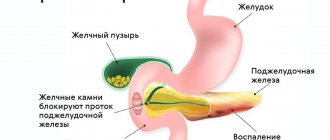
Among all diseases of the gastrointestinal tract (GIT), the incidence of chronic pancreatitis ranges from 5.1 to 9%, and in the last few decades, the incidence of pancreatitis in our country has doubled1.
Chronic pancreatitis is an insidious disease, largely due to its recurrent nature. Patients are often concerned about periods of exacerbation, accompanied by severe pain, nausea, and vomiting.
Where does chronic pancreatitis come from? What causes the disease and is it possible to fight it? Let's try to figure it out.
JUSTIFICATION
The progressive increase in morbidity, development of serious complications and mortality in acute pancreatitis (AP), chronic pancreatitis (CP), pancreatic cancer (PCa) necessitates in-depth study of these diseases.
According to WHO, in the 21st century. There is a trend toward an increase in the number of young and middle-aged patients with AP; the prevalence of CP in Russia has tripled over the past decades [1]. About 338,000 new cases of prostate cancer are diagnosed annually, and among the causes of cancer mortality, prostate cancer ranks 4th in the world [1]. Obese people have an increased risk of developing multisystem organ failure during acute inflammatory conditions [2]. With obesity, the metabolic activity of adipose tissue changes - the balance of adipokines is disrupted: the production of pro-inflammatory adipokines increases and the production of anti-inflammatory ones is inhibited, including in the pancreas (P) [1]. However, information about the relationship between obesity and pancreatic diseases is heterogeneous. Obesity and associated metabolic disorders lead to the development of a number of serious diseases: dyslipidemia, type 2 diabetes mellitus (T2DM), cholelithiasis (GSD), non-alcoholic fatty liver disease (NAFLD), malignant tumors of certain localizations, etc. [2, 3 ]. These diseases, in turn, contribute to an increased risk of AP. Thus, in patients with cholelithiasis, there is a higher likelihood of developing pancreatitis not only with the help of biliary sludge or microlithiasis, which cause biliary-pancreatic reflux and trigger intrapancreatic activation of trypsin, but also due to the formation of metabolic disorders [1]. For example, through hypertriglyceridemia (HTG), more often observed in cholelithiasis, T2DM, NAFLD, which leads to microthrombosis of pancreatic vessels, causing ischemia and necrosis, as well as in patients with HTG-associated pancreatitis, there is a deficiency of lipoprotein lipase (LPL) or apolipoprotein (apo) C and apo E as a ligand for the uptake of TH-rich remnants by the liver [4]. Due to excess circulating triglycerides, there is an uncontrolled release of lipotoxic unsaturated fatty acids (UFAs), which increase the release of proinflammatory cytokines (tumor necrosis factor (TNF-α), CXC ligand 1 (CXCL1) and CXCL2), which in turn causes acinar necrosis. cells by inhibiting mitochondrial complexes I and V [5]. However, other studies have not found an association between obesity and the risk or severity of AP [6].
The mechanism of HTG in T2DM is associated with insulin resistance, which leads to the return of excess free fatty acids (FFAs) to the liver, with increased production of very low density lipoproteins (VLDL) and decreased synthesis of apoB, and with hyperinsulinemia, which promotes de novo synthesis of triglycerides (TGs). , which generally leads to a worsening of the course of AP, but other authors provide evidence that T2DM is associated with a 1.22-fold reduction in the risk of death from AP compared to patients with AP without T2DM [7].
Unlike AP, the number of adipocytes in CP is not associated with body mass index (BMI): in CP, severe fibrosis of the pancreas develops, and since the adipocytes that make up the intrapancreatic fat are surrounded by massive strands of collagen fibers, an obstacle arises to the release of lipotoxic FFAs, which prevents lipolytic cascade between adipocytes and acinar cells, thereby reducing acinar necrosis [8]. The experiment established that the morphological structure of the pancreas of rats with nutritional obesity has signs of productive chronic inflammation [9], however, data on the comorbidity of obesity and CP are very scarce.
