Back to list Previous article Next article
25.06.2013
Tags:
kzhk, microflora
Vote
Synthesis of short chain fatty acids (SCFA)
In the modern understanding of processes in the human body, microflora has already been identified as the most important adaptation system. The main food substrate for normal intestinal flora is dietary fiber, broken down (fermented) by saccharolytic microflora into simple carbohydrates. It is during the hydrolysis of insoluble disaccharides, especially lactitol, that beneficial lacto- and bifidobacteria synthesize SCFAs - short-chain fatty acids. And the whole story with beneficial microflora, in addition to their ability to form biofilms, largely comes down to the role of these metabolites of beneficial bacteria, vital for humans, SCFAs.
In the upper parts of the large intestine, fermentation of food chyme and absorption of amino acids and vitamins synthesized by bacteria, as well as electrolytes and up to 95% of water, occur. In the distal sections, absorption is partial and it is more of a storage organ.
Food components are broken down by a variety of bacterial enzymes - protease and peptidase, polysaccharidase and glycosidase into oligomers (glucose and amino acids), which are further fermented into organic acids, hydrogen, carbon dioxide, methane, water and short-chain fatty acids. When glycoproteins and polysaccharides are broken down by bacterial enzymes - glycosidases, monosaccharides are formed: glucose, galactose, the oxidation of which contributes to the release of heat into the environment in the form of 60% of their free energy.
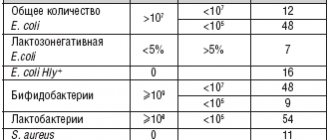
Anaerobic beneficial bacteria hydrolyze simple carbohydrates to form short-chain fatty acids - acetic, propionic and butyric - acetate, propionate, butyrate.
Publications in the media
The gastrointestinal tract (GIT) performs not only a digestive, but also an immune function, in particular, it participates in the implementation of the body’s defense reactions against pathogenic, conditionally pathogenic microorganisms and many inorganic substances.
Local intestinal immunity
About 80% of all immunocompetent cells of the body are localized in the intestinal mucosa; about 25% of the intestinal mucosa consists of immunologically active tissue and cells; Each meter of intestine contains about 1010 lymphocytes [1].
The immunocompetent (lymphoid) tissue of the gastrointestinal tract is represented by organized structures (Peyer's patches, appendix, tonsils, lymph nodes) and individual cellular elements (intraepithelial lymphocytes, plasma cells, macrophages, mast cells, granulocytes). The population of lymphoid tissue cells is heterogeneous and consists of many groups, subgroups and clones of cells with different functional properties and specificity of receptors for antigens [2, 3].
The epithelium of the gastrointestinal tract delimits the tissues of the macroorganism from a huge number of living and non-living antigens - substances that carry signs of foreign genetic information. Oral exposure to an antigen (including microbes and their toxins) usually creates, on the one hand, local “mucosal” IgA protection (secretory immunity) and a cell-mediated reaction, but, on the other hand, systemic tolerance or hyporeactivity - suppression of subsequent the production of antigen-specific antibodies of classes G and M and the development of cell-mediated immunity. In relation to pathogenic and opportunistic microorganisms, the local intestinal immune system must show adequate protective properties, and in relation to normal flora - at least tolerance, and in the best case - actively participate in the processes of adhesion, survival and reproduction of representatives of normal flora.
Specific immune mechanisms are developed by the intestines to protect against potentially dangerous microorganisms throughout life. Undifferentiated lymphocytes, mostly producing secretory IgA or IgM antibodies, are present in the stratum propria or Peyer's patches. Stimulation of B and T lymphocytes in the presence of a foreign antigen occurs following their exit from the mesenteric nodes into the thoracic duct, the bloodstream and return to the intestine, where they also accumulate in the own layer of the mucous membrane. Activated cells produce specific antibodies of the IgA and IgM classes, which are secreted on the surface of the mucous membrane 4–8 days after stimulation. Immunoglobulins form complexes with antigens, neutralize toxins, prevent contact of microorganisms with “target” cells of the macroorganism, and promote the rapid removal of microorganisms from the gastrointestinal tract due to agglutination.
