Preparing for the study
Feces should be donated before starting antibiotics and chemotherapy drugs (if this is not possible, then no earlier than 12 hours after stopping the drug). The use of candles is not permitted. Before the study, it is necessary to avoid taking laxatives, administering rectal suppositories, oils, limit taking medications that affect intestinal motility (belladonna, pilocarpine, etc.) and the color of stool (iron, bismuth, barium sulfate) for 72 hours before donating stool . Preparatory rules for submitting feces for coprogram: 2 days before collecting the material, give up tomatoes, tomato juice, pasta, beets, blueberries, pomegranates and other vegetables and fruits containing coloring substances; 3 days before stool collection, stop taking antibiotics, drugs that cause changes in intestinal motor function, enzyme-based drugs, avoid taking laxatives, medications, do not use rectal suppositories, oils; for several days before the test, limit your diet to a variety of exotic foods. Meals should consist of vegetables, fruits, cereals, and dairy products. The amount of food should be within normal limits. Avoid fatty, smoked, spicy and pickled foods; if you are taking medications containing iron and bismuth, they must be discontinued 2 days before collecting stool for examination; after radiography with a contrast agent (barium), collect feces for coprogram no earlier than 7–10 days after the study; Women are not recommended to take the test during menstruation.
Indications for use
1. Pancreatitis in chronic or acute form (PC - up to 94%) 2. Cystic fibrosis (PC - 100%); 3.Cholelithiasis (stones in the gall bladder or bile ducts); 4. Diabetes mellitus type I and II (insulin-dependent and non-insulin-dependent); 5. Lactose intolerance (lactose deficiency); 6. Crohn's disease; 7. Malignant processes in the pancreas (PM for pancreatic carcinoma - 61%); 8. Mechanical injuries or recent surgery in the pancreas; 9. Abdominal syndrome (acute pain in the epigastric region) or digestive disorders without established causes; 10. Obstructive jaundice (impaired bile outflow caused by a mechanical obstruction (tumor, calculus) that blocked the lumen of the bile duct); 11. Cystic fibrosis of the pancreas 12. Shwachman-Diamond syndrome
Description
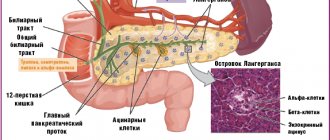
Pancreatic elastase I is a proteolytic enzyme synthesized only in the pancreas and involved in digestion. It is synthesized in the pancreas in the form of a proenzyme - proelastase, which is activated by trypsin. The enzyme is not affected when passing through the intestine, is excreted unchanged in the feces and therefore can reliably reflect the real exocrine capacity of the pancreas. When insufficiency of the exocrine function of the pancreas occurs, the content of pancreatic elastase I in the feces decreases.
Elastase
Elastase pancreatic
(eng.
Elastase
) - a proteolytic enzyme that breaks down peptides and proteins, endopeptidase.
The second name is pancreatopeptidase
E.
To distinguish it from leukocyte elastase, pancreatic elastase is sometimes called elastase 1
. Molecular weight 28,000 daltons. Elastase is synthesized in the pancreas in the form of an inactive form - the proenzyme proelastase, which, as part of pancreatic juice, enters the duodenum, where, under the influence of trypsin, it is converted into elastase.
Elastase, trypsin and chymotrypsin represent a group of serine proteases due to the presence of serine in their active site. They belong to the same family and make up 44% of the total protein of the exocrine pancreas. Elastase has a broader specificity of action than trypsin. Elastase is most active in the cleavage of peptide bonds formed by amino acids with small hydrophobic radicals - glycine, valine, leucine, isoleucine and serine. Elastase is capable of breaking down the protein elastin, which is not hydrolyzed by either trypsin or chymotrypsin.
Elastase can enter the blood in increased quantities during pancreatitis.
Elastase 1 and chymotrypsin remain relatively stable when passing through the gastrointestinal tract. Their determination is easy to perform and relatively inexpensive, but these tests are characterized by low sensitivity for mild and moderate exocrine pancreatic insufficiency and low specificity for certain pathologies of the gastrointestinal tract not related to the pancreas (Maev I.V. et al.).
Stool test for elastase
Elastase is not destroyed when in the intestine, so testing the elastase content in feces is an important indicator of exocrine pancreatic function.