Many studies have examined the associations of overweight and obesity with the risk of developing prostate cancer, but the results of these studies are inconsistent: both direct [10] and gender-mediated associations between obesity and prostate cancer [11] have been identified. And for cancer of the stomach, esophagus, and lungs, an inverse relationship was found between BMI and mortality [12]. In the last decade, the “obesity paradox” has been observed, where in patients with cancer, an increased BMI is associated with improved survival compared with patients of normal weight (BMI 22.5 kg/m2 is accepted as the average reference for normal weight) [13]. In cancer patients with distant metastases, mortality rates were significantly lower than in patients with normal weight: 1.2 times lower in patients with LBW and 1.5 times lower in patients with obesity [12]. In addition, the World Cancer Research Fund International (WCRFI) recommends determining BMI in cancer patients before and after diagnosis [14]. Thus, the heterogeneity of information on the relationship of overweight and obesity with AP, CP and PCa, as well as the limited data on the study of these indicators within a single study, prompted us to carry out this work.
Causes of chronic pancreatitis
One of the most common causes of chronic pancreatitis is poor diet and unhealthy lifestyle. Constant overeating, abuse of fatty foods and alcohol cause blockage of the excretory ducts of the pancreas.
Narrowed excretory ducts provoke the accumulation and premature activation of digestive enzymes. As a result, the pancreas actually begins to digest itself, and inflammation forms1.
In addition, the following factors can lead to chronic pancreatitis3:
- ulcer of the duodenum, stomach and enteritis. Chronic inflammation of the mucous membrane of the gastrointestinal tract makes it difficult to secrete pancreatic juice, which often causes chronic pancreatitis;
- cholelithiasis. After leaving the gallbladder, the stone causes blockage of the common duct and inflammation of the pancreas develops;
- genetic predisposition;
- toxic effects of drugs.
METHODS
Study design
Observational, multicenter, cross-sectional, uncontrolled, case series study. The flow chart of the study is presented in Figure 1.
Figure 1. Study design.
Notes PZ – pancreas, AP – acute pancreatitis, CP – chronic pancreatitis, PC – pancreatic cancer, TNF-α – tumor necrosis factor-alpha.
Eligibility Criteria
Inclusion criteria: male and female patients with AP, CP or PC aged 25–70 years.
Exclusion criteria: severe concomitant pathology.
Conditions
The search for study participants was carried out in medical institutions: the hospital of the State Budgetary Healthcare Institution of the Novosibirsk Region "State Novosibirsk Regional Clinical Hospital", the Research Institute of Therapy and Preventive Medicine - a branch of the Federal State Budgetary Scientific Institution "Federal Research Center Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy science", day hospital and consultative diagnostic center of the State Budgetary Healthcare Institution of the Novosibirsk Region "City Clinical Infectious Diseases Hospital No. 1".
Duration of the study
The study was conducted from 2014 to 2022.
Description of medical intervention
All patients filled out questionnaires on the severity of clinical signs of pancreatic diseases, including changes in body weight in the previous year before the present examination or for prostate cancer in the year before the diagnosis of cancer. All patients underwent anthropometric measurements. All patients underwent analysis of personal data and general clinical examination.
Main outcome of the study
Frequency of obesity in patients with AP, CP, and prostate cancer.
Methods for recording outcomes
To verify the diagnosis, general clinical, laboratory, instrumental and morphological methods of examining the pancreas were used [1]. The diagnosis of AP is based on a combination of at least two of the following signs, subject to the exclusion of other surgical pathology: a typical clinical picture (intense girdling pain not relieved by antispasmodics, uncontrollable vomiting, bloating; consumption of alcohol, spicy food or a history of cholelithiasis, etc. ); characteristic signs according to ultrasound examination: increased size, decreased echogenicity, blurred contours of the pancreas; the presence of free fluid in the abdominal cavity; hyperenzymemia (hyperamylasemia or hyperlipasemia), exceeding the upper limit of normal by three times or more. The diagnosis of CP was established on the basis of a comprehensive assessment of the results of instrumental methods of examining the pancreas (ultrasound, computed tomography or magnetic resonance imaging) and laboratory tests (determination of glycemic levels, fecal elastase-1) taking into account the M-ANNHEIM criteria. Defined CP was defined as the presence of one or more of the following criteria: pancreatic calcification, moderate or severe changes in the pancreatic ducts, severe persistent exocrine pancreatic insufficiency. Probable CP: mild changes in the pancreatic ducts, pseudocyst, pathological results of functional tests (fecal elastase-1), endocrine insufficiency. In the presence of a typical clinical picture in the absence of criteria for probable and definite CP, a diagnosis of “borderline CP” was made (Schneider A., 2007, [1]). The diagnosis of prostate cancer was suspected based on instrumental research methods and verified by morphological examination of surgical material. The evaluation of histological preparations was performed by two pathologists independently, followed by discussion of the results and a collegial conclusion. When neuroendocrine prostate cancer was detected, the diagnosis of neoplasms was carried out in accordance with the classification criteria for tumors of endocrine organs (WHO, 2017).
