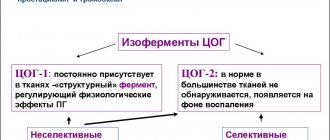
Antienzyme drugs (proteolysis inhibitors) are a group of drugs that block the action of digestive pancreatic enzymes and are used to treat acute pancreatitis.
The pancreas is an organ of mixed secretion. Its endocrine part, which is represented by the islets of Langerhans, produces hormones directly into the blood that affect carbohydrate metabolism - insulin and glucagon. The exocrine part of the pancreas produces pancreatic juice, which is critically necessary for normal digestion, which is secreted through the duct into the lumen of the duodenum.
Pancreatic juice is a mixture of enzymes that can break down all the main components of food - proteins, fats and carbohydrates - into simple compounds that can be further absorbed in the small intestine.
Pancreatic enzymes (pancreatic enzymes) have extremely powerful “digestive” powers, so the pancreas synthesizes them in an initially inactive (zymogenic) form. Under normal conditions, the transition of pancreatic enzymes to an active form occurs only in the lumen of the duodenum, when interacting with food components.
In case of disruption of the outflow of pancreatic juice, for example, in acute diseases of the pancreas, enzymes are activated directly in the tissue of the gland itself. This, in turn, leads to so-called autolysis - “self-digestion” of the pancreas and enzymatic “melting” of surrounding tissues.
Autolysis of the pancreas is an acute condition that threatens human life. In the absence of adequate assistance measures, there is a high risk of death for the patient.
One of the basic groups of drugs that are used for the prevention and treatment of autolysis of the pancreas includes anti-enzyme drugs - proteolysis inhibitors.
Indications for use
Antienzyme drugs are used for diseases that are accompanied by a violation of the outflow of pancreatic juice from the pancreas: acute pancreatitis, exacerbations of chronic pancreatitis, severe injuries of the pancreas, pancreatic tumors, swelling of the walls of the duodenum with long-term active intake of alcohol.
Anti-enzyme drugs are also used to prevent severe bleeding and severe blood loss in case of operations on the heart, cardiac (coronary) vessels, lungs, etc.
Modern enzyme therapy of chronic pancreatitis with a predominance of pain
The high socio-economic significance of the problem of chronic pancreatitis (CP) is associated with its high prevalence, the worldwide increase in incidence, and the increasing incidence of temporary disability and disability of patients. In recent decades, in developed countries, CP has become noticeably “younger”: the average age since diagnosis has decreased from 50 to 39 years. This trend is associated with the deterioration of the environmental situation, increased consumption of alcohol, including low quality, and changes in dietary patterns. Among the sick, the proportion of women increased by 30%, and primary disability of patients reaches 15%.
The leading symptoms in the clinical picture of CP are pain and signs of insufficiency of the excretory function of the pancreas (PG) - diarrhea, polyfecal matter, steatorrhea, weight loss [1, 4].
Important clinically and socially are such features of CP as a progressive course with a gradual increase in exocrine insufficiency, persistence of pain and dyspeptic syndrome, the need to follow a diet, and constant, even lifelong, use of enzyme preparations.
The main indication for the use of enzymatic agents in CP is the state of impaired digestion and absorption of nutrients - maldigestion and malabsorption syndrome.
Of all the symptoms of CP, pain is the most difficult to eliminate, often leading to disability of patients and frequent re-hospitalization. The development of pain during exacerbations of the disease is associated with an increase in pressure in the pancreatic ducts. Pancreatic enzymes are also used to relieve pain during exacerbation of CP. Their analgesic effect is due to the fact that the entry of pancreatic enzymes, primarily trypsin, into the duodenum leads to the destruction of regulatory proteins - secretin releasing peptides and cholecystokinin. The destruction of these proteins by exogenous enzymes stops the release of the corresponding hormones and reduces pancreatic secretion, which reduces the pressure in the ducts and parenchyma of the pancreas and relieves pain.
In the last decade, enzyme preparations in the treatment of exacerbations of CP have become much more widely used, which provides not only compensation for impaired digestion, but also relief of clinical manifestations of the disease, primarily pain, which, in turn, helps to improve the quality of life of patients.
