Analysis of stool for dysbiosis in children is considered the main method that can determine the immaturity of the gastrointestinal tract, as well as a violation of healthy intestinal microflora. Dysbacteriosis in an infant can be a consequence of many pathologies. Dysbacteriosis does not have a specific clinical picture, so it can occur, accompanied by various symptoms. Many manifestations of this pathology are similar to signs of other diseases of the digestive tract. For this reason, in order to diagnose dysbiosis, it is necessary to undergo appropriate laboratory tests. The correct collection of material for analysis deserves special attention. This process can be difficult for parents, since with dysbiosis the child may experience constipation or difficulty defecating. What do you need to know when collecting biomaterial for analysis?
What does the analysis for dysbacteriosis show?
Dysbacteriosis is an imbalance of intestinal microflora: a condition in which the number of pathogenic and opportunistic bacteria exceeds the number of beneficial ones.
According to the provisions of the regulatory document of the World Health Organization, it is not considered a disease, but many pediatric doctors prescribe tests in the first year of a child’s life to identify dysbiosis and its causes. With this diagnosis, the baby may experience prolonged constipation, diarrhea, abdominal pain, and allergic reactions.
Experts believe that stool analysis for dysbiosis in infants should reflect information about the biocenosis in the intestines. In fact, the analysis only reflects the microbial composition of stool. From it you can judge what the current state of the microflora is, but how normal it is for a particular child cannot be understood from it.
On the issue of intestinal dysbiosis and its correction in children
The intestinal microbiocenosis plays a vital role in the life of the human body. The entire intestinal microflora can be divided into three groups: the main one - bifidobacteria (Bifidobacterium (B.) bifidum, B. brevis, B. longum, B. adolescentis, etc.) and lactobacilli (Lactobacillus (L.) acidophilus, L. fermentum, L. brevis, L. lactis, etc.); concomitant - E. coli with typical biological properties and enterococci (Enterococcus (E.) fecalis, E. faecium) and residual - opportunistic bacteria of the Enterobacteriaceae family: Klebsiella, Citrobacter, Proteus, etc., as well as yeast-like fungi.
Normal microflora performs important functions - protective, metabolic and immuno-inducing, participating in the maintenance of homeostasis. Violation of any of them is accompanied by changes in metabolism, the occurrence of micronutrient deficiency, and a decrease in immune status.
Disturbances of intestinal microbiocenosis are associated with the nature of nutrition, age, antibacterial, hormonal, radiation therapy, chronic diseases of the gastrointestinal tract, changes in immune status, and environmental conditions; they occur long before clinical manifestations. Changes in normal microflora are called dysbiosis, or dysbiosis. Dysbacteriosis is a microbiological concept and in no case can be an independent diagnosis.
A microecological classification of the severity of intestinal dysbiosis was proposed in 1998 by S. D. Mitrokhin.
I degree of severity - the total number of E. coli is increased or decreased. There are no E. coli with atypical biological properties. The number of bifidobacteria and lactobacilli was not changed. Changes in the general indicators of the microbial metabolite passport of feces are characteristic only in relation to the pool of volatile fatty acids (VFA), the content of phenylpropionic acid, skatole and methylamine. The total content of VFA in patients with the first degree of severity of dysbiosis may be slightly lower or higher than in healthy individuals. Skatole content is reduced. The content of phenylacetic acid and methylamine is increased. Changes in the specific gravity of oxaloacetic acid in the dicarboxylic acid (DCA) profile are noted. Dysbacteriosis is latent, compensated, intestinal dysfunctions are not recorded.
II degree of severity - the number of bifidobacteria and lactobacilli is slightly reduced. Quantitative and qualitative (appearance of forms with atypical biological properties) changes in E. coli are observed. Opportunistic intestinal microorganisms are sown in moderate quantities. Changes are noted in both general and specific indicators of the microbial metabolite passport of feces (decreased excretion of phenolic compounds in feces: p-cresol, indole, and skatole). The amount of phenylpropionic acid is an order of magnitude higher than that in healthy individuals. The profile of phenolic compounds (PCs) also changes: the specific gravity of indole increases by more than 2 times, the specific gravity of p-cresol decreases slightly, and the specific gravity of skatole decreases by more than 10 times. In general, fecal excretion of carbonic and aromatic amino acids, phenolic and indole compounds (with the exception of phenylalanine), as well as histamine and serotonin is reduced in grade II dysbiosis. The profile of complex and simple amines (AMPs) has been changed: the specific gravity of histamine and serotonin is lower, and the representative of simple amines - methylamine - is higher. Dysbacteriosis - local (local), subcompensated; intestinal dysfunctions are usually not observed.
III degree of severity - a significant decrease in the number of bifidobacteria (105–106) combined with a decrease in the number of lactobacilli and a sharp change in the typical properties of Escherichia coli (a significant predominance of hemolytic, lactose-negative forms). A significant increase in the number of opportunistic bacteria with pathogenic properties (hemolytic forms) and pathogenic yeast-like fungi (genus Candida albicans, Geotrichum, etc.). Characterized by even more pronounced changes in both general and specific indicators of the microbial metabolite passport of feces. The amount of excretion with feces of PS: p-cresol and indole is reduced. Skatole is practically absent in feces. On the contrary, the content of phenylpropionic acid in feces increases sharply. The PS profile changes in such a way that the specific gravity of indole increases significantly and the specific gravity of p-cresol decreases significantly. The content of histamine and serotonin in feces may be higher or lower than normal (depending on the nosological form of the underlying disease). The content of carboxylic acids changes as follows: the VFA pool sharply decreases, oxaloacetic acid is practically not detected, and excretion of alpha-ketoglutaric acid with feces increases significantly. The LFA profile has been changed. In case of stool disorders such as diarrhea, the specific gravity of acetic acid is lower, and propionic and butyric acids, on the contrary, are increased; with constipation, the opposite picture is observed. There is a decrease or increase in the specific gravity of lactic acid and similar changes in the specific gravity of alpha-ketoglutaric acid in the DKC profile.
