The ability to metastasize is one of the main features of malignant tumors, which, in fact, makes them deadly. Cancer cells are able to separate from the primary lesion, penetrate the blood or lymphatic vessels and spread to various parts of the body, giving rise to new lesions.
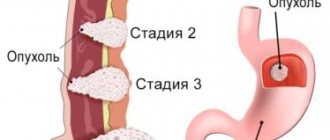
The course of most oncological diseases is usually divided into five main stages, they are designated by the numbers 0, I, II, III, IV. Gastric cancer with metastases is stage 4 cancer. In the TNM classification, the letter M stands for distant metastases. It can take two meanings:
- M0 - there are no distant metastases; in such cases, stage 0, I, II or III gastric cancer will be diagnosed.
- M1 - distant metastases are present. In such cases, stage IV cancer is diagnosed.
How often is gastric cancer with metastases diagnosed?
Since in the early stages the tumor has no symptoms or is disguised as other diseases (for example, gastritis), it is often diagnosed at later stages. In about four out of five patients, the tumor has already spread throughout the body at the time of diagnosis. These are American statistics; in Russia things are no better.
Since the appearance of distant metastases sharply worsens the prognosis, the issue of early diagnosis of gastric cancer is extremely relevant. For this purpose, screening studies are used, in particular gastroscopy . The most successful example of mass screening can be observed in Japan: despite the high prevalence of stomach cancer in this country, the mortality rate from it is lower than in many Western countries. This was achieved due to the fact that in many patients the tumor is detected in the early stages.
Why does stomach cancer metastasize?
Many molecular mechanisms are involved in the process of metastasis; there are still many gaps in current knowledge about them. It is known that malignant stomach tumors develop from special stem cells . Certain subtypes of stem cells make cancer more likely to metastasize.
In general terms, the process of metastasis occurs as follows:
- The primary tumor in the stomach gradually grows and invades surrounding tissue.
- Some cancer cells break away from the primary site and enter the blood or lymphatic vessels.
- With the flow of blood or lymph, tumor cells migrate to other organs, settle in small blood vessels, and penetrate through their walls into the surrounding tissues.
- Such a “journey” is dangerous for cancer cells, many of them die. But, if conditions are favorable, the cancer cell attaches itself to a new location and forms a microscopic secondary focus.
- Metastasized cells can remain inactive for a long time, for years. At a certain point in time, they can begin to actively multiply and secrete substances that stimulate the formation of new blood vessels necessary for the growth of tumor tissue.
Even after the patient has undergone treatment and is in remission, some microscopic metastases may remain in the body. Over time, they can cause a relapse.
How Cancer Spreads
Doctors identify the following ways of tumor metastasis:
- lymphogenous;
- hematogenous;
- implantation
There is also a mixed type of tumor spread, when malignant tissues spread throughout the patient’s body in different ways.
Lymphogenic
Lymph nodes become the first “targets” on the path of cancer spread. Lymph flowing from the stomach accumulates in them. Along with it, altered cells “settle” in the lymph nodes. First, the disease affects the lymph nodes located at the pylorus, then at the thoracic aorta and beyond.
There are two types of lymphogenous metastasis:
- orthograde - along the lymph flow;
- retrograde - against him.
Primary gastric tumors quickly spread throughout the abdominal organs in small “dust” nodules.
These neoplasms have characteristic lymph nodes, the presence of which simplifies diagnosis. The main ones:
- Virchow's metastasis - localized above the left clavicle;
- Schnitzler metastasis - in the pelvic area;
- Irish metastasis - in the axillary lymph nodes;
- Krukenberg metastasis - tumors in the ovaries;
- sister Mary Joseph's metastasis - in the navel.
Schnitzler nodes and Virchow metastases are determined during ultrasound or palpation. Secondary Krukenberg cancer is detected by ultrasound or laparoscopic examination. The affected ovaries greatly increase in size, become denser, and acquire a whitish tint.
Of particular diagnostic value is the detection of foci in regional (closest) lymph nodes.
