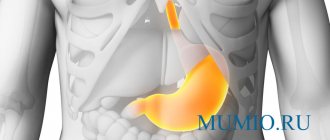
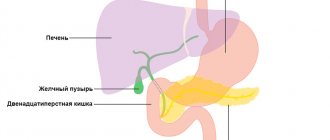
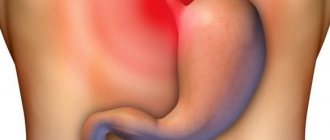
Alcoholic gastritis (otherwise known as alcoholic reactive gastropathy, alcoholic gastropathy, acute alcoholic erosive gastritis) is damage to the mucous membrane of the human stomach caused by excessive alcohol intake. Often this process is accompanied by the appearance of surface defects (erosions), from which blood can be released.
This disease is the only one from the group of gastritis, the occurrence of which is not associated with the development of an inflammatory process in the gastric mucosa. Under the influence of ethanol, the secretion of hydrochloric acid increases, and food is retained in the stomach. As a result, blood circulation in the stomach walls is disrupted, the formation of protective mucus is inhibited, and cell regeneration is interrupted.
Symptoms of alcoholic gastropathy
All the symptoms of this disease can be summarized into two groups. Disorders of the digestive system include:
- • The appearance of aching pain in the upper abdomen immediately after eating.
- • Often a person begins to feel nauseous on an empty stomach. This is accompanied by the manifestation of general malaise and discomfort in different places of the abdomen.
- • Over a short period of time, the patient may vomit several times, and the vomit contains inclusions of bile and mucus.
- • Regardless of the type of food eaten, heartburn and belching occur.
- • There is a feeling of fullness in the stomach.
- • In the chronic course of the disease, symptoms of constipation appear.
- • As alcohol is consumed, all negative manifestations of the disease stop.
Systemic factors for manifestations of alcoholic gastropathy include:
- • Loss of sensitivity and limited movement due to the development of peripheral polyneuropathy.
- • Tachycardia.
- • Muscle atrophy.
ASSESSMENT OF PORTAL HYPERTENSION
The shortest, albeit indirect, method of assessing portal pressure involves wedged hepatic venous pressure (WHVP), which is determined by inserting a balloon catheter (from the jugular, brachial or stegnosus access) under the fluid. oroscopic guidance of the hepatic vein and “jamming” it in the small hilt (introduced all the way) or (which is still sticky) - by inflating the balloon and occluding the larger hilt of the liver vein. The correction of WHVP will ultimately involve the improvement of the internal hepatic vein pressure (for example, with ascites) by releasing the pressure in the hepatic vein - VTPV (free hepatic vein pressure - FHVP) or the internal hepatic vein pressure pressure in the empty vein, which is respected by the internal zero level. They survive when the balloon is deflated. The hepatic venous pressure gradient (HVPG) is determined by the pressure gradient in the hepatic vein. The indicator, as a matter of course, dies three times; If the values are technically correct, they are also well-designed and reliable. Although there is a gradient of pressure between the sinusoids (and not the portal vein veins) and the hepatic vein, HPVT will be advanced in case of intrahepatic causes of portal hypertension, such as cirrhosis, however normal for prehepatic causes, such as portal vein thrombosis. Normal HPVT readings are 3–5 mmHg. Art. It was found that WHVP closely correlates with portal pressure in both alcoholic and non-alcoholic cirrhosis. HPVT and changes in its symptoms, which are observed over time, may have a transferable value to the development of varicose veins in the vein, the risk of varicose bleeding, the development of non-varicose folds of portal hypertension And death. Single extinction is useful for the prognosis of both compensated and decompensated cirrhosis, while repeated extinction is useful for monitoring the effectiveness of drug therapy and the reversal of liver disease. The main considerations for the widespread viability of HPVT include the lack of evidence from practicing physicians and their continued recommendations on how to eliminate reliable and effective indicators, as well as other the simplicity of the procedure.
Duplex Doppler ultrasonography
Ultrasonography is a safe, inexpensive and effective method for screening for PG. The knobby structure of the liver, splenomegaly and the presence of colateral circulation give rise to suspicion of liver cirrhosis and PG. The diameter of the splanchnic vessels, directly and the fluidity of the bloodstream, changes in the diameter of the vessels during breathing, the index of portal venous stagnation, the pulsativity index and the resistance index of the liver, upper pars and glands also depend. Hepatic artery and hepatic vessel index. Normally, the diameter of the portal vein does not exceed 13 mm during quiet breathing, but during deep breathing it can increase by 50%. Expand a vein with a diameter of >15 mm while breathing calmly. When the pressure of the hemorrhage at the portal vein is increased and becomes monotonous (without breathing sounds) and resolves completely in the portal vein, straightened at the non-functioning portocaval shunts.