Elastase of animal origin supplied with food does not affect the analysis result. In newborns, the elastase content is low and reaches adult levels by two weeks of age.
A stool test for elastase is mandatory if there is a suspicion of cystic fibrosis. In addition, indications for the study are:
- chronic and acute pancreatitis
- cholelithiasis
- diabetes mellitus types I and II
- lactose intolerance
- pancreas cancer
- pancreatic injury
- Crohn's disease
- obstructive jaundice
- abdominal pain or indigestion of unknown origin
Interpretation of analysis results:
- more than 200 mcg of elastase per 1 g of feces is normal
- from 100 to 200 mcg of elastase per 1 g of feces - mild to moderate severity of exocrine pancreatic insufficiency
- less than 100 mcg of elastase per 1 g of feces - severe form of exocrine pancreatic insufficiency
The elastase test indicators do not depend on age, gender, previous treatment with enzyme preparations and proteolysis inhibitors. Affect the results of the analysis: castor and mineral oils, bismuth, magnesium or antidiarrheal drugs, irrigoscopy.
Publications for healthcare professionals addressing the role of elastase in gastroenterology
- Sablin O.A., Grinevich V.B., Uspensky Yu.P., Ratnikov V.A. Methods for studying exocrine function // In the book. Functional diagnostics in gastroenterology. St. Petersburg, 2002.
- Golubev N.N. Disorders of cavity digestion in patients with peptic ulcer and their correction with the help of polymer preparations. Abstract of dissertation. PhD, 14.00.05 - internal diseases. MGMSU, Moscow, 2008.
- Mokrousova N.V. Connective tissue metabolites in assessing the course and prognosis of gastroesophageal reflux disease. Abstract of dissertation. PhD, 14.00.05 - internal diseases. Saratov, 2002.
- Maev I.V., Kucheryavyi Yu.A., Andreev D.N., Dicheva D.T., Gurtovenko I.Yu., Baeva T.A. Chronic pancreatitis: new approaches to diagnosis and therapy / Educational manual for doctors. M.: FKUZ "GKG Ministry of Internal Affairs of Russia". 2014. 32 p.
- Maev I.V., Samsonov A.A. Chronic duodenitis (Algorithm for diagnosis and treatment tactics) / Manual for general practitioners, therapists, gastroenterologists: Textbook. - M: GOU VUNMTs MH and SR RF, 2007. 80 p.
On the website GastroScan.ru in the “Literature” section there is a subsection “Secretion, digestion in the gastrointestinal tract”, containing articles for healthcare professionals on this topic. Back to section
Important information
- The information on the site is for reference purposes and is not a public offer. For up-to-date information, contact the contractor’s medical center or the STK0LAB call center at: 8-800-505-90-13
- The service catalog indicates the maximum possible period for completing the study. It reflects the time it takes to complete the study in the laboratory and does not include the time for delivery of the biomaterial to the laboratory.
- Please check the deadlines for tests taken at regional medical centers by calling the contact numbers of the centers. To view the list of tests offered, click on the section of the catalog that interests you.
- Please note that collection of biomaterial for research from children under 15 years of age is possible only in the presence of parents or legal representatives. After 15 years of age, the presence of parents or legal representatives is not required.
Patient preparation rules
feces
Standard preparation conditions (unless otherwise determined by the doctor):
14 days in advance Avoid instrumental studies of the gastrointestinal tract or medical procedures that can cause mechanical damage to the mucous membrane (for example, colonoscopy, sigmoidoscopy, bowel cleansing with enemas, etc.).
Notes:
Collect a sufficient amount of stool (1/3 container) after involuntary bowel movement (more than 5 g or ml if the sample is liquid). Feces should not contain foreign impurities. Material for research collected after an enema, taking medications that affect peristalsis, taking castor or vaseline oil, after administering suppositories, or medications that affect the color of stool (iron, bismuth) is not accepted. You should not collect biomaterial during menstruation, in the presence of bleeding hemorrhoids, blood in the urine, or after significant effort during defecation.