IV degree of severity - a sharp decrease or absence of bifidobacteria, a significant decrease in the number of lactobacilli, a sharp decrease in the number or absence of E. coli with typical properties, a significant increase in the number of both obligate and facultative species (not normally found) of intestinal bacteria and yeast-like fungi with pathogenic properties . Pathogenic intestinal bacteria (Salmonella, Shigella, Yersinia) are detected. Qualitative changes in the microbial metabolite passport remain the same as in grade III, but their quantitative characteristics are even more changed; characterized by a deep imbalance in the biochemical regulatory mechanisms of the microbial ecosystem, combined with a similar imbalance in the intestinal microbial infrastructure. Dysbacteriosis - widespread (with bacteremia), decompensated (with the threat of generalization of infection, sepsis or septicopyemia); Severe intestinal dysfunctions are observed.
In clinical practice, three main groups of intestinal microflora are conventionally distinguished: obligate - constantly occurring (resident, autochthonous, indigenous); additional (accompanying) and transitory (random, allochthonous) (Table).
Depending on the clinical manifestations, various degrees of dysbacteriosis are distinguished. In particular, one of these classifications belongs to V. A. Tabolin (1998).
I degree - the latent phase of dysbiosis, manifests itself only in a decrease by one or two orders of magnitude in the amount of protective microflora - bifidobacteria, lactobacilli, as well as full-fledged E. coli (up to 80% of the total amount). The remaining indicators correspond to the physiological norm (eubiosis). As a rule, the initial phase does not cause intestinal dysfunction and occurs when exposed to unfavorable factors (malnutrition, etc.). In this phase, it is possible for a small number of individual representatives of the opportunistic flora to grow in the intestines. There are no clinical manifestations of dysbacteriosis in this phase.
Stage II is the starting phase of more serious disorders, characterized by a pronounced deficiency of bifidobacteria against the background of a normal or reduced number of lactobacilli or a decrease in their acid-forming activity, an imbalance in the quantity and quality of E. coli, among which the proportion of lactose-negative or citrate-assimilating variants is increasing. At the same time, against the background of a deficiency of protective components of the intestinal microbiocenosis, the proliferation of either plasma-coagulating staphylococci, or Proteus, or fungi of the genus Candida albicans occurs. Vegetation in the intestines of Proteus or plasma-coagulating staphylococci in this phase of the development of dysbacteriosis is often transient rather than permanent. Functional digestive disorders are not clearly expressed - sporadically loose stools of a greenish color with an unpleasant odor, with a shift in pH to the alkaline side, sometimes - stool retention; Nausea may occur.
III degree - phase of aggression of the aerobic flora, characterized by a clear increase in the content of aggressive microorganisms; at the same time, Staphylococcus aureus and Proteus, hemolytic enterococci multiply up to tens of millions in association; replacement of full-fledged Escherichia with bacteria of the genera Klebsiella, Enterobacter, Citrobacter, etc. is observed. This phase of dysbiosis is manifested by intestinal dysfunction with disorders of motility, enzyme secretion and absorption. Patients experience frequent, loose stools, often green in color, deterioration in health, children become lethargic and moody.
IV degree is a phase of associative dysbiosis, characterized by a deep imbalance of intestinal microbiocenosis with a change in the quantitative ratios of the main groups of microorganisms, a change in their biological properties, and the accumulation of toxic metabolites. Vegetation of enteropathogenic serotypes of E. coli, Salmonella, Shigella and other pathogens of acute intestinal infections is characteristic. Clostridia may multiply. This phase of dysbiosis is characterized by functional disorders of the digestive system and disturbances in general nutritional status, body weight deficiency, pale skin, frequent stools mixed with mucus, greens, sometimes blood, with a sharp putrid or sour odor.
To confirm the diagnosis, the following research must be performed:
- study of clinical signs;
- examination of the upper gastrointestinal tract, including endoscopic with aspiration of contents or biopsy of the jejunum, including bacteriological examination of aspirate or biopsy (this is the most accurate method, but due to technical difficulties it cannot be used on a daily basis);
- stool culture for dysbacteriosis;
- study of coprogram after preliminary food load.
Determining the composition of fecal microflora is the most accessible method, but it is not informative enough, since it reflects the microbial composition of only the distal parts of the intestine. An important diagnostic test for bacterial overgrowth syndrome is an excretory breath test with the identification of various metabolites that are produced with the participation of intraintestinal bacteria (lactulose test with determination of H2 in exhaled air). The latter seems to be the most acceptable for everyday pediatric practice. Gas-liquid chromatography allows you to evaluate chemical compounds associated with the life of normal microflora. In some cases, it is advisable to study lipopolysaccharides (LPS)-O-antigen and the level of enterotoxins. To determine the degree of dysbacteriosis, you can use the standard method of R. B. Epstein-Litvak and F. L. Vilshanskaya (1969).
Correction of the normal composition of the intestinal flora should be comprehensive and aimed primarily at treating the underlying disease that caused the microflora imbalance; a complex of therapeutic and protective measures is needed for the general health of the child’s body in general and for the correction of its microflora in particular.