However, the creation of many ultrasonic parameters, their accuracy is insufficient, lies both in the tracking technology and in the form of additional rhythms, the development of the sympathetic nervous system, medicine, etc. So, for example, the diameter of the portal vein may increase in case of severe congestive heart failure (water from the inferior empty vein). Therefore, duplex Doppler ultrasonography itself is not recommended for establishing an accurate diagnosis of PG; it is unclear that its diagnostic value for the early stages of PG is lost (in most of these studies, patients were monitored with severe cirrosis and severe PG). Nevertheless, this method is valuable for primary closure, and also helps to identify the etiology of PG, for example, obstruction of blood vessels at different levels, highly sensitive to the identification of functional portocaval anastomoses. After placement of TIPS or portocaval anastomoses, Doppler ultrasonography is of great importance in monitoring shunt patency.
Computed tomography is a test with clear (not sharp) values, whereas ultrasonography did not give clear results. Apart from ultrasonography, CT results do not indicate the presence of gas in the intestine. Reconstruction of the vascular tree with the help of 3-D technology makes it possible to accurately create the actual portal venous system and collaterals. A common finding in portal hypertension during CT scanning is dilatation of the inferior vein. However, CT does not allow assessing the flow of blood through the venous and arterial vessels. MAR makes it possible, in addition to clear information, to obtain detailed information, for example, to assess venous flow in the portal and azygos veins. However, this does not exclude the need for invasive surveillance.
In cases of doubt about the genesis of liver disease, puncture biopsy may be necessary: necrosis of the third zone, for example, indicates portal hypertension, secondary to cardiac failure, and normal liver parenchyma - about subhepatic cause of PG.
Contraindications for liver biopsy include coagulopathy and ascites. Table 1. Causes of portal hypertension and pressure gradient in the hepatic vein
| GTPV | |||
| Rhubarb occlusion | Normal | Normal or movement | Advances |
| Subhepatic syndromes of PG | Portal vein thrombosis Thrombosis of the splenic vein Splanchnic arteriovenous fistula | ||
| Intrinsic hepatic syndromes of PG More important than the level of presinusoidal level | Schistosomiasis, primary biliary cirrosis, idiopathic PG (early stage)* Vuzlova regenerative hyperplasia* | Myeloproliferative diseases*† Polycystic disease*† Liver metastases*† Granulomatous disease † (sarcoidosis, tuberculosis)*† | |
| More important is the level on the sinusoidal or postsinusoidal level | Budd-Chiari syndrome‡ | Cirrosis of the liver Schistosomiasis, primary biliary cirrosis, idiopathic PG (late stage) Hospital and fulminant hepatitis Gostria alcoholic hepatitis Wilson's illness Veno-occlusive disease Budd-Chiari syndrome‡ | |
| Post-hepatic syndromes of PG | Obstruction of the inferior venous vein Right heart failure Clutching pericarditis Tristucular valve insufficiency | ||
* Rare causes of PG. † For the mind of the sinusoids. ‡ Sinusoidal symptoms are weak.
Forms, stages and causes of alcoholic gastropathy
There are acute and chronic gastropathy. In the first case, even a single intake of an excessive dose of alcohol manifests itself in pronounced symptoms. The most characteristic feature of acute gastropathy is frequently repeated painful vomiting. If gastric erosion occurs, vomiting is accompanied by the release of blood.
Chronic alcoholic gastritis is associated with systematic abuse of alcohol-containing drinks. Symptoms are moderate, but are characterized by morning vomiting on an empty stomach, as well as constant thirst and lack of appetite. Experts distinguish two stages of the chronic form: remission (significant weakening or complete disappearance of symptoms) and exacerbation (repeated, often violent manifestation of signs of the disease).
The immediate causes of alcoholic gastropathy are:
- • One-time intake of excessive amounts of alcohol, calculated individually for each person. The risk of developing gastritis increases approximately threefold when consuming more than 60 g of ethanol in one day.
- • Systematic inhibition of the motor and secretory functions of the stomach under the influence of alcohol, as well as its breakdown products. Chronic alcoholism inevitably leads to atrophy of the gastric glands and destruction of the mucous membrane.