The main function of intestinal antibodies is immune rejection at the mucosal surface. It is known that IgA predominates among immunoglobulins in all secretions and in the intestinal lamina propria. Secretory IgA, which plays the role of the main “cleaner” and immunomodulator of the gastrointestinal mucosa, is retained near epithelial cells as a result of interaction with the glycocalyx, largely due to the presence of normal flora. IgA occupies a favorable position that prevents the absorption of antigens. The two-dimensional IgA molecule can function as an agglutinin, reducing bacterial adherence to enterocytes [4].
In the intestinal mucosa there are also cells that produce immunoglobulins of other classes, but there are much fewer of them. Thus, the ratio of plasma cells producing IgA, IgM, IgG is 20:3:1, respectively [2].
The most important property of the intestinal local immune system is the phenomenon of lymphocyte recycling. Sensitized by antigens (both food and infectious), lymphocytes of Peyer's patches migrate to the mesenteric lymph nodes, and from there through the lymphatic vessels through the thoracic duct and the circulatory system they are sent to the own layer of the intestinal mucosa, mainly as cells secreting IgA. This mechanism ensures the formation of lymphocyte clones and the formation of specific antibodies in areas of the mucous membrane remote from the site of primary sensitization. In the process of sensitization of plasma cells followed by cloning of lymphocytes that produce antibodies with certain properties (similar to those that acted as the matrix), not only native immunoglobulin molecules, but also active Fc- and F(ab')2-fragments are involved.
Cellular immunity of the intestine, in contrast to the system of antibodies secreted by it, has not been studied enough. It is known that systemic cellular immune responses are rarely detected after oral exposure to antigens. Obviously, when healthy people receive harmless antigens (for example, normal flora antigens), cellular immunity reactions do not develop in the intestinal mucosa [2].
The local intestinal immune system works as follows. Microorganisms that enter the intestinal lumen or mucous membranes are recognized by memory immunoglobulins (IgG), after which the information is transmitted to the immunocompetent cells of the mucous membrane, where plasma cells responsible for the synthesis of IgA and IgM are cloned from sensitized lymphocytes. As a result of the protective activity of these immunoglobulins, the mechanisms of immunoreactivity or immunotolerance are activated. The immune system “remembers” normal flora antigens, which is facilitated by genetic factors, as well as class G antibodies transmitted from the mother to the fetus during pregnancy, and immunoglobulins entering the baby’s gastrointestinal tract with breast milk. As a result of lymphocyte recycling and cloning, the immune response covers all mucous membranes of the gastrointestinal tract.
The regulation of immune responses of the intestinal mucosa is a complex process that can change in various situations, such as: the presence or absence of mucosal damage, maintenance of biofilm integrity and functionality, the presence of acute or chronic infections, the maturity of the immune system, the nutritional status and genetic potential of the individual . Changes in immunological reactivity may occur as a result of mucosal damage, although in this situation it is difficult to distinguish between primary and secondary effects.
The role of intestinal microflora in immune reactions
The intestinal microflora protects humans from colonization by exogenous pathogens and inhibits the growth of pathogens already present in the intestine through competition for nutrients and binding sites, as well as the production of certain pathogen growth-inhibiting substances. In addition, bacteria are involved in the implementation of immunological defense mechanisms [5].
It is known that one of the functions of normal flora is immunotropic, which consists in stimulating the synthesis of immunoglobulins, potentiating the mechanisms of nonspecific resistance, systemic and local immunity, properdin, complement, lysozyme, as well as stimulating the maturation of the system of phagocytic mononuclear cells and the intestinal lymphoid apparatus [6, 7]. Normoflora activates not only local intestinal immunity, but also the immune system of the whole organism, which is confirmed in experiments on germ-free animals [8]. The main activities of indigenous (normal) microflora in ensuring a normal immune response: changing the immunogenicity of foreign proteins by proteolysis; decreased secretion of inflammatory mediators in the intestine; decreased intestinal permeability; direction of antigen to Peyer's patches. The same effects are realized in probiotic preparations [5].