All enzyme preparations presented in pharmacies can be divided into three groups:
- tableted pancreatins;
- medications that, in addition to pancreatin, contain bile components or adsorbents (dimethicone or simethicone);
- drugs in capsules containing pancreatin microgranules coated with an enteric coating.
Mikrasim® - microgranulated pancreatin in capsules - is a representative of the latest generation of enzyme preparations. It is produced from a high-tech German substance in accordance with GMP standards and is available in two dosages - 10,000 units and 25,000 units for lipase. The Micrasim® capsule dissolves in the stomach within 1–2 minutes, releasing pancreatin microgranules. Microgranules in the stomach are evenly mixed with food and, due to their small size, quickly penetrate the duodenum. This ensures the reproduction of the natural digestion process and the maximum speed of onset of the effect.
The acid-resistant shell of the microbeads allows the enzymes to be kept completely intact before they begin to work in the intestines, which ensures their maximum digestive activity.
Purpose of the study
In an open study, based on clinical and biochemical data, the effectiveness and safety of microgranulated pancreatin in Mikrasim® 10,000 units capsules was assessed in patients diagnosed with CP accompanied by pain, and also provided a comparative assessment of the effectiveness of therapy with Mikrasim® 10,000 units and pancreatin tablets 0.25 mg (10,000 units for lipase).
Study design
The study included a screening phase, which was conducted 1–2 days before the first dose of study drug to determine whether the patient met the inclusion/exclusion criteria.
After randomly assigning patients to groups, they were prescribed the drug for two weeks according to the manufacturer's recommended dosage: Micrasim® 10,000 units, 2 capsules 3 times a day, tableted pancreatin, 4 tablets 3 times a day.
Criteria for inclusion of patients:
- CP with pain syndrome;
- men and women from 18 to 65 years old;
- availability of documented, signed informed consent to participate in the study;
- patient's ability to perform study procedures.
Exclusion criteria:
- pregnancy or breastfeeding;
- lack of informed consent to participate in the study or to fulfill the requirements of the study;
- acute pancreatitis;
- severe forms of concomitant diseases;
- marked deterioration of the patient’s condition due to increasing severity of the disease or the addition of another disease;
- withdrawal of patients' consent to take the drug;
- individual intolerance to the drug and/or its components, a history of clinically significant allergic reactions;
- patient participation in other studies.
Material and research methods
50 patients were observed - 15 (30%) women and 35 (70%) men aged from 21 to 60 years. The main group A consisted of 30 patients who received the drug Mikrasim® 10,000 units. Control group B consisted of 20 patients who received pancreatin tablets 0.25 mg (10,000 units for lipase).
The clinical picture of the disease upon admission was typical in both compared groups and was characterized primarily by aching pain in the upper abdomen, loose stools, and flatulence.
Study safety
The safety of taking the drugs was monitored throughout the entire period of taking the drug and subsequent observation. Possible side effects of enzyme therapy were taken into account [4,6], which included:
- pain in the oral cavity;
- skin irritation in the perianal area;
- abdominal discomfort;
- allergic reactions to pork protein.
The following clinical adverse events (AEs) were also taken into account:
- all serious AEs;
- non-serious AEs of special interest;
- AEs dictating the need to interrupt the study drug. An additional criterion for premature discontinuation of the drug was the refusal of patients to take the drug.
The effectiveness of the therapy
The effectiveness of the therapy was assessed based on clinical symptoms (abdominal pain, stool consistency, stool frequency) on a 4-point scale (1 point is normal, 4 points is the maximum severity of the disorder).
The data from the initial (1st day), intermediate (7th day) and final (14th day) clinical and laboratory examinations were compared.
At the end of the study, the doctor assessed the clinical effectiveness of the drug according to the following criteria:
- pronounced effect (4 points) - a decrease in the number of points by 50% or more;
- good effect (3 points) - reduction in the number of points from 25% to 45%;
- satisfactory effect (2 points) - reduction in the number of points by less than 25%;
- unsatisfactory effect (1 point) - lack of positive dynamics or deterioration of the patient’s condition.
During the randomization period and on the 14th day of treatment, all patients underwent clinical and biochemical blood tests, a general urine test, and a stool test (coprogram). During hospitalization and before discharge from the hospital, anthropometric measurements (height, weight, Quetelet index) were performed. Systolic and diastolic blood pressure, pulse were determined daily, and the abdomen was palpated. The frequency of stool and the presence and/or absence of flatulence were determined. During the first days of hospital stay, all patients underwent ultrasound of the pancreas.