The following factors contribute to the formation of alcoholic gastropathy:
- • Hereditary predisposition.
- • Harmful effects of professional working conditions.
- • Physical and neuropsychic fatigue.
- • Smoking.
- • Over or under nutrition.
- • Previous illnesses.
Clinical manifestations
PG can occur in various clinical variants, most often asymptomatic or with minimal manifestations (complaints of epigastric discomfort, heartburn, belching, slight weakness). In the absence of pathogenetic treatment, PG progresses to the development of bleeding directly from the gastric mucosa and manifests clinically as hypochromic IDA, which in cirrhosis is aggravated by impaired coagulation [1, 3]. Pathology of the blood system is a common complication of chronic hepatitis and is observed in more than half of patients [3].
Acute bleeding in patients with PG is indicated by complaints of vomiting with blood (coffee grounds) or black stools (melena) with mandatory laboratory confirmation (decrease in hemoglobin content in the peripheral blood, iron in the blood serum). The final diagnosis is established after excluding bleeding from esophageal varices or other sources of blood loss. Chronic bleeding associated with PG is most often detected during routine examination for IDA. After which the patient is referred to a gastroenterologist, whose qualifications determine the correct interpretation of gastric pathology. Often years pass before the diagnosis of chronic hepatitis is established and, accordingly, the beginning of correct etiotropic and pathogenetic treatment [2, 3, 5]. However, in most cases, the diagnosis of PH and, as a consequence, hypochromic IDA is made against the background of known chronic hepatitis [6, 15].
Management of patients with chronic hepatitis involves treatment of the underlying disease and prevention of complications, including PG and IDA, for which compliance with examination standards is mandatory. All patients with newly diagnosed or suspected cirrhosis should undergo esophagogastroduodenoscopy (EGD) to detect PG. The further frequency of endoscopy depends on the endoscopic picture and the degree of liver damage according to the Child–Pugh criteria [1] (Table 1).
Optimal results of prevention and treatment of PG are achieved at the stage of cirrhosis corresponding to class A.
The key method in diagnosing PG is endoscopic examination of the stomach. At the Consensus Conference on Gastric Endoscopy for Portal Hypertension (Milan, Italy, September 19, 1992), PG was defined as changes in the gastric mucosa in the form of small polygonal pink areas, slightly protruding towards the center in a “mosaic” manner.
Three main endoscopic signs have been identified:
1 – small red dots on the gastric mucosa with a diameter of less than 1 mm;
2 – red spots with a diameter of more than 2 mm;
3 – black-brown spots resulting from intramucosal hemorrhages [6, 9, 14].
K. Tanoue et al. PG was classified into three degrees of severity:
• 1st Art. – slight hyperemia of certain areas of the gastric mucosa; the mucous membrane has a “stagnant” appearance.
• 2nd Art. – pronounced redness with areas of raised, swollen gastric mucosa, separated by a thin white network, “mosaic” type changes.
• 3rd Art. – point hemorrhages against the background of macroscopic changes characteristic of the 2nd stage. [7].
The simplest classification (TT McCormack et al.) assumes two degrees of damage to the gastric mucosa. In mild cases of PH, a mosaic pattern of the mucous membrane is endoscopically revealed; in severe cases, in addition to the mosaic pattern, diffuse dark red spots and submucosal hemorrhages appear. This classification is useful for predicting the likelihood of bleeding. Thus, with a mild degree of PH, which is detected in 75% of cases in all patients with PH, the risk of bleeding is up to 25%. In severe PH, which occurs in 25% of cases, the incidence of bleeding increases to 60%, which can be fatal (see figure) [9].
Complications of alcoholic gastropathy
The prognosis for treatment of this disease is favorable if it occurs in conditions of total cessation of alcohol consumption. Otherwise, the patient faces the following complications:
- • Inflammatory process. It can affect other digestive organs and lead to diseases such as cholecystitis and pancreatitis.
- • Stomach ulcer. Loss of integrity of the mucous membrane leads to internal bleeding, which poses a threat to human life.
- • Cancer. May develop from an ulcer and spread to surrounding tissue.