You can add this study to your cart on this page
Sample result (PDF)
References
- V.T. Ivashkin, I.V. Maev, A.V. Okhlobystin, Yu.A. Curly, A.S. Trukhmanov, A.A. Sheptulin et al. Recommendations of the Russian Gastroenterological Association for the diagnosis and treatment of chronic pancreatitis. RZHGGK. 2014
- Domínguez-Muñoz JE, D Hardt P, Lerch MM, Löhr MJ. Potential for Screening for Pancreatic Exocrine Insufficiency Using the Fecal Elastase-1 Test. Dig Dis Sci. 2017
- Vanga RR, Tansel A, Sidiq S, El-Serag HB, Othman MO. Diagnostic Performance of Measurement of Fecal Elastase-1 in Detection of Exocrine Pancreatic Insufficiency: Systematic Review and Meta-analysis. ClinGastroenterolHepatol. 2018
Principles of diagnosis and correction of exocrine pancreatic insufficiency
Questions regarding the diagnosis of exocrine pancreatic insufficiency (PIN), assessment of the severity of this condition and methods for its correction quite often arise before pediatricians, therapists, gastroenterologists, and surgeons.
Although congenital diseases with isolated loss of lipase, amylase or protease functions of the pancreas are currently known, in clinical practice we often have to deal with mixed deficiency with a predominance of lipase dysfunction.
Significant exocrine insufficiency of the pancreas with loss of predominantly lipase activity is manifested by undigested frequent, sometimes copious, stools with a characteristic greasy sheen and a peculiar odor. The described clinical picture is typical for such severe diseases as cystic fibrosis, Shwachman-Diamond syndrome and congenital lipase deficiency. At the same time, moderate or minor pancreatic insufficiency, often detected only during a special examination, can accompany many gastroenterological diseases (for example, celiac disease) and be a symptom of chronic pancreatitis.
Cystic fibrosis is an autosomal recessive disease caused by a mutation in the transmembrane chloride channel regulator gene; characterized by damage to almost all exocrine glands, severe course and fatal outcome. The degree of damage to the respiratory system and the severity of exocrine pancreatic insufficiency determine the prognosis of the disease. Rational pancreatic replacement therapy with pancreatic drugs, which is vital for patients with cystic fibrosis, helps to increase the patient’s life expectancy and improve its quality.
Shwachman-Diamond syndrome is a congenital condition that is characterized by pancreatic insufficiency (mainly lipase) against the background of pancreatic hypoplasia, hematological changes, growth retardation, and bone abnormalities. The clinical picture is polymorphic and depends on the predominant syndrome. In the case of dominance of pancreatic insufficiency, the disease is manifested by fatty stools and malnutrition of varying degrees of severity, which requires replacement therapy with highly active drugs of pancreatic enzymes. The prognosis of the disease is determined to a greater extent by the severity of hematological changes, especially neutropenia, and, as a consequence, the frequency of infectious complications.
Congenital lipase deficiency is manifested from birth by frequent, fatty stools and the presence of corresponding laboratory signs. Previously, the difficulty of diagnosing this disease was associated with the need to exclude all other diseases manifested by pancreatic insufficiency. With the advent of the test for pancreatic elastase-1 in stool, solving this problem has become much easier. In the case of adequate correction of impaired pancreatic function with highly active pancreatic enzyme preparations, the prognosis can be considered relatively favorable.
Among the acquired forms of pancreatic insufficiency, one should note pancreatic insufficiency in chronic pancreatitis, as well as pancreatic insufficiency due to resection of the pancreas, for example, with nisidioblastosis or a tumor. In this case, in addition to correcting the exocrine function of the pancreas, replacement therapy with insulin preparations under the supervision of an endocrinologist is also required.
To assess the exocrine function of the pancreas, direct (usually probe) and indirect (probeless) methods are used. Direct methods are associated with the direct determination of enzyme activity in the duodenal contents, and indirect methods are associated with the assessment of the processes of digestion of standard substrates.
The simplest indirect method is scatological research. An increase in the amount of neutral fat, connective tissue, muscle fibers and/or starch indicates a decrease in the exocrine function of the pancreas. The accuracy of this method is influenced by a large number of factors, sometimes not directly related to the exocrine function of the pancreas, in particular the volume of bile secreted into the intestinal lumen, its qualitative composition, the state of intestinal motility, the presence of inflammatory processes in the small intestine, therapy with enzyme preparations, etc. Based on this, the method can be considered only indicative. Nevertheless, a scatological examination is recommended for all patients with gastroenterological pathology at the initial stage of examination.