Quetelet body mass index II (BMI) was calculated using the formula: BMI (kg/m2) = body weight (kg)/height2 (m2). We used the classification of obesity according to BMI (WHO, 1997): underweight (BMI<18.5% kg/m2), normal (BMI=18.5–24.9 kg/m2), overweight (BMI=25.0–29, 9 kg/m2), obesity (BMI≥30 kg/m2) [2].
Subgroup analysis
To analyze the results obtained, three groups were identified: patients with AP, patients with CP and patients with PCa. The AP group was divided into subgroups by gender (men and women), etiology (alcoholic, biliary, idiopathic) and form (edematous AP, pancreatic necrosis). The group of patients with CP was divided into subgroups by gender (men, women), etiology (alcohol, biliary-dependent, idiopathic) and form (“definite”, “probable”, “borderline”). The group of patients with prostate cancer was divided into subgroups by gender (men, women), form (prostate adenocarcinoma, neuroendocrine prostate cancer) and stage (stages 1–2 and stages 3–4).
Ethical review
The study was approved by the ethical committee of the Research Institute of Therapy and Preventive Medicine - a branch of the Federal State Budgetary Scientific Institution "Federal Research Center Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences" (No. 38 of 09.23.14), State Budgetary Health Institution of the Novosibirsk Region " City Clinical Hospital No. 7" (No. 1 dated 03/31/2014), State Budgetary Healthcare Institution of the Novosibirsk Region "State Novosibirsk Regional Clinical Hospital" (No. 1 dated 01/29/2014). Patients were informed about the possible use of their data for scientific purposes. All patients remained anonymous during subsequent data analysis. Written voluntary informed consent to participate in the study was obtained from each patient.
Principles for calculating sample size . No preliminary sample size calculation was performed.
Statistical analysis
Principles for sample size calculation : The sample size was not pre-calculated.
Methods of statistical data analysis : statistical data processing was carried out using the SPSS (13.0) software package. The normality of the distribution of the trait was checked using the Kolmogorov–Smirnov test. Comparison of frequencies of qualitative characteristics was carried out using the χ2 test and Student's t-test (t). Odds ratios (OR) with 95% confidence intervals (CI) were calculated using a contingency table with the Mantel–Haenszel correction. When comparatively assessing the average values of the quantitative characteristics of the studied indicators, one-way anova analysis of variance was used between two groups, and between three groups, with Bonferroni correction for post hoc multiple comparisons. The results are presented as the arithmetic mean ± error of the arithmetic mean (M±m). Multivariate analysis of the relationships of the main characteristics was carried out using logistic regression analysis (Enter method). Exp (B) (exponent B) reflects the risk ratio [15], shows how many times the risk of an outcome changes if the value of the predictor changes by one. Differences were considered statistically significant at p<0.05.
3. Diagnosis and treatment for weight loss
Weight loss screening should cover all possible causes. Diagnostics may include:
- study of hormone levels and diagnosis of the thyroid gland;
- tests to detect parasites;
- tests for window markers;
- CT, MRI, ultrasound diagnostics;
- fluorography;
- clinical blood and urine tests;
- examination by a neurologist and psychiatrist.
Therapy is prescribed taking into account the results obtained. Sometimes the development of a treatment regimen requires the participation of doctors from several specialties. Medication, psychotherapy, and physical therapy may be prescribed.
For his part, the patient can take measures to optimize the work and rest regime, review the nutritional schedule and diet, engage in self-analysis and identify stress factors. Good results for weight loss are achieved by sanatorium-resort treatment and a change of stay (vacation by the sea, travel, vacation away from modern civilization).
About our clinic Chistye Prudy metro station Medintercom page!
RESULTS
Objects (participants) of the study
The study included 186 patients with pancreatic diseases (OP, CP, PCa).
- 44 patients with AP: 61.4% men and 38.6% women; average age – 51.1±1.6 years.
- 97 patients with CP: 19.6% men and 80.4% women; average age – 54.5±1.2 years.
- 45 patients with prostate cancer, 48.9% men and 51.1% women, average age – 58.3±1.1 years.