Hematogenous
With the blood flow, the altered tissues of the primary neoplasm begin to spread when the lymphogenous path has already been passed. Such nodes are often multiple and localized far from the primary tumor. The spread of malignant cells passes through the portal vein, first of all penetrating into the liver. Organ damage occurs in 30-50% of patients. In some cases, they almost completely replace natural liver tissue. The liver significantly increases in size due to multiple nodes, in some patients it reaches a weight of 10 kg.
Secondary lesions are subject to necrosis, decay, and cause intraperitoneal bleeding and peritonitis.
The second largest number of diagnosed cases are metastatic nodes in the lungs. They affect the respiratory system and provoke complications such as miliary carcinomatosis.
With the blood, malignant tissues also enter the pancreas, kidneys, bones, and adrenal glands.
Implantation
Implantation (contact) metastasis occurs when a primary malignant neoplasm grows completely into the gastric wall, spreading to nearby organs.
Doctors find multiple nodes in:
- esophagus;
- pleura;
- liver;
- pericardium;
- pancreas;
- diaphragm;
- spleen;
- intestines;
- gland;
- gallbladder;
- peritoneum.
The colonization of the peritoneum by malignant cells is called carcinomatosis. The condition indicates the final stage of cancer and is accompanied by numerous complications.
So, when a neoplasm grows into the head of the pancreas or the patient’s liver, the node compresses the bile ducts, causing jaundice, ascites, and portal hypertension. Damage to any part of the intestine provokes its obstruction.
Primary neoplasms of the cardiac part of the organ often spread to the esophagus, cause a narrowing of the lumen, and make it difficult to swallow food and water. Contamination of the diaphragm and pleura with malignant tissues provokes various forms of pleurisy.
To which organs does stomach cancer metastasize?
In 2016, a group of scientists from Germany, Sweden and Finland conducted a study, the results of which identified the most common locations of gastric cancer metastases:
- Liver - 48%.
- Peritoneum - 32%.
- Lungs - 15%.
- Bones - 12%.
The location of metastases depends on the type of tumor. Thus, with cancer of the cardia (the junction of the esophagus with the stomach) in men, tumor cells more often spread to the lungs, nervous system and bones. Tumors in other parts of the organ tend to metastasize to the peritoneum. Signet ring cell carcinoma most often metastasizes to the peritoneum, bones and ovaries, and less often to the lungs and liver. Single metastases are usually found in the liver and peritoneum, while metastases in the lungs are often combined with metastases in the liver.
Diagnosis of liver metastases
To identify this pathology, our oncologists use the entire range of modern laboratory and instrumental techniques. Secondary tumor foci can be visualized using:
- ultrasound examination of the liver;
- computer, magnetic resonance and positron emission tomography combined with computed tomography;
- radioisotope scanning, etc.
An important role among specific methods for diagnosing primary liver cancer is the detection of a tumor marker called alpha-fetoprotein (AFP) in the patient’s blood. It is a protein compound that is normally present only in the circulatory system of the fetus and disappears on its own after the birth of the child. The appearance of AFP in the blood of an adult is evidence of the development of a tumor process in the liver.
Final confirmation of the diagnosis of secondary liver cancer is carried out using a biopsy of the tumor focus. It can be performed by incisional, aspiration or puncture method. The doctor chooses the technique, taking into account all the factors present in a particular patient. The biological material obtained during the biopsy is sent to the laboratory, where its histological and cytological typing is carried out under a microscope.
Metastatic liver cancer does not have its own division into stages. In accordance with the international TNM classification, the presence or absence of metastases in distant organs reflects the “M” parameter. And regardless of where exactly the primary tumor originated, the appearance of its distant metastases is classified as “M1” and automatically transfers the disease to stage IV. Therefore, if the primary tumor has already metastasized to the liver, then this is in any case stage IV cancer.
Symptoms
Often, the first symptoms of stomach cancer appear in the later stages, when metastasis has already occurred. Symptoms depend on which organ the cancer cells have spread to:
- In the peritoneum : abdominal pain, abdominal enlargement due to the accumulation of fluid inside ( ascites ), loss of appetite, causeless severe weight loss.