A more accurate (quantitative) assessment of lipolytic processes in the intestine is provided by a stool lipid profile with determination of the amount of triglycerides in the stool using thin layer chromatography. The method can be recommended to clarify the nature of steatorrhea and to assess the effectiveness of replacement therapy.
An indirect test, the so-called BT-PAVA test, is widely used abroad. The synthetic peptide N-benzoyl-L-tyrosyl-p-aminobenzoic acid (BT-PABA), which is cleaved mainly by chymotrypsin, is used as a substrate. The released para-aminobenzoic acid is absorbed into the blood and excreted by the kidneys. In urine collected over 8 hours, 61% of the ingested para-aminobenzoic acid is normally determined. A decrease in excretion indicates a violation of proteolysis in the small intestine.
The fluorescein dilaurate test is based on the breakdown of the above substrate by pancreatic esterase to free lauric acid and fluorescein, which is absorbed and excreted in the urine. Determination of fluorescein concentration in urine reflects the activity of pancreatic enzymes. Tests with labeled 14C-triolein and 3H-butyric acid have also been proposed, but due to the specific nature of working with radioisotope preparations, they have not become widespread.
The technique of pancreatic intubation is similar to that used for conventional duodenal intubation. A 33% solution of magnesium sulfate can be used as a stimulant. Portions of duodenal contents are collected before and after stimulation, followed by determination of the activity of pancreatic enzymes.
A more specific stimulant is a 0.5% solution of hydrochloric acid (hydrochloric acid test). After acid stimulation, secretion volume and bicarbonate alkalinity increase and enzyme activity decreases. The test characterizes the production of pancreatic bicarbonates in response to acidification of the duodenal contents.
Food products can be used as secretion stimulants to assess the enzymatic function of the pancreas. The most commonly used test is the Lundh test: stimulation of the pancreas with a mixture consisting of milk powder, vegetable oil and glucose dissolved in 300-500 ml of warm water. A mixture of 18 g of vegetable oil, 16 g of cazillac and 40 g of glucose, dissolved in 300 ml of water, contains approximately 6% fat, 5% protein and 15% carbohydrates. After administration of the stimulant, four consecutive 30-minute samples of duodenal contents are collected (that is, collection is carried out within 2 hours), in which the activity of trypsin, lipase, and amylase is determined.
However, the “gold standard” for assessing the exocrine function of the pancreas for many years has been and remains the secretin and pancreasimin (cholecystokinin) tests developed in 1960. Secretin stimulates the release of bicarbonates from the pancreas, and pancreazimin (cholecystokinin) stimulates the release of enzymes. The tests can be performed individually or together. After inserting the probe and obtaining basal portions of the duodenal contents, secretin is injected intravenously, after which three portions of the secretion are collected at an interval of 10 minutes to determine the dynamics of volume and bicarbonate alkalinity. When conducting the secretin-pancreazimin test, after receiving the indicated portions, pancreazimin is administered intravenously and three more portions of duodenal contents are collected to determine enzyme activity.
There is a normosecretory response to the introduction of stimulants, as well as a hypersecretory response (excessive increase in enzyme activity), characteristic of the initial stages of pancreatic damage, a hyposecretory response (decreased enzyme activity), observed with profound changes in the pancreas, and an obstructive response (decreased secretion volume), reflecting blockage of the ducts. .
Secretin and pancreasimin tests are highly accurate, but their widespread use is impossible due to the extremely high cost of secretin and pancreasimin. Disadvantages of the method are also the need to probe the patient, the duration of the procedure and the possibility of adverse reactions associated with intravenous administration of drugs.
The method for determining elastase-1 in feces, which has appeared in recent years and has become widely practiced, is a real alternative to the expensive and invasive secretin-pancreasimin test. The pancreatic enzyme elastase-1 is not metabolized in the intestine, and its activity in feces objectively reflects the exocrine function of the pancreas. Since elastase-1 is organ specific, its determination eliminates the possibility of error associated with the function of intestinal enzymes. Moreover, unlike indirect tests, such as fecal lipid profiles, elastase-1 determination can be performed without discontinuing pancreatic enzyme medications.
The level of elastase-1 in stool is determined by enzyme immunoassay using monoclonal antibodies (Elastase 1 stool test®, ScheBo Biotech, Germany) and is normally at least 200 μg/g of feces. Lower values indicate the presence of exocrine pancreatic insufficiency.
