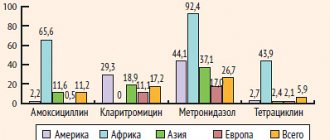
Pharmacological properties of the drug Amoxiclav
Amoxiclav is a combination of amoxicillin (a broad-spectrum penicillin antibiotic) and clavulanic acid (an inhibitor of β-lactamases that forms stable inactive complex compounds with them and prevents the destruction of amoxicillin). Amoxiclav has a wide spectrum of action. The drug is active against both microorganisms sensitive to amoxicillin and bacteria resistant to it that produce β-lactamases, including: gram-positive aerobes ( Streptococcus pneumoniae, Str. pyogenes, Str. bovis, Staphylococcus aureus, S. epidermidis, Listeria spp. , Enterococcus spp. ), gram-negative aerobes ( N. influenzae, Moraxella catarrhalis, E. coli, Proteus spp., Klebsiella spp., N. gonorrhoeae, N. meningitidis , Pasteurella multocida ) and anaerobic ( Peptococcus spp., Peptostreptococcus spp., Clostridium spp., Bacteroides spp., Actinomyces israelii ) bacteria. The basic pharmacokinetic properties of amoxicillin and clavulanic acid are similar and, when used in combination, do not mutually affect the pharmacokinetics of each substance separately. After oral administration, both components of the drug are well absorbed, maximum concentrations in the blood serum are reached after approximately 1 hour. Food intake does not affect the absorption of the active substances. The half-life of amoxicillin is 78 minutes, and clavulanic acid is 60–70 minutes. Both components easily penetrate into most liquid media and tissues of the body (lungs, middle ear, secretion of the maxillary sinuses, pleural and peritoneal fluid, uterus, ovaries, etc.), penetrate the placental barrier, and in case of meningitis - through the blood-brain barrier, and are determined in trace amounts in breast milk. After a bolus injection of Amoxiclav at a dose of 1.2 g, the maximum concentration of amoxicillin in the blood serum is 105.4, clavulanic acid - 28.5 mg/l. The peak concentration in body fluids is observed 1 hour after reaching the maximum concentration in the blood serum. Amoxicillin and clavulanic acid bind to plasma proteins by 17–20 and 22–30%, respectively. Amoxicillin is excreted in the urine mainly unchanged, and clavulanic acid undergoes active metabolic transformations in the liver and is excreted mainly by the kidneys, and is also partially excreted in feces and exhaled air.
Amoxiclav 250mg+62.5mg/5ml 25g 100ml powder for oral suspension
pharmachologic effect
Antibiotic - semi-synthetic penicillin + beta-lactamase inhibitor.
Composition and release form Amoxiclav 250 mg + 62.5 mg/5 ml 25 g 100 ml powder for the preparation of suspension for oral administration
Powder for the preparation of a suspension for oral administration - 5 ml of suspension:
- active ingredients: amoxicillin (in the form of trihydrate) - 250 mg; clavulanic acid (in the form of potassium salt) - 62.5 mg;
- excipients: citric acid (anhydrous) - 2.167 mg; sodium citrate (anhydrous) - 8.335 mg; sodium benzoate - 2.085 mg; MCC and carmellose sodium - 28.1 mg; xanthan gum - 10 mg; colloidal silicon dioxide - 16.667 mg; silicon dioxide - 0.217 g; sodium saccharinate - 5.5 mg; mannitol - 1250 mg; wild cherry flavor - 4 mg.
Powder for the preparation of suspension for oral administration 250 mg + 62.5 mg/5 ml. Primary packaging - 25 g of powder (100 ml of finished suspension) in a dark glass bottle with a ring mark (100 ml). The bottle is closed with a screw-on metal cap with a control ring, inside the cap there is a LDPE gasket.
Secondary packaging - 1 fl. with a dosing spoon with ring marks in the cavity for 2.5 and 5 ml (“2.5 SS” and “5 SS”), a maximum filling mark of 6 ml (“6 SS”) on the handle of the spoon in a cardboard box. Or 1 fl. together with a graduated dosage pipette in a cardboard box.