- In the liver: loss of appetite and weight loss, dark urine, enlarged abdomen, jaundice, pain in the upper abdomen on the right (under the right rib), nausea, vomiting, increased sweating.
- In the lungs: chest pain, persistent chronic cough, blood in the sputum, wheezing, shortness of breath, weight loss.
- In the bones: pain, pathological (from slight effort) fractures.
- In the brain: headaches, nausea, vomiting, weakness, numbness in the arms and legs, impaired coordination of movements, personality and behavior disorders, speech, swallowing, urinary and stool incontinence.
All of these symptoms can be caused by other diseases.
Symptoms of liver metastases
Secondary liver tumor may be accompanied by the following manifestations:
- bloating and associated discomfort;
- the appearance of nausea, sometimes with episodes of vomiting;
- dyspeptic symptoms in the form of constipation or diarrhea;
- decreased appetite;
- sudden loss of body weight;
- slight but persistent increase in body temperature;
- the appearance of general malaise and fatigue.
Unfortunately, all these signs of the appearance of metastases in the liver are not specific to oncological pathologies. The lack of specificity means that these manifestations can be easily confused with symptoms of other liver disorders. This complicates the diagnosis of the disease and worsens the prognosis. However, specialists at the Oncology Center always remember that secondary foci in the liver tissue are one of the most common routes of metastasis in many types of malignant pathologies. Therefore, if a patient has already been diagnosed with a tumor in another organ, our oncologists will always evaluate the results of liver examinations with particular care.
Another common symptom of liver metastases is disruption of the gallbladder. As the size of the secondary lesion grows, it begins to gradually compress the bile ducts, increasingly complicating the flow of bile into the intestinal cavity. At this stage, the patient begins to develop obstructive jaundice, which is due to the fact that bile, unable to be naturally excreted from the gallbladder, begins to enter the bloodstream through its walls. It spreads through the bloodstream throughout the body, which leads to a characteristic icteric coloration of the skin, sclera (protein) and mucous membranes of the eye, lightening the stool and giving the urine a dark tint. In addition, the chemical effect of bile acids on the skin causes itching. At the same stage of development of the disease, patients begin to complain of constant aching pain, which is localized in the right hypochondrium and is caused by increased pressure in the gallbladder.
In the later stages of tumor development, a dense, tuberous neoplasm begins to be palpated in the area of the right hypochondrium. During this period, liver metastases already provoke the development of anemia, the appearance of spontaneous bleeding from internal organs and the occurrence of ascites (fluid accumulation in the abdominal cavity). Finally, the entire liver as a whole gradually loses its ability to neutralize various dangerous chemicals, and the patient’s condition worsens due to increasing intoxication of the body.
How are gastric cancer metastases diagnosed?
To search for metastases in stomach cancer, the following diagnostic methods are used:
- Computed tomography is good at detecting bone metastases, but can also show lesions in soft tissue.
- MRI is a safe examination using a magnetic field that helps detect metastases in soft tissues. In this regard, it is more accurate than CT.
- Positron emission tomography is a study during which a special mark is introduced into the body - safe radioactive sugar. Since tumor cells are actively multiplying and need a lot of energy, they accumulate this sugar, making them visible in pictures taken with a special apparatus. There are devices with which you can perform PET and CT simultaneously, this helps to get a more detailed picture.
- Chest X-ray is used to look for metastases in the lungs.
- Sometimes there is a need for diagnostic laparoscopy - a procedure during which the doctor makes a puncture in the wall of the abdominal cavity and inserts an instrument with a video camera ( laparoscope ) inside. This helps to assess the extent of tumor spread and detect secondary lesions in the peritoneum and internal organs.
A biopsy of the metastatic lesion can be performed. When examining the tissue under a microscope, tumor cells characteristic of stomach cancer are found in it. In order to select the optimal treatment, molecular genetic analysis is performed for certain marker substances:
- HER2 is a receptor on the surface of cells that stimulates their proliferation. In cancer, HER2 activity may be increased.
- PD-L1 is a protein that can interact with immune cells and suppress their activity. It belongs to a class of substances called checkpoints .