In the intestine, bacteria are the most important component of the biofilm: glycocalyx - mucus - IgA - normal flora. Biofilm covers the intestinal mucosa from the inside, occupies all the bulges formed by enterocytes, and protects the mucous membrane from dehydration, physical and chemical aggression, as well as from attacks by microorganisms, bacterial toxins, and parasites [9].
Against the background of a decrease in bifidobacteria and lactobacilli, the permeability of the intestinal epithelial barrier to food macromolecules and the deficiency of secretory IgA increases [10]. In turn, deficiency of secretory IgA can lead to the development of intestinal diseases and frequent sinubronchial infections, and ultimately to a predisposition to atopy and autoimmune diseases [11].
Animal studies have shown that when biocenosis is disrupted in the gastrointestinal tract, autoimmunization to a complex antigen of the intestinal wall develops, and the use of immunobiological drugs prevents this process [12].
Dysbiosis as immune dysfunction
The immune system regulates the balance of the intestinal biocenosis, i.e. the mechanisms of self-regulation of normal flora are controlled by the local intestinal immunity. Since any microorganism is an antigen, there must be mechanisms for the rejection of foreign microorganisms, as well as tolerance and the creation of favorable conditions for normal flora.
It is known that IgG, that is, immunoglobulins that provide immunological memory, is transmitted through the placenta from the mother to the fetus. Antibodies of classes M and A do not pass through the placenta, which explains the lack of protection of the newborn against gram-negative microorganisms (enterobacteria, salmonella) [13]. In addition, it has been proven that the first microorganisms that enter the intestines appear there during and after the birth of a child and attach to certain receptors [14]. The process of specific adhesion of opportunistic and pathogenic microorganisms to the gastrointestinal mucosa can be blocked, among other factors, by the presence of IgA and lysozyme, which, in turn, promote adhesion to the receptors of representatives of bifido- and lactoflora [15].
Confirmation of the role of IgA in preventing colonization of mucous membranes by foreign microorganisms is the fact that 99% of normal flora bacteria are not covered with secretory immunoglobulins. On the contrary, enterobacteria, staphylococci, and other opportunistic and saprophytic microorganisms are completely covered with IgA [8]. This phenomenon is based on the phenomenon of immunological tolerance to normal flora.
In newborns and young children, transient immune deficiency is a biological pattern, mainly related to humoral immunity [13]. Children of this age group, much more often than children older than one year, experience persistent disturbances of the intestinal biocenosis, which is partly due to a deficiency of the immune system.
The physiological insufficiency of the local intestinal immune system in the first three months of a child’s life is compensated by the intake of IgA and other protective factors with human milk. When breastfeeding, a child receives up to 1.5 g of IgA every day. In children who are on artificial or early mixed feeding, i.e., deprived of the protective factors of human milk, food allergies and intestinal dysbiosis are much more likely to be observed, which is noted by most researchers in this area.
The penetration of infectious agents into the mucous membranes of the gastrointestinal tract and other organs causes a response from the local immune system in the form of an increase in the concentration of IgA, which is produced with the participation of normal flora. Accordingly, a situation may arise when a microbiological imbalance of one type will contribute to the aggravation of microecological disorders. Thus, a decrease in the amount of normal flora entails IgA deficiency, resulting in increased colonization of the mucous membranes with opportunistic pathogenic flora (OPF).
Congenital and transient anomalies of the local intestinal immune system reduce the body's resistance not so much to aggressive virulent microorganisms, but to UPF. The stability of intestinal dysbiosis is associated with them [16].
Almost 100% of people with acquired immunodeficiencies (as a result of radiation exposure and other immunosuppressive factors) have disturbances in the composition of the intestinal microflora, while they experience not only an increased increase in the UPF, but also a sharp decrease in the normal flora [8], that is, the protective function is also impaired local immunity, and immunological tolerance, which may indirectly indicate that the local immune system contributes not only to the elimination of foreign microorganisms, but also creates optimal conditions (and not just immunological tolerance) for normal flora.