Description of the dosage form
Powder for oral suspension: white to yellowish-white powder. The finished suspension is an almost white to yellow homogeneous suspension.
Directions for use and doses
Inside
The daily dose of suspensions is 125+31.25 mg/5 ml and 250+62.5 mg/5 ml (to facilitate correct dosing, a dosage pipette is inserted into each package of suspensions 125+31.25 mg/5 ml and 250+62.5 mg/5 ml, graduated at 5 ml, with a scale of division of 0.1 ml or a dosing spoon with a capacity of 5 ml, with ring marks in the cavity at 2.5 and 5 ml).
Newborns and children up to 3 months - 30 mg/kg/day (for amoxicillin), divided into 2 doses (every 12 hours).
Dosing of the drug Amoxiclav® with a dosing pipette - calculation of single doses for the treatment of infections in newborns and children up to 3 months (Table 3).
Table 3
| Body weight, kg | 2 | 2,2 | 2,4 | 2,6 | 2,8 | 3 | 3,2 | 3,4 | 3,6 | 3,8 | 4 | 4,2 | 4,4 | 4,6 | 4.8 |
| Suspension 156.25, ml (2 times a day) | 1,2 | 1,3 | 1,4 | 1,6 | 1,7 | 1,8 | 1,9 | 2 | 2,2 | 2,3 | 2,4 | 2,5 | 2,6 | 2,8 | 2,9 |
| Suspension 312.5, ml (2 times a day) | 0,6 | 0,7 | 0,7 | 0,8 | 0,8 | 0,9 | 1 | 1 | 1,1 | 1,1 | 1,2 | 1,3 | 1,3 | 1,4 | 1,4 |
Children over 3 months - from 20 mg/kg for mild and moderate infections to 40 mg/kg for severe infections and lower respiratory tract infections, otitis media, sinusitis (amoxicillin) per day, divided into 3 doses (every 8 h).
Dosing of the drug Amoxiclav® with a dosing pipette - calculation of single doses for the treatment of mild and moderate infections in children over 3 months (at the rate of 20 mg/kg/day (for amoxicillin) (Table 4).
Table 4
| Body weight, kg | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| Suspension 156.25, ml (3 times a day) | 1,3 | 1,6 | 1,9 | 2,1 | 2,4 | 2,7 | 2,9 | 3,2 | 3,5 | 3,7 | 4 | 4,3 | 4,5 | 4,8 | 5,1 | 5,3 | 5,6 | 5,9 |
| Suspension 312.5, ml (3 times a day) | 0,7 | 0,8 | 0,9 | 1,1 | 1,2 | 1,3 | 1,5 | 1,6 | 1,7 | 1,9 | 2 | 2,1 | 2,3 | 2,4 | 2,5 | 2,7 | 2,8 | 2,9 |
| Body weight, kg | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | |
| Suspension 156.25, ml (3 times a day) | 6,1 | 6,4 | 6,7 | 6,9 | 7,2 | 7,5 | 7,7 | 8 | 8,3 | 8,5 | 8,8 | 9,1 | 9,3 | 9,6 | 9,9 | 10,1 | 10,4 | |
| Suspension 312.5, ml (3 times a day) | 3,1 | 3,2 | 3,3 | 3,5 | 3,6 | 3,7 | 3,9 | 4 | 4,1 | 4,3 | 4,4 | 4,5 | 4,7 | 4,8 | 4,9 | 5,1 | 5,2 |
Dosing of the drug Amoxiclav® with a dosing pipette - calculation of single doses for the treatment of severe infections in children over 3 months (at the rate of 40 mg/kg/day (for amoxicillin) (Table 5).