VARICOSE VEINS
Varicose veins, the veins are smaller, the lower varicose veins, and are now found in 5–33% of patients with portal hypertension, with a bleeding frequency of about 25%, with a length of 2 lines and with a large amount of bleeding. veins of the bottom of the scutum (Sarin SK et al., 1992). Risk factors for vulvar varicose bleeding include the size of the nodules at the bottom of the vulva (large > medium > small, values >10 mm, 5–10 mm and <5 mm uniform), classification of cyrrhosis as Child (C > B > A) and the presence of worms them signs on the surface of the varix during the hour of endoscopy (Kim T. et al., 1999). The schulci varicose veins are usually divided on the basis of their relationship to the splanchnic veins, as well as their localization in the scutum. Gastroesophageal varices (GEVs) are extensions of varicose veins along the line and are divided into 2 types. The most common types of varix are type 1 (GEV 1 – GOV1), which widen the lesser curve. The stinks are respected by the continued VRV voyage and are guilty of rejoicing in the same way. Type 2 slug ERVs (GEV 2) are expanding at the bottom and tend to be long and sinuous. Isolated hose VRV (ISHV) are identified for the presence of varicose veins in the traveler and are also divided into 2 types. VRV type 1 (ІШВ 1 – IGV1) are localized near the day and are often sinuous and complex, and VRV type 2 (ІШВ 2) are located near the body, antrum and near the hilum. The presence of varicose veins at the bottom of the scutum will require excluding the diagnosis of splenic vein thrombosis.
Treatment of alcoholic gastropathy
Two main rules that will help you prevent the development of such a life-threatening disease are giving up bad habits; organization of balanced nutrition. It is recommended to avoid foods that are too hot and fatty, and to eat frequently in small portions.
If it was not possible to avoid alcoholic gastritis, then the key requirement for its treatment is complete and unconditional abstinence from alcohol. Conservative (without surgery) treatment involves taking the following medications:
- • Proton pump inhibitors, aimed at reducing the secretion of gastric juice.
- • Antacids aimed at neutralizing hydrochloric acid.
- • Gastroprotectors that form a special protective film over the mucous membrane.
- • Antispasmodics, which relax smooth muscles and reduce pain.
- • Prokinetics, ensuring the natural movement of food through the digestive tract.
- • A variety of vitamin and mineral complexes.
Surgical intervention for alcoholic gastropathy is not performed. The key to successful treatment is timely contact with specialists.
CLINICAL DIAGNOSIS OF PORTAL HYPERTENSION
With physical examination, it is easiest to detect ascites, redness, spider-like angiomas on the skin, erythema pubis, testicular atrophy, gynecomastia, Dupuytren's contracture and muscle atrophy. Portosystemic encephalopathy may also manifest itself - in some cases, asterixis (blurring or “purrying” tremor of the hands) and lethargy, in the lungs - restlessness, sleep disturbance. Most patients with PG also exhibit splenomegaly, although the size of the spleen correlates poorly with portal venous pressure and its increase may be associated with other illnesses. A hyperdynamic state can be indicated by a high (jumping) pulse, warm, well-perfused endings in conjunction with arterial hypotension.
Signs of portosystemic collaterals are dilated veins on the anterior surface of the abdomen (umbilical-epigastric shunts), the “jellyfish head” becomes a sinuous colateral vein near the navel. Internal hemorrhoids are not a specific finding, but may also manifest collateralization. The weakness of the visible collateral vessels on the back does not indicate portal hypertension, but obstruction in the level of the inferior venous vein. A rare cause may be compression of the lower empty vein by regenerative nodes in liver cirrhosis.
Ascites is easy to detect when there is a lot of swelling in the stomach - in such cases the life is tight, and swelling of the spine can be detected. However, minor ascites can be diagnosed instrumentally, for example, with the help of ultrasonography. Paracentesis helps to establish the etiology of ascites: the portal (non-peritoneal) etiology indicates a serum ascitic albumin gradient (SAAG) of over 1.1 g/dL (11 g/L). However, this method cannot distinguish between hepatic and suprahepatic PH, for example, in cases of persistent heart failure. Recent studies indicate a decrease in SAAG following the placement of transjugular portosystemic shunts (TIPS).
Laboratory testing of gas is not specific for the detection of PG. Indicates an increase in prothrombin hour and thrombocytopenia. In patients with cirrhosis of the liver, these indicators are considered predictors of the presence of varicose veins (see below). Hypoalbuminemia indicates the importance of liver function and, indirectly, PG. Similarly, hyponatremia and low sodium concentrations in the tissue may contribute to water and sodium retention in portal hypertensive syndrome.