The organization of a protective regime includes a favorable psychological atmosphere, prolonged exposure to fresh air, longer sleep, and an appropriate diet.
Adequate, balanced nutrition in accordance with the child’s age with normal functioning of organs and systems prevents the development of dysbiosis. The most important activity is natural feeding of infants. The ideal food for a newborn baby and children in the first months of life is mother’s milk, which is most suitable for her child, as it has a family connection with his tissues. Breast milk carbohydrates (90% of which are β-lactose) are fermented by bifidobacteria into lactic acid, which maintains a low stool pH in infants. The influence of natural feeding on the state of intestinal microbiocenosis in young children has been repeatedly noted in the literature. Thus, the composition of the microflora of meconium of newborns who received breast milk was characterized by a low content of aerobic microflora, a predominance of bifid flora over aerobic flora and a low content of putrefactive bacteria (clostridium, bacteroides, genus Proteus). With artificial feeding, the growth of bifidobacteria was either completely absent or their content was sharply reduced.
Currently, so-called functional nutrition is becoming widespread. Ready-made food products are consumed, to which biological products, antioxidants, carotenoids, enzymes and other substrates have been added. For infants who are bottle-fed, adapted formulas enriched with representatives of microflora are recommended: “Malyutka”, “Biolact adapted”, “Bifidok”, “Bifilin”, “Bifidolakt”, “Bifilife”, “Vitalakt”, etc. In many cases, the use of the dry mixture “Lactofidus” containing bifidobacteria and streptococci, and the mixture “NAN” with bifidobacteria, is justified. For nursing children of the neonatal period, functional nutrition has been developed in the form of lyophilized breast milk enriched with B. bifidum. Taking into account that one of the most important stages of nursing newborns in intensive care units is the organization of rational feeding, recently at the Department of Neonatology of the Russian State Medical University a new type of functional nutrition was developed specifically for the above-mentioned contingent of children. This type of functional food is lyophilized breast milk containing B. bifidum 791 at a concentration of 108 CFU/ml (Yu. G. Mukhina, 2000). Functional nutrition does not belong to the category of drugs; it is used to improve human health in general.
The diet for children with manifestations of dysbiosis should be complete in terms of calories and the content of basic physiological ingredients. Eating should be done at the same hours; it is advisable to restore the endogenous biorhythm of digestion. Food should be as varied as possible. In this case, it is advisable to exclude or limit the consumption of products that are aggressive towards autoflora, such as pasta and noodles made from premium flour, canned food and semi-finished products from meat, fish, legumes, all types of pork, lamb, liver, kidneys, brains, refractory animal fats, whole and condensed milk, sweet yoghurts, canned vegetables and fruits, sweets, lemonade, ice cream, chocolate. The group of recommended products that stimulate the growth of indigenous intestinal microflora includes lentils, products made from wheat, rye, corn, buckwheat, millet, some vegetables - cabbage, carrots, zucchini, pumpkin, artichoke, Jerusalem artichoke, fresh fruits, non-canned fruit and vegetable juices, nuts, lean meat and fish, fermented milk products, vegetable fats.
In complex diet therapy for patients with dysbacteriosis, mare's kumiss can also be used. Under its influence, putrefactive processes in the intestines in patients are reduced, the number of putrefactive bacteria is reduced, and the biological properties of E. coli and other representatives of indigenous microflora are improved. E. A. Tolmacheva developed a technology for making kumiss from cow's milk, and studied its effect on intestinal microflora. The use of cow koumiss led to a sharp decrease in the growth of spore-bearing anaerobic bacteria in the intestine, mainly B. putrificus, B. perfringens and B. sporogenes.
The presence in the diet of vegetables, fruits and plants that have antimicrobial activity is also important. Many antibacterial substances isolated from plants stimulate the body's immunobiological reactions and inactivate bacterial exotoxins and hyaluronidase.
It seems appropriate to emphasize that only complex correction of dysbiosis provides a more pronounced, persistent clinical and microbiological effect. One of the main components of this comprehensive approach is replacement therapy.
The most important method of treating intestinal dysbiocenosis (dysbacteriosis) is the use of drugs of biological origin that can regulate the balance of intestinal microflora. In order to eliminate the deficiency of indigenous flora, probiotics are used - preparations of living microorganisms (representatives of indigenous intestinal microflora) that have the ability to purposefully regulate intestinal microecology and restore eubiosis. These are lactic acid bacteria, bifidobacteria, and lactic acid streptococci.
Probiotic preparations based on these microorganisms are widely used in Western European countries, Canada and the USA as food additives, as well as in yoghurts and other dairy products. The microorganisms that make up probiotics are non-pathogenic, non-toxic, and remain viable during storage. Probiotics are not considered drugs, but are considered to have a beneficial effect on human health. In pediatric practice, probiotics such as Bifidumbacterin, Lactobacterin, Colibacterin, Bificol, Bifidumbacterin-forte, Acylact, Primadofilus, Linex, Bifiform, etc. have become widespread.