Considering the significant interaction between the intestinal biocenosis and the local intestinal immune system, it is advisable to consider dysbiosis not only a microbiological, but also an immunological problem, which should be reflected in therapeutic tactics.
Immunocorrection for intestinal dysbiosis
The development of dysbiosis indicates a lack of functioning of the local intestinal immune system. Fully supporting the thesis about the secondary nature of biocenosis disorders (dysbacteriosis is always secondary and causally determined), we can assume that one of the reasons for the development of any dysbacteriosis is immunological dysfunction and, above all, insufficiency of humoral immunity.
In children in the first months of life, transient immune deficiency is a biological pattern, mainly related to humoral immunity. That is why, in children of this age group, persistent and transient disturbances of the intestinal biocenosis without any other visible reasons occur much more often than in children older than one year. Then, the causes of immune dysfunction can be chronic sluggish parasitic or microbial infections, acute intestinal infections, acute respiratory diseases, childhood infections, vaccinations; unfavorable environmental factors; stress; the use of antibiotics, etc. Very often, manifestations of dysbacteriosis are observed some time after the listed effects.
The main remedy for the immunocorrection of dysbacteriosis is a complex immunoglobulin preparation (CIP), developed by employees of the Moscow Research Institute of Experimental Medicine named after. G. N. Gabrichevsky [17]. The material for obtaining CIP is donor plasma from several thousand donors, so we can talk about herd immunity. KIP, unlike normal human immunoglobulin, contains immunoglobulins of three classes: 50% IgG, 25% IgM, 25% IgA. CIP is characterized by an increased content of antibodies to enterobacteria (Shigella, Salmonella, Escherichia, Proteus, Klebsiella, etc.), Pseudomonas aeruginosa, staphylococci, and rotaviruses. Thus, the CIP includes immunoglobulins of 3 classes to the main types of pathogenic and opportunistic flora. Specific antibodies contained in the CIP neutralize the effect of enteropathogenic microorganisms, which is achieved by the presence in the preparation of antibodies of the same specificity, but of different classes, promoting agglutination, neutralization and precipitation of infectious agents.
The drug is a lyophilized mixture in vials. 1 standard dose contains 300 mg of protein and trace amounts of preservatives. When administered orally, CIP is partially broken down in the stomach and duodenum into active components: Fc- and F(ab')2-fragments, which retain the serological and antigen-binding activity of immunoglobulins [18]. These fragments have too large a molecular weight to penetrate into the systemic circulation through the intestinal mucosa, so CIP has mainly a local effect in the lumen, on the mucous membranes and in the layer of the mucous membrane, penetrating into the bloodstream in micro quantities by pinocytosis, etc. The action of CIP occurs throughout the gastrointestinal tract, but especially in the large intestine, where a large amount of lymphoid tissue (Peyer's patches) is concentrated.
To understand the mechanism of action of CIP, one should recall the basic principles of classical immunology [13, 16]. It is known that the most abundant IgG (75%) in the blood serum of any person has the simplest structure among antibodies and is the main carrier of immunological memory. Specific monoclonal immunoglobulins are formed in lymphoid tissue; they are synthesized by lymphocytes that have undergone differentiation due to antigen-sensitized antibodies. Despite the short lifespan of class G immunoglobulins (21–28 days), due to the differentiation of lymphocytes, immunological memory is preserved for quite a long time (often lifelong). Immunoglobulin molecules in all people have a similar structure (for example, IgG to Klebsiella is the same in everyone), and therefore are not perceived by the immune system as foreign proteins. “Foreign” antibodies introduced into the body, having reached the intestinal lymphoid tissue, are included in the formation of immunological memory along with their own, which are produced as a result of contact with the antigen. The phenomenon of lymphocyte recirculation promotes the formation of specific antibodies in areas of the mucous membrane distant from the site of primary sensitization. Therefore, immunoglobulins administered enterally not only perform the function of an immune response in the intestine, but also act as a matrix from which plasma cells with desired properties are cloned. The local intestinal immune system acquires the ability to resist those microorganisms to which antibodies are contained in the CIP. Passive immunization of a child receiving breast milk is carried out similarly through the immunoglobulins contained in it. Thus, immunocorrection with a complex immunoglobulin preparation is physiological. CIP stimulates the mechanisms of development of one's own local humoral immunity, which is especially important for children deprived of mother's milk.