First stage of pathology
The first stage of cancer development is the presence of a tumor node. At the same time, the lymph nodes are not yet affected and there are no metastases in the body. Recently, the number of detected and treated tumors has increased significantly. This indicates an increase in people's awareness of this issue and their ability to take care of their health. Competent treatment helps in most cases.
Treatment methods
If distant metastases are detected, remission usually becomes impossible. Treatment is palliative in nature, it is aimed at reducing the size and slowing down the growth of the tumor, prolonging the patient’s life, and combating symptoms. But cancer with metastases is not a death sentence. Modern oncologists increasingly talk about it as a temporary chronic disease. Whatever period of time it is possible to extend the patient’s life, this is in any case a small victory.
Surgery
Sometimes it is possible to remove part of the stomach with a tumor - to perform a subtotal resection. If such surgery is not possible and the tumor creates an obstacle to food, one of the following treatment options is possible:
- Bypass surgery : An opening is connected to the upper part of the stomach to the small intestine.
- Stenting : a frame with a mesh wall is installed at the site of narrowing; it helps restore the lumen of the stomach.
- Gastrostomy or jejunostomy : an opening from the stomach or small intestine is brought to the skin, through which food can be introduced.
- Destruction of tumor tissue with a laser using an endoscope inserted through the mouth.
Chemotherapy
For stomach cancer, the following drugs are used: 5-fluorouracil, capecitabine, carboplatin, cisplatin, docetaxel, epirubicin, irinotecan, oxaliplatin, paclitaxel. They are prescribed in different combinations.
Radiation therapy
Radiation helps reduce tumor size, improve food passage, and reduce pain. Modern methods such as three-dimensional conformal radiation therapy and intensively modulated radiation therapy . They use precise calculations that help concentrate radiation in the tumor area, minimally affecting healthy tissue.
Sometimes radiation therapy is combined with chemotherapy. This helps make treatment more effective, but increases the risk of serious side effects.
Targeted therapy and immunotherapy
For gastric cancer with metastases, some targeted drugs and immunotherapy drugs may be prescribed:
- If the tumor is HER2 positive, trastuzumab ( Herceptin ) is given.
- Ramucirumab ( Cyramza ) blocks VEGF, a substance that cancer cells synthesize to stimulate the growth of new blood vessels and provide themselves with oxygen.
- Pembrolizumab ( Keytruda ) is an immunodrug, a PD-L1 checkpoint blocker. It removes the block from immune cells, as a result of which they begin to attack the tumor tissue.
Managing symptoms
In the later stages of cancer, many patients experience severe pain. Adequate pain relief helps improve quality of life. Both non-narcotic and narcotic analgesics are used. With gastric bleeding, anemia develops. If the levels of red blood cells and hemoglobin in the blood decrease significantly, it is necessary to resort to transfusion of red blood cells.
It is important to assess the patient's nutritional status. If the body does not receive the necessary substances, and the problem cannot be solved with diet and gastrostomy, parenteral nutrition is prescribed: nutrient solutions are administered intravenously, bypassing the digestive system.
Maintenance treatment helps to cope with side effects and comfortably endure the course of radiation therapy and chemotherapy.
Treatment of liver metastases
To combat liver metastases in stomach cancer, there are some special methods:
- Intra-arterial chemotherapy , when the chemotherapy drug is injected directly into the vessel feeding the tumor. In this case, you can greatly increase the dosage and achieve a positive effect without fear of serious side effects.
- Chemoembolization . An embolic drug is injected into the hepatic artery in combination with a chemotherapy drug. The embolic drug consists of emboli - microspheres that block the lumen of small vessels and disrupt the supply of oxygen and nutrients to the tumor.
- Radiofrequency ablation . A thin needle-shaped electrode is inserted into the liver node and radio waves are applied to it, which destroy cancer cells. If necessary, the procedure can be repeated.
Treatment of ascites
Ascites, an accumulation of fluid in the abdominal cavity, occurs with stomach cancer as a result of metastatic damage to the liver and peritoneum. You can fight this condition in different ways:
- Limiting fluid and salt intake, diuretics.