Linex is a complex preparation consisting of live lyophilized bacteria B. infantis v. liberorum, L. acidophilus and Enterococcus faecium, resistant to antibiotics and chemotherapeutic agents. The live bifidobacteria, lactobacilli and non-toxigenic fermented milk streptococcus of group D (isolated from the intestines of a healthy person) included in Linex maintain and regulate the physiological balance of intestinal microflora in all parts of the intestine. Available in capsules. 1 capsule contains 1.2 x 107 live lactic acid bacteria (bifido- and lactoflora). When taken, unfavorable conditions are created for the development of pathogenic and conditionally pathogenic intestinal microflora due to the production of acetic, lactic and propionic acids by the fermented milk flora, which reduces the pH of the intestinal contents. Dosage: adults, 2 capsules 3 times a day, children, 1 capsule 3 times a day. Although Linex bacteria are acid-resistant, it should be remembered that at a pH less than 3.5 they may lose their viability - accordingly, the drug will lose its properties. Therefore, it is recommended to take it after meals. The course of treatment depends on the severity of dysbacteriosis; in any case, replacement therapy should be long-term. The strains of microorganisms that make up Linex are specially bred and antibiotic-resistant. That is why the drug can be prescribed even against the background of antibiotic therapy. As a rule, the microorganisms that make up probiotics perform the function of temporarily filling the eco-niche of the microflora, which is eventually occupied by colonies of strains that initially inhabit the intestinal mucosa. Depending on the degree of dysbiosis, this replacement occurs at different time intervals. That is why replacement therapy with probiotics should be of a fairly long intermittent nature.
To enhance the effect of probiotics, it is recommended to additionally prescribe drugs with anti-inflammatory and immunomodulatory properties: F (Immunal, Echinacea with Golden Seal), complex immunomodulatory drugs and vitamin-mineral complexes.
Optimizing diagnostics, individualizing treatment and preventing intestinal dysbiosis will improve the effectiveness of therapy and ensure a stable bacteriological background of the human intestine, which will help maintain a good quality of life.
P. L. Shcherbakov , Doctor of Medical Sciences, Professor of the Scientific Center for Children's Health of the Russian Academy of Medical Sciences, Moscow
Symptoms and norms
When clostridia, lactose-negative enterobacteria, Klebsiella, Candida fungus, Staphylococcus aureus and other pathogens begin to predominate in the intestine over beneficial microorganisms, dysbiosis develops. Increased proliferation of microbes in a baby is accompanied by the following symptoms:
- regurgitation;
- diarrhea;
- blood, mucus in stool;
- constipation;
- diseases of the digestive system;
- increased accumulation of gases in the stomach (bloating);
- allergic reactions;
- abdominal pain;
- poor appetite;
- there are traces of white coating on the tongue;
- bad breath;
- the baby is often sick.
They will prescribe a dysbiosis test for the baby during or after antibiotic treatment. These medications can cope not only with pathogenic bacteria that have affected the body (clostridium, klebsiella, Staphylococcus aureus, etc.), but also kill beneficial microflora.
Dysbacteriosis can only be detected through stool analysis. Thanks to it, it is possible to determine which pathogenic bacteria have exceeded the norm, which will make it possible to prescribe the correct therapy. This is explained by the fact that to destroy the Candida fungus, different methods are needed than when activating clostridia, Staphylococcus aureus, Klebsiella or other pathogen.
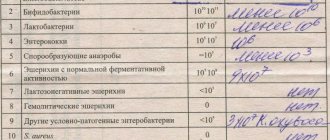
Normally, the number of beneficial and harmful bacteria in the intestines of a baby should fluctuate within the following limits:
| Bacteria | Normal for infants | Children 1 year | After a year | ||
| Form of feeding | |||||
| Chest | Mixed | Artificial | |||
| Bifidobacteria | 10 7 -10 11 | 10 6 -10 9 | 10 6 -10 8 | 10 10 -10 11 | 10 9 -10 10 |
| Lactobacilli | 10 5 | 10 4 -10 6 | 10 4 -10 6 | 10 6 -10 7 | 10 7 -10 8 |
| E. coli | 10 5 -10 8 | 10 6 -10 9 | 10 7 -10 9 | 10 7 -10 8 | 10 7 -10 8 |
| Lactose-negative enterobacteriaceae | 10 3 -10 6 | 10 5 -10 7 | 10 5 -10 7 | up to 10 4 | until 10 7 |
| Enterococci | — | 10 5 -10 9 | 10 6 -10 9 | 10 6 -10 7 | 10 7 -10 8 |
| Staphylococcus | 10 2 -10 4 | 10 3 -105 | 10 3 -10 6 | up to 10 5 | up to 10 4 |
| Clostridia | 10 1 -10 3 | 10 2 -10 4 | 10 3 -10 6 | up to 10 5 | up to 10 5 |
| Candida | 10 2 -10 4 | 10 1 -10 3 | 10 2 -10 4 | up to 10 3 | up to 10 4 |
It is not worth treating a child on your own, having only a transcript of the results in hand: a doctor must prescribe treatment. Each of these pathogens (clostridia, lactose-negative enterobacteria, Klebsiella, Escherichia) requires an individual approach to treatment. Otherwise, you can cause serious harm to the baby and provoke the development of chronic diseases that he will not get rid of even as an adult.
Causes of dysbacteriosis
The most common cause of dysbiosis is considered to be immaturity of the gastrointestinal tract. While the baby is in the mother's womb, its intestines are sterile. The first microorganisms enter it during childbirth, from the mother.
Dysbacteriosis in a child can also appear due to:
- late start of breastfeeding or lack thereof. Mother's milk contains an optimal range of nutrients and is a natural source of bifidobacteria. If a child eats breast milk from the very first day, then by the end of the first week an almost complete complex of necessary bacteria is formed in the intestines;
- use of antibiotics. The biggest risk associated with taking antibiotics is the loss of beneficial microflora. If a nursing mother took antibiotics, the process of deterioration of digestion will also be observed in the baby;
- early introduction of complementary foods. It is not recommended to introduce any complementary foods before six months - the baby’s body is not yet adapted to digest solid food;
- food allergies.