In accordance with the classification of OP Savelyev V.S. (2004) identified 45.5% of patients with edematous AP and 54.5% of patients with pancreatic necrosis. According to the M-ANNHEIM classification (Schneider A., 2007, [1]), in our study, patients with CP were distributed according to clinical forms: “borderline” CP – 45.36%, “probable” CP – 42.27%, “definite” CP - 12.37% and by etiology - alcoholic CP (17.5%), biliary-dependent CP (79.4%), idiopathic (3.1%). In 91.1% of patients with prostate cancer, adenocarcinoma of the prostate was detected, and in 8.9%, neuroendocrine cancer was detected. Distribution of patients by stage of prostate cancer: 57.8% – T1–T2, 28.9% – T3 and 13.3% – T4 stages.
Main results of the study
The average BMI values, the frequency of deficient, normal and overweight, as well as obesity in patients with AP, CP and PCa are presented in Table 1. Pairwise analysis of the average BMI values in patients with AP, CP and PCa revealed that in patients with AP the average BMI was significantly lower than in patients with CP, and in patients with prostate cancer did not differ from that in patients with AP and CP. In addition, an inverse relationship was found between BMI≥22.5 kg/m2 and the risk of AP (OR=0.398; 95%CI 0.195–0.812; p=0.011). Logistic regression analysis (with the inclusion of pancreatic necrosis, “definite” CP or stage 3-4 prostate cancer in the dependent variables and age, gender, BMI in the independent variables) revealed an inverse relationship between BMI and “definite” CP (Exp (B) = 0.772; 95% CI 0.632–0.942; p=0.011), that is, with an increase of 1 BMI unit, the risk of CP decreased by 0.8 times.
Table 1. Body weight characteristics in patients with acute pancreatitis (AP), chronic pancreatitis (CP) and pancreatic cancer (PCa)
| Group of patients | OP, n=44 | HP, n=97 | PCa, n=45 | p (OP and HP) | p (OP and PCa) | p (CP and PCa) |
| BMI, kg/m2, M±m | 24,2±0,7 | 26,3±0,5 | 26,2±0,7 | 0,049 | 0,158 | 1,000 |
| Deficient MT | 3 (6,8%) | 5 (5,2%) | — | 0,693 | — | — |
| Normal BW | 22 (50%) | 37 (38,1%) | 20 (44,4%) | 0,188 | 0,601 | 0,477 |
| Excess BW | 13 (29,5%) | 31 (32%) | 16 (35,6%) | 0,775 | 0,547 | 0,672 |
| Obesity | 6 (13,6%) | 24 (24,7%) | 9 (20%) | 0,138 | 0,425 | 0,535 |
| Obesity (one year before this survey) | 6 (13,6%) | 25 (25,8%) | 25 (55,6%) | 0,000 | 0,001 | 0,107 |
Notes BMI – body mass index; AP – acute pancreatitis; CP – chronic pancreatitis; PCa is pancreatic cancer. The table presents a comparative description of BMI, the frequency of underweight, normal, overweight, and obesity. Differences were considered statistically significant at p<0.05.
The frequency of underweight, normal, overweight and obesity in patients with AP, CP and PCa did not differ significantly. Logistic regression analysis with dependent (OP, CP and PC) and independent variables (age, underweight, normal, overweight, obesity) did not reveal significant associations of these diseases and weight.
Considering the average prevalence of obesity in Russia (29.7%) [16], in patients with AP, obesity (13.6%) was 2 times less common (t=2.3; p=0.020), and the frequency of obesity in patients with prostate cancer ( 20.0%; t=1.4; p=0.155) and CP (24.7%; t=1.1; p=0.286) differed slightly. Among the examined patients, no association was found between obesity and AP (OR=0.522; 95% CI 0.203–1.342; p=0.177), CP (OR=1.622; 95% CI 0.788–3.327; p=0.189) or prostate cancer (OR= 0.925; 95% CI 0.402–2.131; p=0.855). However, in the previous year before the present survey, 68.2% of patients with AP, 45.4% of patients with CP and 84.4% of patients with prostate cancer lost weight, χ2=19.2; p=0.000 when comparing patients with CP and PCa, χ2=3.3; p=0.071 when comparing patients with AP and PCa. Weight decreased on average by 5.1±0.3 kg (patients with AP), by 2.3±0.3 kg (patients with CP) and by 14.0±1.4 kg (patients with prostate cancer); p=0.000 when comparing patients with PCa with patients with AP or CP. Previously (one year before this survey), obesity was more common in patients with PCa (55.6%) than in patients with AP (13.6%; χ2=3.3; p=0.000) and CP (25.8%, χ2 =12.0; p=0.001). Taking this information into account, an association was demonstrated between a history of obesity (in our study, one year before the diagnosis of prostate cancer) and prostate cancer (OR=4.435; 95%CI 2.180–9.025; p=0.000).