Diagnosis of pathology
To detect the disease - even if there are almost no problems with the stomach - make an appointment with a gastroenterologist.
A comprehensive diagnosis is required, since there is no specific clinical picture. A gastroenterologist, after a visual examination and history taking, may prescribe the following:
- general clinical and biochemical blood test;
- general urine analysis;
- general analysis of stool;
- pH-metry;
- PCR testing;
- endoscopic examination of the gastrointestinal tract;
- esophagogastroduodenoscopy;
- X-ray of the stomach with a contrast agent;
- ELISA.
At the discretion of the doctor, the diagnostic program can be supplemented with other laboratory or instrumental research methods.
Diagnosis
The diagnosis of Ménétrier's disease is based on the identification of extreme foveal hyperplasia with glandular atrophy on biopsy in a patient with significant enlargement of the gastric folds observed on endoscopic examination or barium radiography. A full-thickness or loop biopsy is usually necessary [3,22]. Enlarged folds are limited to the body and fundus of the stomach. The folds are usually symmetrically enlarged, although asymmetrical "polypoid" enlargement may rarely occur.
Gastritis, classification, Sydney system, 9th International Congress of Gastroenterologists
In August 1990, in Sydney, Australia, at the 9th International Congress of Gastroenterologists, a new classification of gastritis , called the “Sydney system” (JJ Misiewicz, GNJ Tytgat, P. Sipponen, AB Price, CS Goodwin, RG Strickland). The histological basis of classification consists of three parts: etiology, topography and morphology.
Etiology : Helicobacter pylori (HP) associated gastritis, autoimmune gastritis, idiopathic gastritis, acute drug-induced gastritis, special forms of gastritis (reactive, eosinophilic, lymphocytic, granulomatous, collagenous, lymphoblastic). Hypertrophic gastropathy.
Morphology : tied to the section of the stomach - topography (fundic, antral, pangastritis). Based on the histological picture, the degree of inflammation, activity, atrophy, intestinal metaplasia and Helicobacter pylori contamination is assessed as: absent, mild, moderate, severe. Without assessing the degree, specific and nonspecific changes are distinguished.
The endoscopic section involves indicating the topography of pathological changes (antral, fundal, pangastritis). Manifestations of pathology (edema, erythema, swelling, aphthae, erosion, nodularity, hyperplasia, atrophy, vascular reaction, hemorrhage) are also assessed. The following categories are proposed as a diagnosis for endoscopists: erythematous, exudative, erosive, atrophic, hemorrhagic, reflux gastritis, fold hyperplasia.
Hypertrophic gastropathy (giant hypertrophic gastritis, Menetrier gastritis) is distinguished separately according to the Sydney classification.
Symptoms of the disease
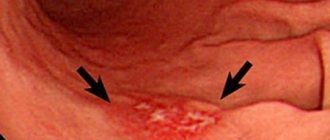
Almost always, concomitant diseases with hyperemia are gastritis, stomach ulcers, and duodenitis. Less commonly, diseases that are not related to the gastrointestinal system are associated with hyperemia (overflow of blood in the vessels of the circulatory system of any organ or area of the body).
Thus, the following symptoms are characteristic of different forms of gastritis:
- Symptoms of hyperemia (overflow of blood in the vessels of the circulatory system of any organ or area of the body) (overflow of blood in the vessels of the circulatory system of any organ or area of the body) of the gastric mucosa. The gastric mucosa is focally hyperemic, there is a coating with whitish foamy mucus on the surfaces of the organ in “mucus lakes”, the folds are compacted and are not completely smoothed out with the help of air.
- As cells die, the surface becomes thinner and turns pale. In this case, the foci of the disease do not become hyperemic, and the vascular web is clearly visible.
- In the superficial form of gastritis, the mucous surface of the stomach (a hollow muscular organ, part of the digestive tract, lies between the esophagus and the duodenum) is hyperemic throughout or only in the body and antrum of the stomach. Sometimes hyperemia is focal in nature or can be diffuse.
- If there is fibrous gastritis, hyperemia is most pronounced, while it is focal and characterized by the presence of pus. This inflammation can be caused by measles or scarlet fever infection. The patient may vomit blood frequently.
- The phlegmonous form of the disease can be triggered by trauma to the stomach with sharp objects, such as fish bones. In such cases, this indicates possible hyperemic foci.
- Bulbit is characterized by swelling and redness, thickening of folds in the antrum. Among the reasons are Helicobacter pylori infection of the antrum of the stomach (a hollow muscular organ, part of the digestive tract, lying between the esophagus and the duodenum) and unhealthy diet.