Recommendations for parents
Parents of newborns should be especially attentive to the health of their children. To prevent the development of dysbacteriosis, it is necessary to monitor the baby’s hygiene, proper and timely complementary feeding, and a varied diet.
Breastfeeding plays an important role in the formation of proper microflora. If there are no problems with lactation, there is no need to stop feeding.
During treatment with antibiotics, the baby must take probiotics. What kind of medicine to choose for your child needs to be decided with the attending physician.
Analysis collection rules
The rules for collecting stool for dysbiosis in infants include several important points.
1) 72 hours before the examination you should not:
- introduce new foods into the baby’s diet;
- give any medications, including prebiotics, sorbents, laxatives, etc.;
- give enemas or use suppositories.
Before the procedure, the child must empty the bladder so that urine does not mix with stool when a bowel movement occurs.
2) feces for dysbacteriosis in infants should be in the morning as much as possible.
3) the container must be delivered to the laboratory where the research will be carried out no later than 2-3 hours after stool collection. If necessary, the material for analysis can be placed in the refrigerator for a short time. You can't freeze stool! It is unacceptable to provide the laboratory with a jar of material for analysis that has been in the refrigerator all night, or obtained using rectal suppositories - to avoid the ingress of foreign impurities.
4) a stool test for dysbacteriosis in an infant is usually done within 1 week. During this period, data is gradually collected on the state of the baby’s intestinal microflora. Employees of the laboratory where the research is being conducted place feces in a specially created nutrient medium. All organisms contained in feces will grow in this environment. They will then be examined using a microscope.
Bacteriological examination of stool
If dysbacteriosis or intestinal infection is suspected, stool culture for microflora is prescribed. If possible, collection of material should be carried out before antibiotics are prescribed. If this is not possible, then no earlier than 12 hours after discontinuation of the drug.
Feces for examination are collected in a sterile vial with a sterile spoon (they are previously obtained in the laboratory). Feces are taken from different parts of the feces in the amount of 1-2 spoons. If there is pus, mucus, or flakes in the stool, then they must be taken for analysis. The bottle is tightly closed with a stopper. The material is delivered to the laboratory within 3 hours from the moment of collection. It is advisable to store feces in a cool place for the specified time. To do this, you can line the bottle with pre-prepared ice cubes.
How to collect stool for analysis
So, the doctor ordered a test for dysbiosis in the baby. How to collect material for analysis?
Experts do not recommend using a diaper to collect feces. If the toilet is performed at a certain time in the morning, it is necessary to place the child on a clean oilcloth, and then carefully collect what happened from it.
If you have problems with bowel movements, you can help your baby by bending his legs towards the navel or laying him on his tummy. You can also use a rubber gas tube for stimulation, lubricating the tip with Vaseline. It needs to be inserted into the anus - shallowly - and rotated slightly. A few minutes after this procedure everything will happen. Feces need to be collected with a spoon, which comes with the container for testing.
You can also help your baby by giving him a tummy massage. Place your palm on the belly near the navel and gently massage your baby's tummy in a clockwise direction. Periodically press the baby's bent knees toward his stomach to speed up the process.
On the container with the received material, it is necessary to indicate the surname, first name and age of the child, as well as the time of stool collection.
How to properly collect material for analysis - useful tips for parents
How to get tested for dysbacteriosis? Before conducting a study, a number of rules must be followed.
- For 3-5 days, refrain from introducing new foods into the child’s diet.
- Limit consumption of meat and meat broths.
- For 2-3 days before the test, you should not use laxatives, do an enema, or use rectal suppositories. If your baby is on a course of treatment with any medications, you should consult a doctor and discuss the possibility of temporarily discontinuing them.
Here are the rules and useful recommendations for parents on how to collect stool for analysis for dysbacteriosis.
- Defecation should occur naturally without the use of enemas, laxatives, or suppositories.
- In the morning, the toilet is performed with thorough washing of the anus.
- The baby's feces are collected from a previously laid clean diaper or oilcloth.
- The biological material is placed in a sterile container (purchased from a pharmacy).
- The amount of feces should be 10-15 g.
- The collection takes place with a special spatula attached to the cup.
After collection, stool must be delivered to the laboratory within two hours , but stool collected the night before can be stored in the refrigerator. Only fresh biological material (in no case frozen) is allowed for research.
Where can I get tested? Stool collection is carried out not only at home, but also in hospital settings. To do this, the laboratory assistant places a sterile cotton swab shallowly into the baby's anus.
Important! If parents have expressed a desire to test their child’s stool for dysbacteriosis within the walls of a medical institution, then it is necessary to find out in advance about the laboratory’s work schedule.
Types of bacteria in the results of stool analysis for dysbacteriosis
Pathogenic and opportunistic bacteria
Pathogenic bacteria are bacteria that parasitize other organisms and can cause infection. Under favorable conditions they reproduce very quickly. Pathogenic bacteria are extremely harmful bacteria that are the causative agents of infectious diseases: typhoid fever, salmonellosis, dysentery, etc. Normally, these bacteria should not exist at all.
Opportunistic bacteria are bacteria that, under normal conditions, do not cause harm to human health. However, if health deteriorates and immunity decreases, they become pathogenic, can cause inflammation and lead to the development of certain diseases.