- Laparocentesis is a procedure during which a puncture is made in the abdominal wall and excess fluid is removed. peritoneal catheter can be installed to drain the fluid.
- Surgical interventions aimed at preventing fluid accumulation in the abdominal cavity: omentohepatophrenopexy, peritoneovenous shunt, deperitonization of the abdominal walls.
- Intraperitoneal chemotherapy to combat peritoneal metastases.
Despite the global decline in cancer incidence (the current level does not exceed 1/4 of the indicators of the 50s of the 20th century), gastric cancer accounts for about 10% of the total number of malignant neoplasms. In recent years, about 800,000 new cases of stomach cancer have been reported worldwide. In the Russian Federation, stomach cancer ranks fifth in the structure of cancer incidence. Late diagnosis and unsatisfactory treatment results lead to the fact that the mortality rate of patients within a year from the moment of diagnosis remains very high and amounted to 53.3% in 2010 [1].
Despite the fact that surgical treatment of gastric cancer, as well as numerous attempts to use various types of adjuvant interventions in order to increase its effectiveness, goes back more than one century, the problem of successful treatment of this pathology remains very far from being resolved [2]. Randomized trials and meta-analyses, the results of which have been published in recent years, show that the optimal amount of intervention on the stomach and lymphatic collectors now and in the foreseeable future is gastrectomy or subtotal D2 gastrectomy [3-5]. However, the issues of safety and reproducibility of this volume of surgical intervention, as well as the possibility and feasibility of its combination with various options for neoadjuvant and adjuvant therapy continue to be the subject of debate.
The purpose of this work was to analyze the immediate results of treatment, as well as prognostic factors for the development of relapses and metastases in patients with gastric cancer operated on with D2 lymph node dissection at the MRRC over the past 10 years.
Material and methods
From 2002 to 2011, surgical treatment with D2 lymph node dissection was performed on 220 patients, with 114 patients undergoing surgery within the last three years. The ratio of men to women was approximately equal; the age of the patients varied from 20 to 80 years, averaging 60 years. Complications of the underlying disease were identified in 60% of patients; anemia and stenosis of the gastric outlet were predominant. In more than half of the cases there was pronounced clinically significant concomitant pathology of the cardiovascular, bronchopulmonary and endocrine systems. Polyneoplasia was diagnosed in 17 (7.7%) patients, including 7 synchronous ones. Among the second tumors, neoplasms of the urinary system (7 cases) and gastrointestinal tract (6 cases) predominated. For all second tumors, radical treatment was carried out in accordance with their location and morphological structure.
By location, tumors of the lower (73 patients) and middle (65) thirds of the stomach predominated; quite often there was subtotal (49) and total (10) organ damage. In most cases (142 patients - 65%), the tumor was represented by poorly and undifferentiated cancer, including 22% - signet ring cell carcinoma. 46 patients had early stomach cancer (damage to the mucous layer - 20, submucosal layer - 26). Most often, the tumor grew beyond the serous membrane (82 patients). Metastatic lesions of regional lymph nodes were morphologically confirmed in 105 (48%) patients. Distant metastases were detected in 6 patients: damage to the peritoneum - in 3, liver - in 1, ovaries - in 1, para-aortic lymph nodes - in 1. The distribution of patients by stage of the tumor process is presented in Table. 1;
the number of patients with stage I, II and III gastric cancer was almost the same.
Surgical interventions involving gastrectomy were performed in 143 patients, distal subtotal resection - in 74, proximal subtotal resection - in 2 patients. In all cases, the volume of lymph node dissection corresponded to D2. Combined operations were performed in 32 patients, of which splenectomy was performed in 15 patients. The continuity of the gastrointestinal tract after gastrectomy was restored by forming a double-row esophageal-intestinal anastomosis end to side with the interintestinal anastomosis according to Roux; after distal subtotal resection of the stomach - retrocolic gastroenteroanastomosis with interintestinal anastomosis according to Brown; after proximal subtotal resection of the stomach - submersible esophagogastroanastomosis.