Table 5
| Body weight, kg | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| Suspension 156.25, ml (3 times a day) | 2,7 | 3,2 | 3,7 | 4,3 | 4,8 | 5,3 | 5,9 | 6,4 | 6,9 | 7,5 | 8 | 8,5 | 9,1 | 9,6 | 10,1 | 10,7 | 11,2 | 11,7 |
| Suspension 312.5, ml (3 times a day) | 1,3 | 1,6 | 1,9 | 2,1 | 2,4 | 2,7 | 2,9 | 3,2 | 3,5 | 3,7 | 4 | 4,3 | 4,5 | 4,8 | 5,1 | 5,3 | 5,6 | 5,9 |
| Body weight, kg | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | |
| Suspension 156.25, ml (3 times a day) | 12,3 | 12,8 | 13,3 | 13,9 | 14,4 | 14,9 | 15,5 | 16 | 16,5 | 17,1 | 17,6 | 18,1 | 18,7 | 19,2 | 19,7 | 20,3 | 20,8 | |
| Suspension 312.5, ml (3 times a day) | 6,1 | 6,4 | 6,7 | 6,9 | 7,2 | 7,5 | 7,7 | 8 | 8,3 | 8,5 | 8,8 | 9,1 | 9,3 | 9,6 | 9,9 | 10,1 | 10,4 |
Dosing of the drug Amoxiclav® with a dosing spoon (in the absence of a dosing pipette) - recommended doses of suspensions depending on the child’s body weight and the severity of the infection (Table 6).
Table 6
| Body weight, kg | Age (approx.) | Mild/moderate course | Severe course | ||
| 125+31.25 mg/5 ml | 250+62.5 mg/5 ml | 125+31.25 mg/5 ml | 250+62.5 mg/5 ml | ||
| 5–10 | 3–12 months | 3 × 2.5 ml (½ spoon) | 3 × 1.25 ml | 3 × 3.75 ml | 3 × 2 ml |
| 10–12 | 1–2 years | 3 × 3.75 ml | 3 × 2 ml | 3 × 6.25 ml | 3 × 3 ml |
| 12–15 | 2–4 years | 3 × 5 ml (1 spoon) | 3 × 2.5 ml (½ spoon) | 3 × 7.5 ml (1½ spoons) | 3 × 3.75 ml |
| 15–20 | 4–6 years | 3 × 6.25 ml | 3 × 3 ml | 3 × 9.5 ml | 3 × 5 ml (1 spoon) |
| 20–30 | 6–10 years | 3 × 8.75 ml | 3 × 4.5 ml | — | 3 × 7 ml |
| 30–40 | 10–12 years | — | 3 × 6.5 ml | — | 3 × 9.5 ml |
| ≥40 | ≥12 years | Amoxiclav® tablets | |||
Daily dose of suspension 400 mg+57 mg/5 ml
The dose is calculated per kg of body weight depending on the severity of the infection. From 25 mg/kg - for mild and moderate infections to 45 mg/kg - for severe infections and lower respiratory tract infections, otitis media, sinusitis (in terms of amoxicillin) per day, divided into 2 doses.
To facilitate correct dosing, a dosage pipette is inserted into each package of the 400 mg + 57 mg/5 ml suspension, graduated simultaneously into 1, 2, 3, 4, 5 ml and into 4 equal parts.
Suspension 400 mg+57 mg/5 ml is used in children over 3 months.
Table 7
Recommended dose of suspension depending on the child’s body weight and severity of infection
| Body weight, kg | Age (approx.) | Recommended dose, ml | |
| Severe course | Moderate course | ||
| 5–10 | 3–12 months | 2×2,5 | 2×1,25 |
| 10–15 | 1–2 years | 2×3,75 | 2×2,5 |
| 15–20 | 2–4 years | 2×5 | 2×3,75 |
| 20–30 | 4 years - 6 years | 2×7,5 | 2×5 |
| 30–40 | 6–10 years | 2×10 | 2×6,5 |
Exact daily doses are calculated based on the child's body weight, not his age.
The maximum daily dose of amoxicillin is 6 g for adults and 45 mg/kg for children.
The maximum daily dose of clavulanic acid (in the form of potassium salt) is 600 mg for adults and 10 mg/kg for children.
In patients with impaired renal function, the dose should be adjusted based on the maximum recommended dose of amoxicillin.
Patients with creatinine Cl >30 ml/min do not require any dose adjustment.
Adults and children weighing more than 40 kg (the indicated dosage regimen is used for moderate and severe infections)
For patients with creatinine Cl 10-30 ml/min - 500/125 mg 2 times a day.