Gram-positive and gram-negative bacteria
They are named after the Danish scientist Gram, who first introduced these concepts into use. When staining microorganisms with a special dye—gentian violet—Gram noticed that one group of bacteria (Gram-positive) was stainable, while the other (Gram-negative) was not. The reason for this is the thickness of the cell wall.
Gram-positive bacteria produce spores and exotoxins. They pose a danger to the body. But their shell allows antibacterial drugs to pass through.
Gram-negative bacteria can also cause serious illness. They do not form spores, but under certain conditions their shell, when destroyed, releases endotoxins, which can lead to various serious diseases. Unlike gram-positive bacteria, their shell is insensitive to antibiotics.
The correct ratio of gram-positive and gram-negative bacteria maintains normal internal microflora in the body.
Bifidobacteria
Bifidobacteria are gram-positive anaerobic bacteria that are shaped like curved rods. They make up the bulk of the intestinal microflora. To date, more than 30 species of bifidobacteria are known. They inhabit the large intestine. In the body, bifidobacteria perform the following functions:
— ensure normal functioning of the intestines by producing organic acids;
- control the proliferation of bacteria and viruses in the body;
- fight allergens;
— ensure the absorption of microelements, break down proteins, fats and carbohydrates;
— stimulate intestinal motility, ensure the processes of protein and fat metabolism;
- support immunity.
Lactobacilli
Lactobacilli are also gram-positive bacteria. They are located in many parts of the gastrointestinal tract, including the oral mucosa. Their main function is to maintain a normal acid-base balance in the body: lactobacilli convert lactose that enters the body with food into lactic acid. Lactobacilli also actively fight harmful microorganisms and create optimal conditions for successful digestion.
Eschejeria
Eschericheria (non-pathogenic Escherichia coli) is a type of gram-negative rod-shaped bacteria that is part of the normal microflora of the gastrointestinal tract. The main functions are to ensure digestion, activate the immune system, destroy pathogenic bacteria, produce vitamins B1, B1, B3, B5, B6. A decrease in the number of Eschecheria may indicate the presence of worms.
As a percentage of other representatives of the intestinal microflora, Escherichia coli does not exceed 1%.
Bacteroides
Bacteroides is a genus of gram-negative anaerobic rod-shaped bacteria. They belong to opportunistic bacteria. They are representatives of normal intestinal microflora. Their main function is the breakdown of fats. A child who was recently born does not have them in the body; they appear after six months.
Enterococci in stool analysis for dysbacteriosis
Enterococci are a genus of gram-positive bacteria of the Enterococcus family (Enterococccaceae), the most important representative of the body's microflora. They have a round or slightly oval shape and multiply quickly at temperatures of 35-37 degrees. Enterococci exist even with a small amount of oxygen.
Enterococci are opportunistic bacteria; excess levels of these bacteria can cause inflammation.
When performing an analysis for dysbacteriosis, among other bacteria, the number of enterococci is also examined. Enterococci in an analysis for dysbacteriosis in an infant should not exceed the norm - from 105 to 107 per 1 gram of feces.
The main therapy for fecal enterococcus is bacteriophages. They are less harmful than antibiotics in terms of the number of side effects, and they are often prescribed to children when an excess of the permissible limit is detected.
Microbes of the genus Protea
Microbes of the genus Protea are a genus of gram-negative bacteria, facultative anaerobes. The average size of an individual is 0.6 * 2.5 microns. They belong to opportunistic bacteria. They can provoke the development of many serious diseases requiring hospitalization.
Klebsiella
Also included in the list of opportunistic microorganisms. If the body is weakened, it can lead to pneumonia, urinary tract infections, and diarrhea.
What does Klebsiella lead to? Read personal experience here
Staphylococcus aureus
Staphylococcus aureus is an opportunistic microorganism. Refers to pathogens that can cause sore throats, folliculitis and other acute pathologies.
Staphylococcus epidermidis
Also refers to opportunistic bacteria. Lives in the surface layer of the skin - the epidermis. Can cause cystitis, urethritis, kidney pathology, tonsillitis, laryngitis, damage to the reproductive system and respiratory tract.
Enterobacter
It is an opportunistic bacteria that lives in the large intestine. Can cause nosocomial infections, blood poisoning, damage to the respiratory and genitourinary systems.
Pseudomonas aeruginosa
Gram-negative bacterium, opportunistic organism. One of the most common pathogens of nosocomial infections. Retains viability even in disinfectant solutions. It can affect various organs - skin, eyes, ears, gastrointestinal tract, urinary tract.
Clostridia
Gram-positive bacteria. They live in the mycophore of the gastrointestinal tract, on the skin, and in the oral cavity. May cause tetanus, gas gangrene, colitis.
Culture of milk for sterility
When inoculating milk for sterility, in 50-70% of cases various microorganisms are sown, most often Staphylococcus aureus and Staphylococcus epidermidis. Very often, when these organisms are detected, the mother is prescribed a course of antibiotic treatment, during which time the child is fed formula for a week, after which he usually refuses to breastfeed. This is the worst option, but common. Either mother and child begin to be treated using biological products, or mother and child drink chlorophyllipt. Meanwhile, the presence of staphylococci in milk does not mean anything! Both Staphylococcus aureus and Staphylococcus epidermidis live on human skin and are also present on most objects surrounding it. For example, staphylococcus has an affinity for cotton fabric. When moving a stack of diapers, the number of staphylococci in the air increases dramatically! Together with mother's milk, the child receives specific antibodies that help him cope with staphylococcus if necessary.