Determination of elastase-1 in stool is indicated in all cases where there is a suspicion of exocrine pancreatic insufficiency and the use of pancreatic enzyme preparations is being discussed, since the use of this method avoids their unreasonable prescription.
With the advent of an accessible and highly accurate test for assessing exocrine pancreatic secretion, doctors have the opportunity to make a reasonable diagnosis of isolated pancreatic insufficiency: until recently, this diagnosis was made by exclusion. A normal level of elastase-1 in the stool along with severe steatorrhea due to neutral fat clearly indicates isolated lipase deficiency. Determination of elastase-1 in patients with severe pancreatic insufficiency (cystic fibrosis, Shwachman syndrome, isolated lipase deficiency) makes it possible, without canceling enzyme therapy, to monitor the condition of the pancreas. Finally, the introduction of this test into everyday practice will dispel the myth about the harmful effects of long-term enzyme therapy on the exocrine apparatus of the pancreas.
At the same time, the appearance of a test for elastase-1 in stool does not exclude the possibility of using indirect methods for studying exocrine pancreatic function, since only they (a coprogram or, preferably, a stool lipid profile) make it possible to assess the degree of adequacy of replacement therapy and select the dose of the drug (Table 1).
| Table 1. Diagnostic value of elastase-1 activity in stool and severity of steatorrhea when simultaneously determined |
The algorithm for studying the exocrine function of the pancreas, as it is seen today, is presented in Table. 2 and in the figure.
| Table 2. General algorithm for studying exocrine pancreatic function |
Correction of exocrine pancreatic insufficiency should be carried out with highly active preparations of pancreatic enzymes. The dose of the drug is selected individually under the control of the nature of the stool, coprogram and stool lipid profile.
The most effective are microspherical and microtablet preparations of pancreatic enzymes with a pH-sensitive shell. The high activity of these drugs is determined by several factors. Firstly, the high degree of activity of the initial substrate (pancreatin) used for their production. Secondly, thanks to the special form of these drugs (microspheres 1-1.2 mm in size), they are evenly mixed with gastric contents and synchronously penetrate into the duodenum. Studies have shown that a microsphere diameter of 1-1.2 mm is optimal in this regard, while spheres with a diameter of 2 mm or more linger in the stomach for at least 2 hours [3, 4]. Third, the pH-sensitive shell of the microspheres protects the enzyme from destruction in the stomach and releases it in the duodenum. In addition, the microspheres themselves are placed in capsules (also pH-sensitive), which protect the microspheres from premature activation in the oral cavity and esophagus, where, as in the duodenum, there is an alkaline environment. In addition, this form makes it easier to take the drug. Thus, the drug in the capsules reaches the stomach, where the capsules dissolve and the microspheres are released and mixed with the gastric contents. In the duodenum, at a pH value of about 5.5, the pH-sensitive shell of the microspheres dissolves and highly active enzymes begin to act.
The microspherical encapsulated preparation of pancreatic enzymes (Creon) is characterized by a rapid and uniform distribution of the active substance in the stomach with complete protection of this substance from inactivation by gastric acid, which is achieved by filling the gelatin capsule with a known amount of microspheres with the pancreatin preparation (diameter 1-1.2 mm) , coated with an enteric coating. Dissolving in the stomach in a few minutes, the capsule releases microspheres that remain resistant to the action of highly acidic gastric juice (pH = 1). The need to protect pancreatic enzymes in the stomach is beyond doubt. It has been shown that only 10% of the lipolytic activity of an unprotected drug persists after passage through the stomach [2]. Due to the fact that Creon microspheres are covered with an enteric protective coating, after passing through the stomach, 98.6% of enzymatic activity is retained. Microspheres are evenly mixed with gastric chyme and evacuated into the small intestine, where they quickly dissolve in an alkaline environment, releasing enzymes. At an environmental pH of 5.5, 90% of the drug is released in 45 minutes, and at pH = 6 - in 15 minutes, which ensures adequate digestive processes [1, 5]. This ensures the possibility of rapid and highly effective action of the drug.