With Cl creatinine
For patients on hemodialysis, the recommended dose is 500/125 mg every 24 hours, plus 500/125 mg during dialysis and another dose at the end of dialysis (since serum concentrations of amoxicillin and clavulanic acid are reduced).
Children weighing less than 40 kg
With creatinine Cl 10–30 ml/min, the recommended dose is 15/3.75 mg/kg 2 times a day (maximum 500/125 mg 2 times a day).
With Cl creatinine
For hemodialysis, the recommended dose is 15/3.75 mg/kg once a day. Before hemodialysis - 15/3.75 mg/kg. To restore appropriate concentrations of the drug in the blood, it is necessary to take another dose of 15/3.75 mg/kg after hemodialysis.
The course of treatment is 5–14 days. The duration of treatment is determined by the attending physician. Treatment should not continue for more than 14 days without repeated medical examination.
Pharmacodynamics
Mechanism of action
Amoxicillin is a semisynthetic broad-spectrum antibiotic that is active against many gram-positive and gram-negative microorganisms. At the same time, amoxicillin is susceptible to destruction by beta-lactamases, and therefore the spectrum of activity of amoxicillin does not extend to microorganisms that produce this enzyme.
Clavulanic acid is a beta-lactamase inhibitor, structurally related to penicillins, and has the ability to inactivate a wide range of beta-lactamases found in microorganisms resistant to penicillins and cephalosporins. Clavulanic acid is sufficiently effective against plasmid beta-lactamases, which most often cause bacterial resistance, and is not effective against type I chromosomal beta-lactamases, which are not inhibited by clavulanic acid.
The presence of clavulanic acid in the drug protects amoxicillin from destruction by enzymes - beta-lactamases, which expands the antibacterial spectrum of amoxicillin.
Below is the activity of the combination of amoxicillin and clavulanic acid in vitro.
Grampore-positive aerobes: Bacillus Anthracis, Enterococcus Faecalis, Listeria MonocyTogenes, NoCardia Asteroides, Streptococcus Pyogenes1,2, Streptococcus Agalactiae1,2, other beta-heemolytic statococci1, 2, Staphylococcus aureus (sensitive to methicillin) 1, Staphylococcus saprophyticus (sensitive to methicillin), coagulase-negative staphylococci (sensitive to methicillin).
Gram-negative aerobes: Bordetella pertussis, Haemophilus influenzae1, Helicobacter pylori, Moraxella catarrhalis1, Neisseria gonorrhoeae, Pasteurella multocida, Vibrio cholerae.
Other: Borrelia burgdorferi, Leptospira icterohaemorrhagiae, Treponema pallidum.
Gram-positive anaerobes: species of the genus Clostridium, Peptococcus niger, Peptostrepiococcus magnus, Peptostreptococcus micros, species of the genus Pepto streptococcus.
Gram-negative anaerobes: Bcicteroides fragilis, species of the genus Bacteroides, species of the genus Capnocytophaga, Eikenella corrodens, Fusobacterium nucleatum, species of the genus Fusobacterium, species of the genus Porphyromonas, species of the genus Prevotella.
Bacteria for which acquired resistance to the combination of amoxicillin and clavulanic acid is likely
Gram-negative aerobes: Escherichia coli1, Klebsiella oxytoca, Klebsiella pneumoniae, species of the genus Klebsiella, Proteus mirabilis, Proteus vulgaris, species of the genus Proteus, species of the genus Salmonella, species of the genus Shigella. Streptococcus pneumoniae1,2, streptococci of the Viridans group.
Gram-positive aerobes: species of the genus Corynebacterium, Enterococcus faecium.
Bacteria that are naturally resistant to the combination of amoxicillin and clavulanic acid
Gram-negative aerobes: species of the genus Acinetobacter, Citrobacter freundii, species of the genus Enterobacter, Hafhia alvei, Legionella pneumophila, Morganella morganii, species of the genus Providencia, species of the genus Pseudomonas, species of the genus Serratia, Stenotrophomonas maltophilia, Yersinia enterocolitica.
Other: Chlamydia pneumoniae, Chlamydia psittaci, species of the genus Chlamydia, Coxiella burnetii, species of the genus Mycoplasma.