It turns out that staphylococcus in mother's milk comes to the baby along with protection from it. It is not dangerous for the child! Moreover, the child needs, in the first hours after birth, to be colonized with the mother’s staphylococcus. He will be protected from this staphylococcus by his mother's antibodies, which he will receive with colostrum and milk, and which he has already received transplacentally! The entire microflora of the mother’s body is already “familiar” to the child’s immune system, thanks to antibodies that penetrate transplacentally. It is dangerous for a child to be colonized by the microflora of the maternity hospital, including hospital strains of staphylococcus that are resistant to antibiotics! He is not familiar with these microorganisms and their colonization of his skin and gastrointestinal tract is dangerous for the baby. If the child does not have the opportunity to “populate” with the mother’s microflora, he is populated by what is around. As they say, a holy place is never empty. If it is not possible for the “domestic” strain of Staphyloccocus aureus to settle on the baby, the hospital strain will take its place. But this is not scary for a breastfed child; the mother’s body, by producing appropriate antibodies, will help the child. If, of course, there is a place for breastfeeding in the child’s life.
The presence of staphylococcus in milk does not affect its quality in any way. Staphylococcal enterocolitis, which is often used to scare mothers, convincing them to stop feeding their “poisonous” milk, is an extremely rare condition that occurs in diseases of the immune system, and the occurrence of which is facilitated by artificial feeding! It must be assumed that even if the baby, for some internal reason, has a weakened immune system, he will still receive significant support from mother’s milk. When transferred to artificial feeding, he loses this support.
Features of therapy
Treatment largely depends on which microorganisms have deviated from the norm, whether there is mucus, blood and other particles in the stool that should not be there. The doctor, studying the results, makes a diagnosis no earlier than he analyzes all the indicators.
If the transcript shows a decrease in the amount of E. coli, this may indicate the presence of worms in the intestines. Sometimes the cause may be a decrease in enzymatic activity, which is why this bacterium does not bring any benefit (although it does not harm). Despite all the benefits of E. coli, its number should not exceed the norm. If this happens, it means that dysbiosis is developing in the body.
As for hemolytic E. coli, in young children they should be completely absent. These pathogens produce toxins that negatively affect the nervous system and intestines, and can also cause various intestinal diseases and allergies.
Deficiency of bifidobacteria and bacteroides leads to long-term intestinal disorders in both children and adults. These bacteria appear in the baby on the tenth day of life. At the same time, children born through cesarean section have significantly fewer of them than babies born naturally.
Lactose-negative enterobacteria in children and adults should not exceed the norm. If the analysis shows their increased proliferation, this can explain the baby’s heartburn, regurgitation, belching, and increased gas formation. Enterococci normally do not harm the body and are even beneficial. But if their number is higher than normal, they will cause the development of infectious diseases of the pelvic organs and urinary tract.
While non-pathogenic staphylococci do not particularly harm the body (within normal limits), the presence of Staphylococcus aureus is dangerous for children. It causes diarrhea, vomiting, abdominal pain, high fever in the baby, mucus and blood are present in the stool. Therefore, Staphylococcus aureus should be absent in the feces of infants . If Staphylococcus aureus is present in the body, then its effect depends on beneficial bacteria . If their number is normal, the body is not afraid of Staphylococcus aureus and the baby does not need treatment. In severe cases, hospitalization is required.
When the doctor prescribes treatment, his instructions must be followed. To destroy fungus, clostridia, Klebsiella, lactose-negative enterobacteria, Staphylococcus aureus, normalize the digestive system, and get rid of mucus in the feces, special medications designed for small children are needed.
During treatment, special attention should be paid to the nutrition of children, since in many cases it is an incorrectly selected diet that causes the growth of clostridia, enterobacteria, Klebsiella and other pathogens. The diet must be agreed with your doctor. If the baby is breastfed, the mother must follow the diet.
When testing for dysbacteriosis in a child’s body, a tendency is immediately revealed that confirms the immaturity of the organs of the digestive system. At the same time, this type of analysis confirms the existing imbalance in the intestinal microflora. Most often, with dysbacteriosis in infants or newborns, there is a risk of having any diseases, as a result of which a malfunction occurred in the body. In most cases, dysbiosis does not have pronounced symptoms and is usually similar to any other disease of the digestive tract. In this regard, it becomes quite difficult to determine and identify dysbiosis in the child’s body. Therefore, submitting stool for analysis is one of the reliable and accurate laboratory methods that can confirm this diagnosis.
Appeal to pediatricians
Dear Colleagues! If a somatically healthy breastfed child you are observing is not gaining weight well, has green, unstable stools, skin problems, before transferring him to artificial feeding, prescribing examination and treatment, try to find out whether breastfeeding is organized correctly for this child. baby? Breastfeeding is a very simple process, if not elementary. But! Only if the breastfeeding mother follows a few simple rules and actions.
These rules and practices related to the culture of motherhood have been universally known and used for thousands of years. And now they are almost lost. Without knowledge of these rules, full breastfeeding cannot take place.
If a mother feeds the baby 6-7 times a day, uses a pacifier, gives the baby tea or water, pumps, or does not feed at night, she is committing actions that neither the child nor she herself are designed for by nature. It is impossible to establish a natural process by acting unnaturally! If a child has an incorrect attachment to the breast, and no one notices it, this is very sad, because... Nature did not count on the fact that a woman starting to breastfeed would not accumulate experience in observing other breastfeeding women throughout her life and would not have an experienced mother nearby who could correct her.