In addition to its effect on intestinal immunity, KIP has a direct antimicrobial effect due to the content of antibodies of classes M and A. These immunoglobulins, by binding to complement, cause lysis of bacteria. Therefore, CIP can be used without the addition of other antibacterial drugs [19].
To correct microbiological disorders, CIP is prescribed in a course of 5–10 days, 1 dose 1 time per day (in the morning 30 minutes before meals). A five-day course is recommended for the following types of dysbiosis:
- dysbiosis with the absence of UPF in the study, compensated;
- dysbacteriosis with the amount of UPF ≤ 50%;
Extended instrumentation courses (ten-day or two five-day courses with an interval of 5 days between them - 5+5 scheme) are shown:
- for any decompensated dysbacteriosis;
- with dysbacteriosis with the amount of UPF > 50%).
In the described situations, prolonged courses turned out to be more effective than the traditional five-day course, which was confirmed by a special study [20].
In addition to CIPs, there are suppository forms in vials, as well as combinations of CIPs with interferon (Kipferon). Kipferon in suppositories has a local effect in the distal parts of the rectum and a general immunostimulating effect due to absorption in the hemorrhoidal plexus of the rectum (inferior vena cava system).
CIP in suppositories is used in children with the following indications: constipation accompanied by the development of rectal fissures; symptoms of colitis; prevention and treatment of respiratory infections in children over 1 year of age; and also together with CIP in vials, used per os, to enhance the immunostimulating effect in children with severely weakened immunity.
The course of treatment for KIP in suppositories is 5–10 days, 1/2–1 suppository once at night, after bowel movement. The child’s well-being improves during treatment or at the end of the course. The effect of using instrumentation in candles is confirmed by laboratory studies.
In addition to correcting dysbiosis, CIP is used in combination with traditional etiotropic and pathogenetic therapy for the treatment of acute intestinal infections of established or unclear etiology, especially in young children [21, 22]. In patients, on days 2–3, intoxication decreases, the frequency of stools decreases, its consistency improves, pathological impurities disappear, and on days 5–6, stool normalization occurs. A study of the intestinal microflora shows the sanitization of the body from the pathogen, while, unlike the use of antibiotics, a decrease in the amount of normal flora is not observed. Suppositories with CIP are indicated for the treatment of acute intestinal infections in a selected group of children (for vomiting, intolerance to oral administration, etc.).
Safety of instrumentation use
TPIs should be used with caution in children with an allergy to protein, a history of reaction to the administration of immunoglobulins, as well as in other situations fraught with the development of adverse reactions during use, and contraindications to the use of immunoglobulins.
The technology for obtaining CIP, including alcohol fractionation of serum with subsequent precipitation of the immunoglobulin fraction with polyethylene glycol, eliminates the possibility of transmission of hepatitis B viruses, HIV and other pathogenic microorganisms with the drug. In addition, donor or placental blood from which plasma is obtained for the preparation of CIP, as well as batches of the finished drug, are carefully checked. Therefore, fears of infection through the use of CPIs are not justified [23].
Clinically significant allergic reactions when taking CIP were observed extremely rarely. In some cases (especially when used together with bacteriophages), a short-term deterioration in well-being and an increase in symptoms that existed before treatment were noted, which is apparently associated with lysis of the UPF. Some children experienced a decrease in appetite while taking CIP, but it always recovered quickly and independently.
The use of CPI in prolonged courses did not increase the incidence of side effects compared to traditional regimens. To be on the safe side, in some cases, antihistamines can be prescribed simultaneously with taking CIP.