Research results
In both groups, no cases of side effects were recorded while taking the drugs.
Dynamics of patient complaints and assessment of the effectiveness of treatment with Micrazim® 10,000 units in main group A
The severity of symptoms in the main group before treatment and on day 14 is presented on
,
Mikrazim® 10,000 units effectively had a statistically significant effect on all considered symptoms of CP (abdominal pain, number of bowel movements, stool consistency). Both on the 7th day and on the 14th day, the change in symptoms towards improvement was significant compared to the previous period and was noted in all 30 patients of the group. During treatment with Mikrasim® 10,000 units, the pathogenetic relationships between symptoms were destroyed. The change in cumulative symptoms already on the 7th day of treatment with Micrazim® 10,000 units in a positive direction was significantly significant (Wilcoxon test, z = 3.5, p = 0.0034). The percentage of patients with a “significant effect” rating on day 14 of treatment exceeded the percentage of patients with the same rating on day 7 (77% vs. 34%, p = 0.0016, Fisher test 2x2) (
).
Dynamics of patient complaints and assessment of the effectiveness of treatment with pancreatin tablets in control group B
The severity of symptoms in group B before treatment and on the 14th day of treatment is presented in
,
.
Pancreatin tablets during treatment on day 7 had a statistically significant effect on all considered symptoms of CP (abdominal pain, number of bowel movements, stool consistency). However, on day 14 of treatment compared to day 7, pancreatin tablets had a significant effect only on pain. The percentage of patients with a “significant effect” rating on day 14 of treatment did not exceed the percentage of patients with the same rating on day 7 (10% vs. 5%, p = 1, Fisher test 2x2), and 10% of patients did not note any effect from treatment (
). Comparative analysis of the effectiveness of treatment with microgranulated pancreatin in capsules Mikrasim® 10,000 units and pancreatin in tablets in patients with CP
When assessing the results, it is worth considering that the daily dose of Pancreatin in tablets 0.25 mg (10,000 units for lipase) (4 tablets 3 times a day) was 2 times higher than the daily dose of Micrazim® 10,000 units (2 capsules 3 times a day) . A comparative analysis of the data showed that Mikrasim® 10,000 units had a more pronounced (statistically significant) effect on reducing abdominal pain (
).
Mikrazim® 10,000 units had a more pronounced (statistically significant) effect on the normalization of stool: reduction in bowel movements, normalization of stool consistency (
,
).
When comparing the study results in both groups, it was revealed that the overall effectiveness of therapy in group A (during treatment with Mikrasim® 10,000 units) was significantly (p < 0.05) higher than in group B (during treatment with pancreatin tablets) both on the 7th day and on the 14th day of treatment for CP. On the 14th day of treatment with Mikrasim® 10,000 units, a good and pronounced effect of therapy was noted in 97% of cases (20% and 77% of patients, respectively). In group B, on the 14th day of treatment with Pancreatin tablets, only 10% of patients noted a pronounced effect of treatment. However, in 10% of patients in group B there was no effect of therapy (
).
conclusions
- The study revealed high (reliably significant) therapeutic efficacy of Mikrasim® 10,000 units in patients with CP with pain. A positive effect of therapy was observed in 100% of patients, in 77% of cases it was characterized as “pronounced”.
- The drug Mikrasim® 10,000 units had a more pronounced (statistically significant) effect on reducing abdominal pain and normalizing stool (reducing bowel movements, normalizing stool consistency) compared to pancreatin tablets, both on the 7th day and on the 14th day of treatment for CP.
- The study revealed good tolerability of the drug Mikrasim® 10,000 units. No cases of adverse events were noted.
- The drug Mikrasim® 10,000 units can be recommended for the treatment of patients with CP with pain syndrome as an effective drug with good tolerability.
- The affordable cost of Micrasim® allows us to prescribe high-quality and modern treatment to a large number of patients, including those under the DLO program. This is an essential factor in choosing a drug for any duration of use.
Literature
- Gastroenterology and hepatology: diagnosis and treatment / ed. A. V. Kalinina and A. I. Khazanov. M.: Miklos; 2007. 602 p.