1For these bacteria, the clinical effectiveness of the combination of amoxicillin with clavulanic acid has been demonstrated in clinical studies.
2 strains of these types of bacteria do not produce beta-lactamases. Sensitivity during amoxicillin monotherapy suggests similar sensitivity to the combination of amoxicillin and clavulanic acid.
Pharmacokinetics
Suction
The active ingredients of the drug are quickly and completely absorbed from the gastrointestinal tract (GIT) after oral administration. Absorption of active ingredients is optimal when the drug is used with food.
The following are the pharmacokinetic parameters of amoxicillin and clavulanic acid after administration at a dose of 45 mg/6.4 mg/kg, divided into two doses, by patients under 12 years of age.
Average value of pharmacokinetic parameters
| Cmax (mg/ml) | T max (h) | AUC (mg in h/l) | T1/2 (h) | |
| Amoxicillin | 11,99±3,28 | 1,0 (1,0-2,0) | 35,2±5,0 | 1,22±0,28 |
| Clavulanic acid | 5,49±2,71 | 1,0 (1,0-2,0) | 13,26±5,88 | 0,99±0,14 |
- Cmax - maximum concentration in blood plasma;
- Tmax is the time to reach the maximum concentration in the blood plasma;
- AUC—area under the concentration-time curve;
- T1/2 - half-life.
Metabolism
About 10-25% of the initial dose of amoxicillin is excreted by the kidneys in the form of an inactive metabolite (penicillic acid). Clavulanic acid in the human body undergoes intensive metabolism with the formation of 2,5-dihydro-4-(2-hydroxyethyl)-5-oxo-1H-pyrrole-3-carboxylic acid and 1-amino-4-hydroxy-butan-2-one and is excreted by the kidneys, through the gastrointestinal tract, and also with exhaled air in the form of carbon dioxide.
Distribution
As with intravenous administration of a combination of amoxicillin and clavulanic acid, therapeutic concentrations of amoxicillin and clavulanic acid are found in various tissues and interstitial fluid (gallbladder, abdominal tissue, skin, adipose and muscle tissue, synovial and peritoneal fluids, bile, purulent discharge) .
Amoxicillin and clavulanic acid have a weak degree of binding to plasma proteins. Studies have shown that about 25% of the total amount of clavulanic acid and 18% of amoxicillin in the blood plasma is bound to plasma proteins.
The volume of distribution is approximately 0.3-0.4 L/kg for amoxicillin and approximately 0.2 L/kg for clavulanic acid. Amoxicillin and clavulanic acid do not penetrate the blood-brain barrier when the meninges are not inflamed. Amoxicillin (like most penicillins) is excreted in breast milk.
Trace amounts of clavulanic acid may also be found in breast milk. With the exception of the possibility of sensitization, diarrhea and candidiasis of the oral mucosa, there are no other known negative effects of amoxicillin and clavulanic acid on the health of breastfed infants.
Animal reproductive studies have shown that amoxicillin and clavulanic acid cross the placental barrier. However, no negative effects on the fetus were detected.
Removal
Amoxicillin is eliminated primarily by the kidneys, while clavulanic acid is eliminated through both renal and extrarenal mechanisms. After a single oral dose of 875 mg/125 mg or 500 mg/125 mg, approximately 60-70% of amoxicillin and 40-65% of clavulanic acid are excreted unchanged by the kidneys during the first 6 hours. The average half-life (T1/2) of amoxicillin/clavulanic acid is approximately 1 hour, and the average total clearance is approximately 25 L/h in healthy patients. In various studies, it was found that amoxicillin excretion by the kidneys within 24 hours is approximately 50-85%, clavulanic acid - 27-60%. The largest amount of clavulanic acid is excreted during the first 2 hours after administration.
The pharmacokinetics of amoxicillin/clavulanic acid does not depend on the gender of the patient.
Patients with impaired renal function
The total clearance of amoxicillin/clavulanic acid decreases in proportion to the decrease in renal function. The decrease in clearance is more pronounced for amoxicillin than for clavulanic acid, because Most amoxicillin is excreted by the kidneys. Doses of the drug for renal failure should be selected taking into account the undesirability of amoxicillin accumulation while maintaining normal levels of clavulanic acid.