The study used the TNM classification (7th edition, 2009) and the Japanese classification of gastric cancer (third English edition, 2011). For statistical processing, the licensed biomedical package Prism 3 (GraphPad Software, Inc., USA) was used. The significance of the differences between the indicators was assessed using Fisher's exact test and the χ2 test; a two-sided test was used. Differences were considered significant at p<0.05.
results
Complications and mortality
In 206 (94%) patients, the postoperative period was smooth. Early postoperative complications occurred in 13 (6%) patients: pneumonia (2 cases), pleurisy (2), thromboembolism of small branches of the pulmonary artery (1), myocardial infarction (1), acute coronary insufficiency (1), pancreatitis (1) , suppuration of a laparotomy wound (1), subcutaneous eventeration (1), adhesive small intestinal obstruction (3). In the last 4 cases (1.8% in relation to all operations), repeated surgical interventions were performed: suturing the wound of the anterior abdominal wall, dissection of adhesions. We consider it necessary to pay attention to the absence of such serious complications as failure of anastomotic sutures and duodenal stump, as well as pancreatic necrosis, which to date, according to various authors, are observed in 2-6% of patients. In the early postoperative period, 1 patient died, the mortality rate was 0.45%. Patient B., 65 years old, diagnosed with gastric antral cancer pT2N2M0G2, died 6 hours after distal subtotal gastrectomy from acute coronary insufficiency (the diagnosis was confirmed by autopsy). Over the past three years, there have been no deaths in 114 surgical interventions with D2 lymph node dissection. It should be noted that the structure of surgical interventions on the stomach has changed significantly: if in 2002-2008. the number of gastrectomies and subtotal resections was almost equal - 56 and 50, then in 2009-2011. this ratio was 88 and 26 (77 and 23%), the difference was statistically significant (p = 0.0002).
Thus, at present, the main surgical intervention for gastric cancer is D2 gastrectomy.
Patterns of lymphogenous metastasis
Metastases in regional lymph nodes were detected in 105 (48%) patients. In early (pT1a-b) gastric cancer, the incidence of lymphogenous metastasis was 13%. Further, it naturally increased with increasing depth of invasion into the stomach wall, exceeding 70% when the tumor grew beyond the serous membrane; the difference in the pN category depending on the pT category is statistically significant (p<0.0001). In general, the frequency of metastases in the lymph nodes increased by 2 times or more with tumor invasion into each subsequent layer of the stomach wall (Table 2).
Metastases in the lymph nodes of the celiac trunk and its branches were detected in 25% of patients, including in every third patient when the tumor grew into the subserous and serous layers. The above-mentioned dynamics of increase in their frequency with increasing depth of tumor invasion into the stomach wall remained, however, there were no significant differences in the germination of the subserous layer and serosa (33 and 37%). However, the difference by pT category was also significant with a high degree of statistical significance (p<0.0001).
Thus, the use of D2 lymph node dissection shows a high frequency of lymphogenous metastasis, including to the second lymphatic drainage collector, especially when the tumor grows beyond the subserous layer, which allows us to consider a smaller volume of intervention as non-radical.
Relapses and metastases
During the observation period, relapses and metastases, as well as their main characteristics (time of development, localization, extent) were established in 37 patients. The main methods for diagnosing relapses and metastases were ultrasound, SCT, MRI, radionuclide studies, endoscopy, laparoscopy, puncture biopsy, relaparotomy, and autopsy. The timing of detection of relapses and metastases varied from 2 to 54 months, with an average of 15.5 months. They were diagnosed within 1 year in 20 patients, from 1 to 3 years in 15 patients, and over 3 years in 2 patients (Table 3).
Isolated relapses: local (intraluminal), as well as regional (in the soft tissues of the celiac trunk area) with morphological confirmation were diagnosed in 1 patient each. The vast majority of patients (35) had distant metastases. Among them, peritoneal carcinomatosis predominated (23 patients), including in combination with ovarian damage (4). Hematogenous metastases developed in 11 patients, with damage to the liver (7), skeletal bones (3), and lungs (1). Only in one case was a combination of peritoneal and hematogenous metastases recorded.