One capsule of the drug Creon 10,000 contains 150 mg of highly purified pancreatin, obtained from porcine pancreas and consisting of lipase (10,000 U), amylase (8000 U) and proteases (600 U) (E - Ph. Eur. units). The drug Creon 25,000 contains 300 mg of highly purified pancreatin with high activity of lipase (25,000 U), amylase (18,000 U) and proteases (1000 U). The dose of the drug is selected individually. Initially, the patient is prescribed one or two capsules of the drug, which should be taken with food, and then the dose is adjusted depending on individual needs. The daily dose of the drug depends on the severity of exocrine pancreatic insufficiency. In pediatric practice, to facilitate administration of the drug, the capsule can be carefully opened, and the microspheres can be taken without chewing and washed down with a small amount of water or juice. If microspheres are mixed with food, they should be taken immediately; otherwise, damage to the enteric coating may occur.
The drug has a wide range of indications for which its effectiveness has been demonstrated. The main area of its use is conditions with exocrine pancreatic insufficiency. Numerous studies have shown the high effectiveness of microspheres in patients with malabsorption, in the broad sense of the word. In particular, the feasibility of using the drug as an adjuvant therapy for malabsorption in children with celiac disease, as well as with acute intestinal infections, has been shown. Thus, the administration of Creon during the period of convalescence of an acute intestinal infection made it possible to significantly reduce the number of bowel movements, the stool becomes more formed, and the phenomena of flatulence, steatorrhea, creatorrhoea, and amilorrhea decrease. In general, when correcting malabsorption in patients with acute intestinal infection, a good effect is achieved in 87% of sick children, satisfactory in 10%, and no effect is observed in only 3% of patients. Using radioimmune methods, it was shown that Creon significantly improves nutrient absorption (compared to the control group, where treatment was carried out with regular pancreatin).
Among the advantages of modern pancreatic enzyme preparations, it should be noted the absence of side effects and good tolerability, due to which these drugs can be used in all age groups. If necessary, drugs are prescribed for a long time, without fear that any negative consequences will arise. Patients with cystic fibrosis, Shwachman-Diamond syndrome, and congenital lipase deficiency receive replacement therapy for life. For patients with transient pancreatic insufficiency, pancreatic enzyme preparations are prescribed for a period of several weeks to several months under the supervision of appropriate research methods. At the same time, the unreasonable prescription of enzyme preparations, which has been quite widely practiced in recent years, should also be avoided. The use of modern methods for diagnosing exocrine pancreatic insufficiency makes it possible to really narrow the range of patients who require expensive replacement therapy.
In conclusion, I would like to note that a rational approach to the selection of methods for diagnosing and correcting exocrine pancreatic insufficiency ensures the greatest effectiveness of treatment at an optimal cost.
For questions about literature, please contact the editor
Note. The frequency of control studies of elastase-1 and fecal lipidogram is determined individually, however, approximately in case of severe exocrine insufficiency of the pancreas, the fecal lipidogram should be repeated at least once every 3 months, and determination of elastase-1 in stool should be carried out at least once a year.
Norms of elastase in feces
- The norm is 200 or more mcg/g of feces;
- Moderate (mild to moderate degree of pancreatic secretory insufficiency) – from 100 to 200 µg/g of feces;
- Critical (severe degree of impairment of the exocrine function of the pancreas) – up to 100 µg/g of feces.
Factors influencing the result
- Violation of the material collection algorithm;
- Violation of the rules for storing feces;
- Conducting X-ray, CT, irrigoscopy and other studies using contrast the day before;
- Taking magnesium and bismuth supplements;
- Ingestion of oils (mineral or castor);
- Treatment with antidiarrheal drugs;
- The use of laxatives, rectal suppositories or enemas on the eve of the stool collection procedure.
The results are NOT influenced by the following factors:
- pancreatic enzyme replacement therapy;
- treatment with proteolysis inhibitors;
- age and gender of the patient;
- genetic factors.
Algorithm for collecting biomaterial
- Stool collection is performed in the morning after emptying the bladder and hygienic washing of the external genitalia and anal area.
- After the act of natural defecation, feces are collected with a special spatula in a plastic container. Glassware for analysis can be freely purchased at any medical center. institution.
The amount of feces required for research is up to 30% of the container volume.
- The following information is indicated on the container: name and age of the patient, date and time of stool collection.
- The container with the biomaterial is sent to the laboratory immediately after collection. If it is not possible to do this immediately, then the material can be stored in the refrigerator for about 5-8 hours at a temperature of 4-6°C.
Other stool tests
- Coprogram
- Feces for occult blood
- Pancreatic elastase
- Carbohydrates in feces