Functions of SCFA (short chain fatty acids)
SCFAs are quickly absorbed into the blood and are the main source of energy for the cells of the colon mucosa. They stimulate the growth and renewal of mucosal cells, mucus formation, blood flow in the mucosa, increase the absorption of water and salts, regulate the acid-base balance, and maintain microbial balance. SCFA is the main source of respiratory substrate and acetyl-coenzyme A, necessary for metabolism in mucosal cells, for the synthesis of lipids and the construction of cell membranes, to maintain the integrity of mucosal cells and the regeneration of tissue cells.
The main functions of SCFA (effect on the intestinal mucosa):
- The main source of energy for the intestinal mucosa
- Stimulation of proliferation and differentiation of mucosal cells
- Stimulation of blood flow in the mucosa
- Stimulates mucus production
- Stimulates the absorption of sodium chloride, potassium, magnesium and water
- Decreased pH in the colon
- Maintaining the integrity of the mucosa.
Critical role of Paneth cells in gut health identified
Scientists at Sechenov University analyzed everything that is known about the functions of Paneth cells contained in the mucous membrane of the small intestine. Researchers described how the same cells can fight bacteria, fungi and viruses, regulate the immune response and the growth of stem cells in the journal BioEssays.
The intestinal mucosa is constantly faced with dangerous influences: bacteria that can cause intestinal infections, food antigens, mechanical and chemical damage associated with the digestive process and bacterial metabolism. To protect themselves from them, the mucous membranes have developed an entire defense system, in which Paneth cells play an important role. They are found at the bottom of the crypts - depressions in the intestinal mucosa - and are involved in many processes necessary for the normal functioning of the intestinal microbiota and the fight against pathogenic microorganisms.
Although Paneth cells were first described back in 1872, scientists are still investigating their functions and roles in various processes. Interest in them continues, including because disruption of these cells can lead to intestinal diseases such as Crohn's disease and ulcerative colitis. The authors of the article analyzed dozens of publications over the past few years and summarized everything that is known about these cells.
“As part of this interdisciplinary work, we carried out a unique systematization of data on Paneth cells. These cells are the most important regulators of the intestinal microbiome and the vital activity of local stem cells, which determines their key role in the development of many diseases of the gastrointestinal tract. The most promising result of our work is the streamlining of data on the mechanisms of regulation of stem cells, the health of which is the key to a healthy intestine. Having studied the latest aspects of the functions of Paneth cells, we have built a significant basis for further research and development of innovative methods for treating intestinal diseases,” said one of the authors of the work, Mikhail Sinelnikov, junior researcher at the Laboratory of Bioengineering and Prototyping of Organs and Tissues at Sechenov University.
Paneth cells actively “communicate” with their immediate environment – stem cells. At the bottom of each crypt there are from 5 to 15 Paneth cells, and they are arranged in a strict geometric order - so as to interact with the surrounding stem cells as much as possible (in terms of area and number of contacts). And this is no coincidence: the behavior of stem cells, which in the future will become epithelial cells, is regulated by growth factors - signaling molecules synthesized by Paneth cells. They are necessary for the growth of stem cells, their movement, division and specialization, and therefore the regeneration of the epithelium.
One of the most important functions of Paneth cells is the synthesis of proteins with antimicrobial activity. They accumulate in granules contained in the cytoplasm of cells, and when pathogens appear, they are released into the lumen of the crypts. These include lysozyme, which destroys the cell membrane of microorganisms, α-defensins, which are effective against both bacteria and fungi and viruses, and cathepsin G, which is involved in the digestion of pathogens captured by the cell. Antimicrobial compounds prevent pathogens from multiplying in the crypts and prevent inflammation.
Scientists also know that Paneth cells participate in the work of humoral (based on the activity of chemical compounds, not specialized cells) immunity. So, in the 1970s, researchers discovered that these cells contain antibodies of types A and G (IgA and IgG); subsequently, scientists proved that IgA can be synthesized in Paneth cells, and not just enter them from the outside, for example, when absorbing bacteria . Thus, these cells secrete antibodies into the lumen of the crypts and prevent infection of the mucosal cells.