Factor analysis showed that patients who developed peritoneal and hematogenous metastases had significant differences in clinical and morphological parameters. Thus, in the structure of the development of metastases (37 patients), damage to the peritoneum was significantly more common compared to hematogenous metastases with tumor invasion into the serous membrane and surrounding organs (57 and 18%), with total damage to the stomach (22 and 0%), with cricoid cellular and undifferentiated cancer (57 and 18%); all differences are close to the limit of statistical significance (p = 0.055—0.065). At the same time, the frequency of involvement of regional lymph nodes did not differ (65 and 64%); damage to the lymph nodes of the second collector was more common in patients with hematogenous metastases (55 and 35%). However, the difference due to the small number of observations is statistically insignificant (p = 0.45). Also, a very important factor in the development of both types of metastases was subcircular and circular damage to the stomach by the tumor, which occurred in 73-83% of cases.
The only patient with synchronous metastases in the peritoneum, liver and lungs had a combination of the prognostic factors described above: tumor invasion into the serous membrane (pT4a), multiple metastases in regional lymph nodes (pN3a), including three lymph nodes of the left gastric and general hepatic arteries, the presence of moderately differentiated adenocarcinoma with subcircular lesions of the stomach.
Thus, the incidence of isolated local and regional relapses was extremely low. Distant metastases predominate, among which peritoneal carcinomatosis predominates. The combination of clinical and morphological parameters is a reliable factor in predicting the path of metastasis.
Discussion
In recent years, in leading clinics, surgical treatment of gastric cancer with D2 lymph node dissection is accompanied by the following indicators of the early postoperative period: postoperative mortality is 0-7%, postoperative complications develop in 16-29% of patients (including anastomotic suture failure in 0-5 %, abdominal abscess and peritonitis - in 2-6%); relaparotomies are performed in 3-5% of patients [2, 3, 6-8]. In a 2010 population-based study conducted in a number of European countries, the postoperative mortality rate was significantly higher and ranged from 5.2-6.4% (Italy, France, England) to 11-16% (Spain, Holland, Slovenia, Poland ) [10]. In a published analysis [11], the 30-day mortality rate after more than 9000 gastrectomies and gastrectomies in Nordic countries was 3.5–6.9%. Thus, the results we obtained (mortality rate - 0.45%; complication rate - 6%, including the absence of anastomotic suture leakage, abdominal abscesses and pancreatic necrosis; number of relaparotomies - 1.8%) are quite consistent, and in many cases exceed the data of domestic and foreign surgical and oncology centers. Many authors [7–9] emphasize the critical importance of specialization and accumulation of surgical experience in reducing postoperative mortality and complications, as well as performing operations for gastric cancer in multidisciplinary, well-equipped institutions.
Our data on the frequency of lymphatic metastasis when performing D2 lymphadenectomy, taking into account a fairly large number of patients with early stage gastric cancer, are quite consistent with similar indicators in leading domestic and foreign clinics [6, 12, 13]. So, according to M.I. Davydova et al., A.F. Chernousova et al. [13], based on a significant number of our own observations, as well as an analysis of world literature, the frequency of damage to the lymph nodes of the 2nd collector is 23-26% (in our study - 25%). In general, the dynamics of the increase in the frequency of their lesions coincides with the increase in the category of pT. Thus, when a tumor grows into the serous membrane, the frequency of metastatic lesions of the lymph nodes of the celiac trunk and its branches, according to various authors, is 31-42% (in our study - 37%).
Thus, for a more precise diagnosis of the prevalence of lymphogenous metastasis of gastric cancer, the value of using D2 lymph node dissection is beyond doubt. With regard to its therapeutic significance and influence on long-term treatment results, everything is not so clear. This is evidenced by both the ongoing randomized European D1 versus D2 trial [4] and attempts to answer the question of whether adjuvant chemoradiotherapy could be an alternative [14]. It is clear that expanding the scope of lymph node dissection cannot lead to an increase in survival rate in all patients with gastric cancer, especially with a high risk of early implantation metastasis [15]. Moreover, it may promote the dissemination of cancer cells in the abdominal cavity [16].