Patients with liver dysfunction
In patients with impaired liver function, the drug is used with caution. It is necessary to constantly monitor liver function.
Both components are removed by hemodialysis and minor amounts by peritoneal dialysis.
Indications for use Amoxiclav 250 mg + 62.5 mg/5 ml 25 g 100 ml powder for the preparation of suspension for oral administration
Infections caused by sensitive strains of microorganisms:
- upper respiratory tract and ENT organs (including acute and chronic sinusitis, acute and chronic otitis media, retropharyngeal abscess, tonsillitis, pharyngitis);
- lower respiratory tract (including acute bronchitis with bacterial superinfection, chronic bronchitis, pneumonia);
- urinary tract (eg cystitis, urethritis, pyelonephritis);
- in gynecology;
- skin and soft tissues, including human and animal bites;
- bone and connective tissue;
- biliary tract (cholecystitis, cholangitis);
- odontogenic.
Contraindications
- hypersensitivity to the components of the drug;
- history of hypersensitivity to penicillins, cephalosporins and other beta-lactam antibiotics;
- history of cholestatic jaundice and/or other liver dysfunction caused by taking amoxicillin/clavulanic acid;
- infectious mononucleosis and lymphocytic leukemia;
With caution: history of pseudomembranous colitis, gastrointestinal diseases, liver failure, severe renal impairment, pregnancy, lactation, simultaneous use with anticoagulants.
Application Amoxiclav 250mg+62.5mg/5ml 25g 100ml powder for the preparation of suspension for oral administration during pregnancy and breastfeeding
During pregnancy and lactation, Amoxiclav® is used only if the expected benefit to the mother outweighs the potential risk to the fetus and child.
Amoxicillin and clavulanic acid pass into breast milk in small quantities.
special instructions
During a course of treatment, it is necessary to monitor the state of the function of the hematopoietic organs, liver, and kidneys.
In patients with severe renal impairment, adequate dose adjustment or increased intervals between doses is required.
It is possible that superinfection may develop due to the growth of microflora that is insensitive to it, which requires a corresponding change in antibacterial therapy.
In patients who are hypersensitive to penicillins, cross-allergic reactions with cephalosporin antibiotics are possible.
In women with premature rupture of membranes, it was found that prophylactic therapy with amoxicillin + clavulanic acid may be associated with an increased risk of developing necrotizing colitis in the newborn.
Crystalluria very rarely occurs in patients with reduced diuresis. During the use of large doses of amoxicillin, it is recommended to take sufficient fluids and maintain adequate diuresis to reduce the likelihood of amoxicillin crystal formation.
Lab tests. High concentrations of amoxicillin give a false-positive reaction to urine glucose when using Benedict's reagent or Fehling's solution. It is recommended to use enzymatic reactions with glucosidase.
Impact on the ability to drive a car or perform work that requires increased speed of physical and mental reactions. Due to the possibility of developing side effects from the central nervous system, such as dizziness, headache, convulsions, during treatment, care should be taken when driving and other activities that require concentration and speed of psychomotor reactions.
Overdose
There are no reports of death or life-threatening side effects due to drug overdose.
Symptoms: in most cases - gastrointestinal disorders (abdominal pain, diarrhea, vomiting), anxiety, insomnia, dizziness are also possible, and in isolated cases - seizures.
Treatment: in case of overdose, the patient should be under medical supervision, treatment should be symptomatic.
In case of recent use (less than 4 hours) of the drug, it is necessary to perform gastric lavage and prescribe activated charcoal to reduce absorption. Amoxicillin/potassium clavulanate is removed by hemodialysis.
Side effects Amoxiclav 250mg+62.5mg/5ml 25g 100ml powder for suspension for oral administration
From the digestive system: loss of appetite, nausea, vomiting, diarrhea, abdominal pain, gastritis, stomatitis, glossitis, black “hairy” tongue, darkening of tooth enamel, hemorrhagic colitis (can also develop after therapy), enterocolitis, pseudomembranous colitis, disorder liver function, increased activity of ALT, AST, alkaline phosphatase and/or bilirubin levels in the blood plasma, liver failure (more often in the elderly, men, with long-term therapy), cholestatic jaundice, hepatitis.