- Grinevich V. B., Bogdanov I. V., Sablin O. A. Clinical and pharmacoeconomic aspects of multienzyme replacement therapy // Klin. prospects for gastroenterol., hepatology. 2004. No. 2. P. 16–23.
- Korotko G.F. Secretion of the pancreas. M.: “Triad-X”, 2002. 224 p.
- A short guide to gastroenterology / ed. V. T. Ivashkina, F. I. Komarova, S. I. Rapoport. M.: LLC “Izdat. House "M-Vesti", 2001. 458 p.
- Loginov A.F. Enzyme replacement therapy for pancreatic insufficiency and disorders of cavity digestion // Pharmateka, 2005, No. 1. - P. 29–35.
- Okhlobystin A.V., Buklis E.R. Digestive enzymes in gastroenterology // Consilium Medicum, 2003, volume 5, no. 6, pp. 322–327.
T. N. Popova , Candidate of Medical Sciences E. A. Janashiya , Candidate of Medical Sciences A. F. Loginov , Candidate of Medical Sciences,
Associate Professor A.V. Kalinin , Doctor of Medical Sciences, Professor of the State Military Clinical Hospital named after. N. N. Burdenko, State Administration of Internal Affairs of the Ministry of Defense of the Russian Federation , Moscow
pharmachologic effect
Antienzyme drugs block the most powerful pancreatic enzyme, trypsin. Trypsin breaks down proteins (proteins) through a biochemical process called proteolysis. That is why antienzyme drugs are also called proteolysis inhibitors. At the same time, the processes of self-digestion in the pancreas stop and toxins - autolysis products - stop being released into the blood.
Antienzyme drugs additionally have a hemostatic effect by blocking the action of other protease enzymes in the blood responsible for the breakdown of proteins. In particular, antienzyme drugs inhibit the activity of the enzyme fibrinolysin (plasmin), which is responsible for the destruction of fibrin threads that stabilize blood clots, thereby preventing bleeding from stopping.
In addition, antienzyme drugs have a nonspecific anti-inflammatory effect.
Ingitril: effective dosages, indications and contraindications
Contrical is an anti-enzyme drug
This substance is obtained by processing the lung tissue of cattle. Inhibits the production of plasmin, kininogenase, trypsin, chymotrypsin, kallikrein.
The drug is used in the treatment of pancreatitis, for prophylactic purposes during surgical interventions on the gastrointestinal tract, and for nonspecific inflammation of the salivary glands.
In addition, Intrigil is indicated for relieving shock and massive blood loss. The active substance is excreted within 7–10 hours through the urinary system.
At the initial stage of therapy, the patient is administered 100 units, then another 200 units during the day. Then the dose is increased to 300 units. Treatment is carried out for 5 days. The drug is administered exclusively intravenously.
Contraindications for use:
- pregnancy;
- lactation;
- DIC syndrome;
- individual intolerance to components.
Before 1 use of the drug, it is advisable to conduct skin tests. Use with caution when treating patients who have recently taken muscle relaxants.
Basics of treatment with antienzyme drugs
Considering the peculiarities and difficulties in selecting an individual dose of antienzyme drugs for a particular patient, as well as a wide range of side effects and interactions with other drugs, proteolysis inhibitors are prescribed only by an experienced doctor in a hospital setting.
Aprotinin can cause a severe allergic reaction, including anaphylactic shock. It is strictly contraindicated to prescribe the drug to patients who have been administered aprotinin over the past year.
Gordox: indications and recommended dosages
Antienzyme drugs for pancreatitis: a wide choice
The drug is a complete analogue of Kontrikal. The main active ingredient is aprotinin.
Indicated in the treatment of various forms of pancreatitis, including those caused by trauma and surgery.
To relieve a primary attack in adult patients, 0.5 million units of the drug are used. The antienzyme is administered by infusion.
When the patient's condition normalizes, the maintenance dose is 200 thousand units every 6 hours, but not less than 1 million per day. If general indicators improve, the dosage regimen is 0.5 million units per day.
Side effects during treatment with Gordox develop extremely rarely. Most often, hypersensitivity reactions occur, a decrease in blood pressure, and the appearance of dyspepsia are possible.