Studying the patterns of development of gastric cancer recurrence is a key point both in terms of assessing the possibilities of surgical treatment and developing optimal adjuvant therapy regimens. Since surgical intervention, including lymph node dissection, is a method of locoregional intervention, the main indicator of the quality of their implementation is this type of control. We completely share the opinion of V.I. Chissova et al. [12], S.N. Nereda et al. [15] that the development of distant metastases is associated primarily with the clinical and morphological characteristics of the tumor process, and not with the type and extent of surgical treatment. Data regarding the predominant influence of the biological characteristics of signet ring cell and undifferentiated gastric cancer on the patterns of metastasis were obtained at the MRRC and previously published [17, 18].
Of the 220 operated patients and 37 registered cases of tumor progression, only 1 patient had a local relapse and 1 patient had a regional relapse. Distant metastases were detected in 35 patients, of which more than 2/3 were cases of peritoneal carcinomatosis. According to leading domestic and foreign authors, the number of locoregional relapses is currently 16-20% [2, 7, 8, 12]. We attribute the exceptionally low incidence of isolated local and regional relapses in our study to two main reasons: the implementation of an adequate volume of surgical intervention on the stomach and lymph collectors; early manifestation of distant metastases, primarily peritoneal, in patients with locally advanced, low- and undifferentiated gastric cancer. In the latter case, identifying regional relapse is extremely difficult and has no practical significance.
Thus, the main objective of clinical trials conducted throughout the world is to develop optimal neoadjuvant and adjuvant therapy regimens aimed at preventing the development of distant metastases, including those caused by the dissemination of cancer cells during surgery [14, 19—21]. Thus, in a randomized study by T. Kim et al. [20] showed that adjuvant chemoradiotherapy compared with chemotherapy after D2 gastrectomy contributes to a statistically significant improvement in relapse-free survival in patients with stage III gastric cancer. A promising direction is the development of neoadjuvant therapy, the goal of which, in addition to systemic effects, is regression of the primary tumor and regional metastases and achieving an increase in R0 of resections [21].
Conclusion
Currently, the main scope of surgical intervention for gastric cancer is D2 gastrectomy, which in specialized centers can be performed with low rates of postoperative complications and mortality. The use of D2 lymph node dissection shows a high frequency of lymphatic metastasis, including to the second lymphatic drainage collector, especially when the tumor grows beyond the subserous layer. Adequate volume of surgical intervention on the stomach and lymph collectors leads to a low incidence of isolated local and regional relapses. The structure of progression of gastric cancer is dominated by distant metastases, among which peritoneal carcinomatosis predominates. To increase resectability rates, locoregional control and prevent the development of distant metastases, an optimal combination of adjuvant systemic (chemotherapy, targeted therapy) and local (radiation therapy) effects is necessary.
Prognosis for gastric cancer with metastases
The five-year survival rate for stage 4 gastric cancer is 5%. This means that only five out of a hundred patients diagnosed with the disease will still be alive after 5 years.
On average, after 3 months from the moment of diagnosis of gastric cancer with metastases, half of the patients remain alive. The prognosis is worse if the cancer has spread to the bones and liver: with such metastases, half of the patients die within 2 months.
| More information about the treatment of stomach cancer at Euroonco clinics: | |
| Treatment of stomach cancer | |
| Oncologist-gastroenterologist | RUB 5,100 |
| Chemotherapy appointment | RUB 6,900 |
| Emergency oncology care | from 12,100 rub. |
| Radiologist consultation | RUB 11,500 |
Book a consultation 24 hours a day
+7+7+78
Complications of metastatic liver cancer
The most common complication of secondary tumor foci in the liver tissue is the growth of the tumor into the gallbladder and biliary tract, which manifests itself:
- pain in the upper and right parts of the abdomen, which is dull and constant;
- increased formation of gases in the intestines;
- a slight increase in body temperature, which stubbornly remains at the same level;
- appetite disturbances and weight loss;
- nausea and sometimes vomiting that does not bring relief;
- icteric coloration of the skin and whites of the eyes;
- increased sweating, especially at night.