- Renal dysfunction (various degrees of swelling).
- Depression and permanent stress also provoke hyperemia.
The main symptoms of gastropathy include:
- hyperemia of the mucous membrane;
- pain in the stomach;
- nausea, vomiting;
- heaviness, weakness;
- loss of appetite.
If such symptoms appear, patients should undergo an examination and consult a doctor - an endoscopist, who will prescribe antigastritis therapy based on the diagnostic results. If necessary, with severe manifestations of pathology in the stomach, endoscopic resection will be prescribed. The prognosis after the operation is quite favorable.
Ménétrier's disease
Ménétrier described two types of disorders associated with polypoid growths in the stomach, which he tried to associate with gastric carcinoma [10].
- The first (called “polyadenomes polypeux”) consists of multiple non-confluent gastric polyps, which are currently classified as hyperplastic polyps [11].
- The second (which he called Ménétrier's polyadenomatosis, or multiple sheet-like adenomas) is a new type associated with foveal hyperplasia and should by law bear his name [8].
Authorized Products
The diet for erythematous gastropathy includes:
- Soups made from non-concentrated broths with the addition of pureed cereals/vegetables.
- Low-fat varieties of red meat/fish - chicken, rabbit, veal, turkey, beef, cod, pike perch, pollock, hake.
- Soft-boiled eggs/steam omelet.
- Dried white bread, white bread croutons.
- Puree porridges cooked in water with milk/cream (oatmeal, semolina, rice), a little later - buckwheat.
- Vegetables/fruits that can be consumed in boiled and pureed form are beets, zucchini, potatoes, carrots, and pumpkin. Fruits - sweet/ripe baked/boiled.
- Whole milk, cream, pureed calcined cottage cheese, curd soufflé.
- Sweet dishes include marshmallows, marshmallows, berry jelly, honey, jam, fruit mousses.
- For drinks, it is recommended to drink infusion of rose hips, juices with pulp (apricot, pear, peach), fruit jelly, weak tea with milk.
Table of permitted products
| Proteins, g | Fats, g | Carbohydrates, g | Calories, kcal | |
Vegetables and greens | ||||
| zucchini | 0,6 | 0,3 | 4,6 | 24 |
| cauliflower | 2,5 | 0,3 | 5,4 | 30 |
| potato | 2,0 | 0,4 | 18,1 | 80 |
| carrot | 1,3 | 0,1 | 6,9 | 32 |
| beet | 1,5 | 0,1 | 8,8 | 40 |
| pumpkin | 1,3 | 0,3 | 7,7 | 28 |
Fruits | ||||
| apricots | 0,9 | 0,1 | 10,8 | 41 |
| bananas | 1,5 | 0,2 | 21,8 | 95 |
| nectarine | 0,9 | 0,2 | 11,8 | 48 |
| peaches | 0,9 | 0,1 | 11,3 | 46 |
| apples | 0,4 | 0,4 | 9,8 | 47 |
Berries | ||||
| strawberry | 0,8 | 0,4 | 7,5 | 41 |
| raspberries | 0,8 | 0,5 | 8,3 | 46 |
Cereals and porridges | ||||
| buckwheat (kernel) | 12,6 | 3,3 | 62,1 | 313 |
| semolina | 10,3 | 1,0 | 73,3 | 328 |
| cereals | 11,9 | 7,2 | 69,3 | 366 |
| white rice | 6,7 | 0,7 | 78,9 | 344 |
Flour and pasta | ||||
| noodles | 12,0 | 3,7 | 60,1 | 322 |
Bakery products | ||||
| white bread crackers | 11,2 | 1,4 | 72,2 | 331 |
Confectionery | ||||
| jam | 0,3 | 0,2 | 63,0 | 263 |
| jelly | 2,7 | 0,0 | 17,9 | 79 |
| marshmallows | 0,8 | 0,0 | 78,5 | 304 |
| paste | 0,5 | 0,0 | 80,8 | 310 |
| Maria cookies | 8,7 | 8,8 | 70,9 | 400 |
Raw materials and seasonings | ||||
| honey | 0,8 | 0,0 | 81,5 | 329 |
| milk sauce | 2,0 | 7,1 | 5,2 | 84 |
Dairy | ||||
| milk | 3,2 | 3,6 | 4,8 | 64 |
| cream | 2,8 | 20,0 | 3,7 | 205 |
| sour cream | 2,8 | 20,0 | 3,2 | 206 |