Without proper attachment, there will not be sufficient stimulation of the breast to produce the required amounts of milk, even with frequent feedings, and it is difficult for a child with incorrect attachment to extract “back”, fatty, thick milk from the breast! In such a situation, it is necessary to teach the mother and child how to properly attach to the breast, establish frequent feedings at the child’s request, eliminate the use of other oral objects and supplementary feeding, establish full night feedings, and eliminate pumping, if any. After 2-4 weeks, look at the child again. In 99% of cases, the child will not need artificial nutrition, examination, or treatment.
Author: Liliya Kazakova, pediatrician, head of the “Breastfeeding and Child Care Consultant Service”
Decoding the analysis for dysbacteriosis
Below is a breakdown of the test for dysbacteriosis in infants . As mentioned earlier, the results of the test for dysbiosis may vary from child to child. The following indicators are considered the recognized norm for infants:
- Bifidobacterium spp. (bifidobacteria) - from 109 to 10 12. A decrease in numbers indicates the presence of dysbacteriosis.
- Lactobacillius spp. (lactobacteria) - from 107 to 109. . A decrease indicates a risk of exposure to allergens.
- E. coli with normal enzymatic activity – from 107 to 108.
- E. Coli with weak enzymatic properties - no more than 105.
- E. Coli lactose-negative - no more than 105. Excess means a violation of the intestinal microflora.
- E. Coli hemolytic – must be absent. The presence indicates a violation of the microflora and a tendency to allergies.
- Enterococcus spp. (enterococci) - from 105 to 107.
- Proteus spp. (microbes of the genus Protea) – no more than 104. Exceeding the norm is a sign of dysbacteriosis.
- Klebsiella spp. (klebsiella) - no more than 104.
- Other opportunistic bacteria - no more than 104.
- Enterobacter spp. (Enterobacter) - no more than 104. Excess can cause disease of the kidneys, urinary tract, genital organs, etc.
- Staphylococcus aureus (Staphylococcus aureus) – must be absent.
- S. epidermis (Staphylococcus epidermidis) – should not exceed 105.
- S. saprophiticus (saprophytic staphylococcus) – should not exceed 105.
- Fungi of the genus Candida are yeast-like fungi. Normally should be absent
- Non-fermentative gram-negative bacteria – should not exceed 104.
- Pseudomonas spp. (Pseudomonas aeruginosa) - should be absent from the analysis.
- Clostridium spp. (clostridia) – must be absent.
- Clostridium difficile – should be absent from the analysis.
The form with the results of the analysis may be different in different medical institutions.
Decoding the analysis in children under one year old
All microorganisms in the analysis form are divided into:
- obligate – permanently inhabiting the body, playing an important role in metabolic processes and protection against infectious diseases;
- facultative - non-permanent residents of the body, which are either conditionally pathogenic (lead to diseases when immunity is reduced) or saprophytes (consume decaying organic substances);
- transient - free-living microorganisms that enter the body from the external environment. They can be both saprophytes and pathogens.
Let us briefly describe the main representatives of the above groups of bacteria.
Bifidobacteria
- Promote the breakdown of fats, carbohydrates, proteins that enter the bloodstream through the intestinal wall.
- Produce organic acids.
- Prevents the proliferation of pathogenic flora in the intestines.
- Promotes the absorption of nutritional elements of food.
- Optimize intestinal motility.
- Activate immunoglobulins and lymphatic elements (provide local immunity).
- Neutralizes toxins and waste.
A lack of bifidobacteria leads to flatulence, diarrhea, and frequent diarrhea.
Lactobacilli
- They convert lactose into lactic acid, which promotes better digestion of food.
- Improves digestion.
- Stimulates metabolic processes.
- They fight pathogenic microflora (especially Helicobacter Pylori strains).
- Some enzymes are activated.
- Participate in the synthesis of vitamins.
If there is a lack of lactobacilli, a child experiences frequent colds, poor digestion of milk, allergic reactions, and constipation.
Video from the Union of Pediatricians of Russia. Dysbacteriosis (dysbiosis).
Bacteroides
They occupy approximately half of the entire intestinal microflora. When maintaining a normal amount, bacteroids perform a number of useful functions - they participate in the utilization of proteins, the breakdown of fats, the fermentation of carbohydrates, and the biotransformation of bile acids. If the levels are exceeded, bacteroids lead to infectious and septic complications and contribute to the destruction of beneficial E. coli (in the fight for oxygen).
Eubacteria
They are more common in formula-fed children and are practically absent in children fed breast milk. Most typical for adults. Their role is not clear enough, but it is known that they are involved in some metabolic processes.
Fusobacteria
More often observed in the intestinal microflora of adults. Some species are sown during purulent-inflammatory processes. Little studied.
Opportunistic bacteria and fungi do not cause intestinal problems in a healthy child until the immune system is weakened or the condition of the intestinal mucosa is disrupted. An increase in the level of opportunistic organisms is also observed against the background of a decrease in indicators of lactobacilli and bifidobacteria. At the same time, the baby experiences flatulence, diarrhea, inflammation of the intestinal mucosa, and increased fermentation processes.
Indications for examination in children
In infants, dysbiosis is most often caused by changes in diet and various diseases. Sometimes, when new products are introduced in children under one year of age, the frequency and nature of stool changes. However, after a few days everything returns to normal. It is worth thinking about the development of dysbiosis and conducting an examination of the baby if the following signs appear:
In older children, the signs of dysbiosis are in many ways similar to those in infants under one year old, only they, unlike babies, may complain of discomfort and abdominal pain. Dysbiosis is caused by poor nutrition, infectious diseases, food poisoning, poor environment, stress, helminthic infestations, and hormonal changes in adolescents.