Allergic reactions: itching, urticaria, erythematous rashes, erythema multiforme exudative, angioedema, anaphylactic shock, allergic vasculitis, exfoliative dermatitis, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis, a syndrome similar to serum sickness, toxic epidermal necrolysis.
From the hematopoietic system and lymphatic system: reversible leukopenia (including neutropenia), thrombocytopenia, hemolytic anemia, reversible increase in PT (when used together with anticoagulants), reversible increase in bleeding time, eosinophilia, pancytopenia, thrombocytosis, agranulocytosis.
From the central nervous system: dizziness, headache, convulsions (may occur in patients with impaired renal function when taking high doses of the drug).
From the urinary system: interstitial nephritis, crystalluria, hematuria.
Other: candidiasis and other types of superinfection.
From the side of the central nervous system: hyperactivity. Feelings of anxiety, insomnia, behavior changes, agitation.
Drug interactions
Antacids, glucosamine, laxatives, aminoglycosides slow down absorption, ascorbic acid increases absorption.
Diuretics, allopurinol, phenylbutazone, NSAIDs and other drugs that block tubular secretion (probenecid) increase the concentration of amoxicillin (clavulanic acid is excreted mainly by glomerular filtration).
The simultaneous use of Amoxiclav® and methotrexate increases the toxicity of methotrexate.
Prescription together with allopurinol increases the incidence of exanthema. Concomitant use with disulfiram should be avoided.
Reduces the effectiveness of drugs, during the metabolism of which PABA is formed; ethinyl estradiol - risk of breakthrough bleeding.
The literature describes rare cases of increased INR in patients with the combined use of acenocoumarol or warfarin and amoxicillin. If simultaneous use with anticoagulants is necessary, PT or INR should be carefully monitored when prescribing or discontinuing the drug.
The combination with rifampicin is antagonistic (mutual weakening of the antibacterial effect). The drug Amoxiclav® should not be used simultaneously in combination with bacteriostatic antibiotics (macrolides, tetracyclines), sulfonamides due to a possible decrease in the effectiveness of the drug Amoxiclav®.
The drug Amoxiclav® reduces the effectiveness of oral contraceptives.
The drug Amoxiclav® and aminoglycoside antibiotics are chemically incompatible.
Amoxiclav® should not be mixed in a syringe or infusion bottle with other drugs.
Avoid mixing with solutions of dextrose, dextran, sodium bicarbonate, as well as with solutions containing blood, proteins, lipids.
Indications for use of the drug Amoxiclav
Infections of the upper and lower respiratory tract caused by microorganisms sensitive to Amoxiclav (including chronic bronchitis and pneumonia); acute and chronic sinusitis and otitis media, retropharyngeal abscess; odontogenic infections (including periodontitis); urinary tract infections; gynecological infections, as well as gonorrhea (including those caused by gonococci producing β-lactamase), chancroid; infections of the skin and soft tissues (including wound infections); bone and joint infections; treatment of mixed infections caused by gram-positive and gram-negative microorganisms and anaerobes (ENT infections, biliary tract infections and postoperative abdominal infections, breast abscess, aspiration pneumonia). Amoxiclav can be used to prevent purulent-septic complications during operations on the abdominal organs, pelvis, heart, kidneys, bile ducts, in orthopedic practice and maxillofacial surgery.