| curdled milk | 2,9 | 2,5 | 4,1 | 53 |
Cheeses and cottage cheese | ||||
| cottage cheese | 17,2 | 5,0 | 1,8 | 121 |
Meat products | ||||
| boiled beef | 25,8 | 16,8 | 0,0 | 254 |
| beef liver | 17,4 | 3,1 | 0,0 | 98 |
| boiled beef tongue | 23,9 | 15,0 | 0,0 | 231 |
| boiled veal | 30,7 | 0,9 | 0,0 | 131 |
| rabbit | 21,0 | 8,0 | 0,0 | 156 |
Bird | ||||
| boiled chicken | 25,2 | 7,4 | 0,0 | 170 |
| turkey | 19,2 | 0,7 | 0,0 | 84 |
Eggs | ||||
| chicken eggs | 12,7 | 10,9 | 0,7 | 157 |
Oils and fats | ||||
| butter | 0,5 | 82,5 | 0,8 | 748 |
Non-alcoholic drinks | ||||
| mineral water | 0,0 | 0,0 | 0,0 | — |
| coffee with milk and sugar | 0,7 | 1,0 | 11,2 | 58 |
| black tea with milk and sugar | 0,7 | 0,8 | 8,2 | 43 |
Juices and compotes | ||||
| apricot juice | 0,9 | 0,1 | 9,0 | 38 |
| carrot juice | 1,1 | 0,1 | 6,4 | 28 |
| pumpkin juice | 0,0 | 0,0 | 9,0 | 38 |
| * data is per 100 g of product | ||||
Fully or partially limited products
The diet for erythematous gastropathy excludes:
- Concentrated meat/fish soups with non-puréed vegetables/cereals, first courses with cabbage.
- Fatty fish/meat (goose, duck, pork, lamb), canned meat, animal fats, smoked meats and sausages.
- Fresh white bread, puff pastry, baked goods, black bread.
- Some types of cereals (barley, millet, corn, pearl barley), coarse large pasta, legumes.
- Products with an irritating effect on the gastric mucosa (white cabbage, radishes, mushrooms, radishes, rutabaga, onions, turnips, beans, sorrel, garlic, peas, canned vegetables), pickled/pickled vegetables.
- Spicy dishes, seasonings/spices, tomato sauce.
- Ice cream, chocolate, pastries, coffee, cakes, nuts, seeds.
- Sour/unripe fruits that are raw/with rough skin.
- Alcohol-containing/carbonated drinks, caffeine-containing drinks (coffee, cocoa, Pepsi, cola).
Table of prohibited products
| Proteins, g | Fats, g | Carbohydrates, g | Calories, kcal | |
Vegetables and greens | ||||
| vegetables legumes | 9,1 | 1,6 | 27,0 | 168 |
| swede | 1,2 | 0,1 | 7,7 | 37 |
| cabbage | 1,8 | 0,1 | 4,7 | 27 |
| green onion | 1,3 | 0,0 | 4,6 | 19 |
| bulb onions | 1,4 | 0,0 | 10,4 | 41 |
| cucumbers | 0,8 | 0,1 | 2,8 | 15 |
| canned cucumbers | 2,8 | 0,0 | 1,3 | 16 |
| white radish | 1,4 | 0,0 | 4,1 | 21 |
| turnip | 1,5 | 0,1 | 6,2 | 30 |
| horseradish | 3,2 | 0,4 | 10,5 | 56 |
| spinach | 2,9 | 0,3 | 2,0 | 22 |
| sorrel | 1,5 | 0,3 | 2,9 | 19 |
Mushrooms | ||||
| mushrooms | 3,5 | 2,0 | 2,5 | 30 |
Cereals and porridges | ||||
| corn grits | 8,3 | 1,2 | 75,0 | 337 |
| pearl barley | 9,3 | 1,1 | 73,7 | 320 |
| millet cereal | 11,5 | 3,3 | 69,3 | 348 |
| barley grits | 10,4 | 1,3 | 66,3 | 324 |
Confectionery | ||||
| candies | 4,3 | 19,8 | 67,5 | 453 |
Ice cream | ||||
| ice cream | 3,7 | 6,9 | 22,1 | 189 |
Cakes | ||||
| cake | 4,4 | 23,4 | 45,2 | 407 |
Raw materials and seasonings | ||||
| mustard | 5,7 | 6,4 | 22,0 | 162 |
| ginger | 1,8 | 0,8 | 15,8 | 80 |
| ketchup | 1,8 | 1,0 | 22,2 | 93 |
| mayonnaise | 2,4 | 67,0 | 3,9 | 627 |
| ground black pepper | 10,4 | 3,3 | 38,7 | 251 |
| chilli | 2,0 | 0,2 | 9,5 | 40 |
| sugar | 0,0 | 0,0 | 99,7 | 398 |
Dairy | ||||
| kefir | 3,4 | 2,0 | 4,7 | 51 |
Meat products | ||||
| pork | 16,0 | 21,6 | 0,0 | 259 |
| ham | 22,6 | 20,9 | 0,0 | 279 |
Sausages | ||||
| dry-cured sausage | 24,1 | 38,3 | 1,0 | 455 |
| sausages | 10,1 | 31,6 | 1,9 | 332 |
| sausages | 12,3 | 25,3 | 0,0 | 277 |
Bird | ||||