The characteristics of MT practically did not differ depending on gender and the etiology of AP; gender, form or stage of prostate cancer. Among patients with CP, significant gender differences were noted: the frequency of deficient MT was 6 times higher in men with CP (15.8%) than in women with CP (2.6%) (χ2=5.5; p=0.019), and IBMI, on the contrary, is 7 times less (5.3 and 38.5%, respectively; χ2=7.7; p=0.005). In patients with biliary-dependent CP, the average BMI was higher (27.2±0.5 kg/m2) than in patients with alcoholic CP (23.5±1.2 kg/m2; p=0.003). MT deficiency was more often observed in patients with alcoholic CP (17.6%) compared to that in patients with biliary-dependent CP (1.3%), χ2=9.1; p=0.003. In patients with “definite” CP, the average BMI was the lowest (22.0±1.6 kg/m2) compared with “probable” (26.0±0.7 kg/m2; p=0.024) and “borderline” CP (27.8±0.6 kg/m2; p=0.000). Patients with “borderline” CP were 5 times more likely to report IBW than patients with “definite” CP (χ2=11.6; p=0.001). However, the frequency of obesity did not differ significantly in patients with “definite” CP (8.3%), “probable” CP (22.0%) and “borderline” CP (31.8%).
Logistic regression analysis (with the inclusion of pancreatic necrosis, “definite” CP or stage 3-4 prostate cancer in the dependent variables and age, deficient weight gain and obesity in the independent variables) revealed a direct connection between deficient weight loss and “definite” CP (Exp (B) = 14.706; 95% CI 1.903–113.647; p=0.010).
How to make an appointment with a gastroenterologist
You can make an appointment with a gastroenterologist by calling +7 (495) 775-73-60. The line operates 24 hours a day and accepts calls from English-speaking citizens. In case of acute attacks, it is recommended to call an ambulance by phone. Calls are accepted around the clock, the clinic is open both on weekdays and on weekends, and there is a hospital on the territory of the medical institution. Doctors with extensive experience work here. You can also use the form to make an appointment with a doctor provided on the website. JSC "Medicine" (clinic of academician Roitberg) is located in the center of Moscow, at 2nd Tverskoy-Yamskaya lane 10.
DISCUSSION
Summary of the main finding of the study
In our study, the incidence of obesity did not differ significantly in patients with AP, CP and PCa. When comparing the frequency of obesity with the average prevalence of obesity in Russia (29.7%) [16], data was obtained that among patients with AP, obesity was 2 times less common; the frequency of obesity in patients with PCa and CP did not differ significantly. Logistic regression analysis did not reveal an association between obesity and pancreatic necrosis, “definite” CP or stages 3–4 PCa.
Discussion of the main result of the study
Previously conducted meta-analyses have shown that obesity is one of the most important negative prognostic factors of AP: it increases the risks of development, correlates with the severity of the disease, the development of local and systemic complications [17–19]. In an experimental study in a mouse model, obesity was also correlated with the severity of AP [20]. However, other researchers have questioned the association of obesity and AP. In the work of Hall (2015), on the contrary, the distribution of fat (subcutaneous, retroperitoneal and intra-abdominal) in patients with AP did not affect the severity and mortality from this disease [21]. A Japanese study by Taguchi (2014) showed that BW deficiency, and not obesity, is a negative prognostic sign of AP mortality [22]. This is not contradicted by the results of our study: an inverse relationship between BMI≥22.5 kg/m2 and the risk of AP was revealed. When reviewing the literature, we did not find convincing epidemiological evidence of the association of obesity and the risk of developing CP. In a study by Ammann (2010), overweight and obesity were found in 54.2 and 15% of cases of alcoholic CP, respectively, which was significantly higher compared to the control group (37.7 and 3.1%) [23]. Masharipova (2019) determined that patients with CP had a higher average BMI level (37.3±4.1 kg/m2) and a high frequency of overweight (75%) [24], in another study, on the contrary, a lower average BMI was noted in patients CP than in the control group (22.9±4.2 kg/m2 and 26.8±5.2 kg/m2, respectively, p<0.0001) and a high frequency of deficient MT (26%) [25]. In a study by Roberts (2019), patients with CP also had a lower BMI compared to the control group (24 and 31 kg/m2; p<0.001) [26]. In addition, cases of sarcopenic and osteosarcopenic obesity have been described in patients with CP, when a decrease in physical activity as a result of sarcopenia leads to an increase in fat mass, and existing obesity aggravates sarcopenia through a