diet. In the long term, the appearance of adhesions is possible, which can lead to intestinal obstruction.
Rehabilitation period after rectal extirpation - what are the features?
The patient is allowed to stand up on the day of surgery, but should not sit for a few more days due to the postoperative wound. On the second day, the bandage is removed from the colostomy and the colostomy bag is fixed. Food is allowed to be taken on the second day, but at first only in liquid form. The diet expands gradually, starting from the second week. In the future, you should eat 5-6 times a day in small portions, excluding foods that irritate the intestines. Suture removal is planned for 7-8 days, discharge is possible for 10-14 days. To reduce tension in the abdominal muscles during the postoperative period, it is recommended to wear a special bandage. For the first two years, the patient must undergo examinations every three months, after five years - once a year.
Why is it better to perform rectal extirpation at the Swiss University Hospital?
- At the Coloproctology Center you can undergo all the necessary examinations: from laboratory tests (more than 5,000 parameters) to studies using the most sophisticated modern computer or video endoscopic equipment.
- Patients of the clinic can count on the help of other specialized specialists; we provide medical consultations in more than 15 specializations. Every year more than 5,000 people come to the clinic for consultation, including those with intestinal diseases.
- All surgical interventions performed at the Center in the treatment of patients with rectal cancer are as radical as possible and are not inferior in effectiveness to open operations, but the emphasis is always on nerve-saving and cosmetically effective technologies.
- We have only an individual approach to each person; treatment is prescribed by an experienced team of specialists, based on the characteristics of the individual patient’s body. The clinic employs doctors of the highest category; some of our specialists are known not only in the country, but also in the best European clinics.