| smoked chicken | 27,5 | 8,2 | 0,0 | 184 |
| duck | 16,5 | 61,2 | 0,0 | 346 |
| smoked duck | 19,0 | 28,4 | 0,0 | 337 |
| goose | 16,1 | 33,3 | 0,0 | 364 |
Fish and seafood | ||||
| dried fish | 17,5 | 4,6 | 0,0 | 139 |
| smoked fish | 26,8 | 9,9 | 0,0 | 196 |
| canned fish | 17,5 | 2,0 | 0,0 | 88 |
Oils and fats | ||||
| animal fat | 0,0 | 99,7 | 0,0 | 897 |
| cooking fat | 0,0 | 99,7 | 0,0 | 897 |
Non-alcoholic drinks | ||||
| bread kvass | 0,2 | 0,0 | 5,2 | 27 |
| * data is per 100 g of product | ||||
Helicobacter pylori
Acute HP-associated gastritis can cause severe inflammation and enlargement of the folds of the stomach, which may even have an appearance similar to that of malignant diseases. In chronic HP associated gastritis, an increase in the folds of the stomach may also be observed, simulating Ménétrier's disease [30,31]. Several reports indicate the presence of enlarged gastric fundus and body folds with glandular hyperplasia, hypersecretion, and the presence or absence of hypoalbuminemia in patients without Zollinger-Ellison syndrome [19,32–34]. In the past, these cases were classified as gastritis with glandular hypertrophy [35], hypertrophic hypersecretory gastropathy [34,36], and Schindler's disease [37]. However, there are two observations indicating the role played by Helicobacter pylori in the development of this pathology:
- An examination of published micrographs of these patients showed that virtually all of them had gastritis consistent with HP infection [34].
- In one study, 18 of 32 patients with thickened gastric folds were infected with Hp [38]. Anti-Hp therapy led to normalization of the size of the gastric folds. Similar findings have been described in other reports [39,40]. It is noteworthy that in two of these reports [38,39], the term “Ménétrier's disease” was inappropriately used to refer to a condition associated with enlarged gastric folds.
Pathogenesis
The pathogenesis of Ménétrier's disease is not entirely understood, but may be mediated by transforming growth factor alpha (TGF-alpha). TGF-alpha increases gastric mucus production and inhibits acid secretion [12-14]. The level of TGF-alpha in the cells of the gastric mucosa in patients with Ménétrier's disease is significantly increased [12]. The role of TGF-alpha is further supported by a mouse study in which overproduction of TGF-alpha is associated with significant hyperplasia of gastric mucus-producing cells comparable to that found in humans with Ménétrier's disease [12]. These mice also had reduced basal and histamine-stimulated acid secretion, similar to that observed in humans with Ménétrier's disease [12,13,15]. TGF-alpha may exert its effect by binding to epidermal growth factor receptors. This was suggested based on a report of a patient who experienced clinical improvement after treatment with monoclonal antibodies directed against epidermal growth factor receptors [16]. A similar connection with cytomegolovirus infection is observed in the so-called childhood Ménétrier's disease. Its mechanism can also be realized through TGF-alpha [17]. However, during various types of inflammation and regenerative responses in the stomach, increases in foveal TGF-alpha may also be observed, demonstrating that these findings may be nonspecific [18]. In addition, this pathology may be an example of the incorrect use of the term Ménétrier's disease to other forms of hypertrophic gastropathy.