decrease in muscle sensitivity to insulin, which developed due to an increase in the production of pro-inflammatory cytokines and impaired regulation of secretion leptin and adiponectin [27]. The work of Gubergrits (2013) showed that in patients with CP against the background of obesity, structural changes in the pancreas typical for CP develop (increase in size, uneven contours, changes in echogenicity, calcification, dilation of the main pancreatic duct, pancreatic pseudocysts) [28]. Data on the effect of obesity on the risks of prostate cancer are ambiguous: some studies found a direct relationship between obesity and prostate cancer [29], while others found an inverse relationship [30]. The results of a pooled analysis of 20 prospective cohort studies by Genkinger J. (2015) confirmed the association between mortality from prostate cancer and central obesity [31]. Shi (2016) conducted a meta-analysis on the prognostic role of BMI on survival in PCa. Results showed that obesity was associated with the risk of developing prostate cancer (OR=1.29) and lower survival from prostate cancer in adulthood (the risk of death from prostate cancer increased by 11% for every 5 units increase in BMI) [32]. In a meta-analysis of 13 studies, Majumder (2016) demonstrated an increase in PCa-related mortality in individuals with LBW (OR = 1.06) and obesity (OR = 1.31) compared with controls. Each 1 kg/m2 increase in BMI was associated with a 10% increase in mortality [33]. According to the World Cancer Research Fund (2007), there is a “strong increased risk” of PCa in obese individuals and a “probable increased risk” in those with abdominal obesity [34]. Our study did not reveal an association between the presence of obesity (at the time of examination) and the development of prostate cancer; however, the association of a history of obesity (one year before detection of prostate cancer) with prostate cancer was confirmed (OR=4.4; 95% CI 2.2–9.0; p=0.000). The fact that previously (before weight loss, possibly as a result of oncogenesis) obesity was more common in patients with PCa (55.6%) compared to patients with AP (13.6%; p<0.05) and CP (25. 8%; p<0.05), may serve as indirect evidence of a more significant role of obesity in prostate cancer than in inflammatory diseases of the pancreas. Currently, researchers are calling for wider use of the opportunity to modify MT in order to improve the course of both inflammatory and oncological diseases of the pancreas.
Limitations of the study
Limitations of the study include the small number of observations, uneven gender distribution among patients with CP (out of 97 patients with CP, 19.6% were men and 80.4% were women). In addition, our study did not study indicators of abdominal obesity such as waist circumference, hip circumference, and waist-to-hip ratio.
ADDITIONAL INFORMATION.
Source of financing. The work was carried out according to the State assignment within the budget theme No. AAAA-A17-117112850280-2.
Conflict of interest. The authors declare that there are no obvious or potential conflicts of interest related to the publication of this article.
Authors' participation. Grigorieva I.N. – concept and design of the study, data analysis, text writing; Efimova O.V. – collection and processing of material, data analysis, text writing; Suvorova T.S. – collection of material. All authors made significant contributions to the research and preparation of the article, and read and approved the final version of the article before publication.
Exacerbation of chronic pancreatitis
Chronic pancreatitis may hardly manifest itself until the period of exacerbation. Relapse of the disease is usually associated with two main reasons3:
- alcohol consumption. Even a small amount of alcohol consumed can provoke the transition of the disease into the acute phase;
- violation of the diet, overeating, a large number of fatty, fried, spicy dishes on the menu.
Other factors can also provoke exacerbation of chronic pancreatitis in adults, for example, chronic stress, physical fatigue, poisoning or the toxic effects of certain medications3.
Exacerbation of the disease is manifested by the following symptoms3:
- attack of acute or dull pain in the hypochondrium. Painful sensations spread to the subscapular region or the entire back;
- progressive diarrhea. In this case, the feces have a characteristic greasy shine. Undigested food remains are often observed in the stool;
- the occurrence of specific bitterness in the oral cavity, nausea and loss of appetite;
- whitish coating on the surface of the tongue;
- weight loss.
Exacerbation of chronic pancreatitis in adults can last for one to two weeks. You cannot fight the disease on your own during this period: the best solution is hospitalization and constant monitoring by specialists.