Finally, Paneth cells can regulate the manifestations of cellular immunity - they secrete substances that attract immune cells (cytokines IL-17 and tumor necrosis factor-alpha TNF-α), and, conversely, suppress inflammation (cathepsin G).
The authors of the study showed that Paneth cells perform several important functions, including those unusual for epithelial cells. They are involved in maintaining a healthy intestinal microbiota, protect the mucous membrane from the effects of pathogens, regulate the growth and division of stem cells in the crypts and the body's immune response. Understanding the role of Paneth cells in these processes is necessary to create new therapies for intestinal diseases.
Information provided by the press service of the First Moscow State Medical University named after. THEM. Sechenov
Photo: https://www.sechenov.ru/pressroom/news/opredelena-reshayushchaya-rol-kletok-paneta-v-zdorove-kishechnika-/
Antibodies to intestinal goblet cells are autoantibodies directed against goblet cell antigens of the small and large intestine, which are a marker of ulcerative colitis.
Synonyms Russian
ABA, antibodies to goblet enterocytes.
English synonyms
Anti-Intestinal goblet cell antibodies, IIFT, goblet-cell antibodies, Anti-Goblet Cell Ab, AGCA, Anti-Enterocyte Antibodies.
Research method
Indirect immunofluorescence reaction.
What biomaterial can be used for research?
Venous blood.
How to properly prepare for research?
Do not smoke for 30 minutes before the test.
General information about the study
Goblet cells (or goblet exocrinocytes) make up 9.5% of all intestinal epithelial cells. They are located singly on the intestinal villi among the bordered enterocytes and are usually not detected at the top of the villi or the bottom of the crypts (tubular recesses of the intestinal lining). The number of goblet cells increases from the small to the large intestine, that is, there are significantly fewer of them in the duodenum than in the rectum. Goblet cells accumulate mucin, which, being the main component of mucus, moisturizes the surface of the mucosa, maintains water-salt balance, promotes the movement of chyme (bolus of food), and participates in parietal digestion. According to the results of recent studies, goblet cells are able to interact with antigen-presenting cells in the wall of the gastrointestinal tract and play an important role in the formation of oral tolerance to food antigens and intestinal microbiota, which is of particular importance in studying the mechanisms of development of autoimmune intestinal pathology and food allergies.
Antibodies to intestinal goblet cells are normally absent in the blood. The autoantigen for them is probably mucin, but this fact has not been definitively established. They are a serological marker of nonspecific ulcerative colitis and may be important in the pathogenesis of this disease.
Nonspecific ulcerative colitis (UC) is a chronic intestinal disease of unknown etiology, which is characterized by ulcerative-inflammatory damage to the mucous membrane of the colon. Typically, the lesion begins in the rectum, spreads to all parts of the colon, and in 18-30% of cases affects the terminal ileum. Inflammatory damage in the intestinal wall coincides with the distribution of goblet cells in the mucous membrane of the digestive tract: UC is not characterized by damage to the proximal parts of the small intestine, duodenum, where there are few goblet cells, but inflammation of the rectum is specific, where the number of goblet cells is maximum.
Antibodies to goblet cells are detected in 30% of patients with ulcerative colitis and are absent in people with Crohn's disease and other intestinal diseases. The specificity of the assay for ulcerative colitis is 100%. Simultaneous determination of antineutrophil cytoplasmic antibodies (ANCA) increases the sensitivity of the test to 70-83%.
What is the research used for?
- For the diagnosis of nonspecific ulcerative colitis;
- for differential diagnosis of chronic inflammatory bowel diseases (Crohn's disease, ulcerative colitis);
- for examining close relatives of patients with chronic inflammatory bowel diseases.
When is the study scheduled?
- For clinical and laboratory signs of chronic inflammatory bowel disease (diarrhea, blood in the stool, weight loss, fever, loss of protein and electrolytes);
- when examining close relatives of a patient with ulcerative colitis.
What do the results mean?
Reference values:
Normally, there are no antibodies to intestinal goblet cells.
Reasons for increased levels of antibodies to intestinal goblet cells:
- nonspecific ulcerative colitis.