Use of the drug Amoxiclav
Amoxiclav, Amoxiclav 2X Orally in tablets, the drug is recommended for adults and children weighing more than 40 kg, in the average daily dose - 1 tablet 375 mg 3 times a day (every 8 hours) or 1 tablet 625 mg 2-3 times a day day, depending on the severity of the infectious disease. Amoxiclav 2X 1000 mg tablets are used in adult patients with severe infections or respiratory infections, 1 tablet 2 times a day. For children aged 3 months and older with a body weight of less than 40 kg with moderate infectious diseases, Amoxiclav is prescribed in a daily dose of 25 mg/kg, divided into 2 doses (every 12 hours), or 20 mg/kg, divided into 3 doses (every 8 h), and for severe infections - 45 mg/kg, divided into 2 doses (every 12 hours), or 40 mg/kg, divided into 3 doses (every 8 hours). For newborns and children up to 3 months, Amoxiclav is prescribed in a daily dose of 30 mg/kg (in terms of amoxicillin), divided into 2 equal doses. The maximum daily dose of amoxicillin for adults is 6 g, for children - 45 mg per 1 kg of body weight; the maximum daily dose of clavulanic acid (in the form of potassium salt) for adults is 600 mg, for children - 10 mg per 1 kg of body weight. For otitis media, sinusitis, lower respiratory tract infections and other severe infections, the recommended dose for children is 45 mg/kg per day (for amoxicillin) every 12 hours. For moderate infections, the daily dose is 25 mg/kg (every 12 hours). The exact dose of the suspension for a child can only be calculated taking into account body weight. Patients with severe renal failure (creatinine clearance less than 10 ml/min) require dose adjustment or an increase in the interval between doses (for anuria - up to 48 hours or more). For patients with moderate renal failure (creatinine clearance 10–30 ml/min), the dose should be adequately reduced or the interval between doses of the drug should be increased:
Creatinine clearance, ml/min | 80 | 80–50 | 50–10 | ≤10 |
| Interval between doses, h | 8 | 8 | 12 | 24 |
To prepare the suspension, shake the bottle well (until the powder separates from the walls and bottom of the vessel), then add 86 ml of water (Amoxiclav suspension) to the bottle in 2 additions, shaking thoroughly each time. One measuring spoon for taking the suspension (attached) contains 5 ml, 3/4 spoon - 3.75 ml; 1/2 spoon - 2.5 ml of suspension. Parenterally - intravenously for adults and children over 12 years of age (with body weight more than 40 kg) - 1.2 g every 8 hours, for severe infections - every 6 hours; children aged 3 months to 12 years - 30 mg/kg every 8 hours, for severe infections - every 6 hours; children under 3 months - 30 mg/kg every 8 hours; for newborns, including premature babies, 30 mg/kg every 12 hours. 30 mg of Amoxiclav for intravenous administration contains 25 mg of amoxicillin and 5 mg of clavulanic acid. After achieving a therapeutic effect with intravenous use of Amoxiclav, you can switch to taking the drug orally. Treatment with Amoxiclav for adults and children can be carried out for 14 days. Adult dosage for renal failure:
- if creatinine clearance is more than 30 ml/min, there is no need to reduce the dose;
- with a creatinine clearance of 10–30 ml/min, treatment begins with an intravenous injection of 1.2 g, and then 600 mg intravenously at intervals of 12 hours;
- in case of severe renal failure (creatinine clearance less than 10 ml/min), treatment begins with 1.2 g intravenously, and then 600 mg intravenously at 24-hour intervals. In children with renal failure, it is also necessary to reduce the dose of the drug.
For peritoneal dialysis, no dose adjustment is required; during hemodialysis, about 85% of the drug is excreted from the body, so after the procedure the drug is administered intravenously at a dose of 600 mg. To prepare a solution for intravenous use, the contents of the bottle, which contains 600 mg of Amoxiclav, are dissolved in 10 ml of water for injection; the contents of the bottle, which contains 1.2 g of Amoxiclav, in 20 ml of water for injection; the resulting solution is administered intravenously slowly (over 3–4 minutes). For intravenous infusion, to 0.6 g (dissolved in 10 ml of water for injection) or 1.2 g (dissolved in 20 ml of water for injection) of the drug, add 50 or 100 ml of infusion solution, respectively; administered intravenously over 30–40 minutes. IV bolus injections must be performed within 20 minutes after preparing the injection solution. Do not freeze the solution. For prophylactic purposes in surgery, adults are administered 1.2 g of Amoxiclav intravenously before anesthesia in connection with short surgical interventions; during long-term operations (more than 1 hour), repeated administration of the drug is required (1.2 g up to 4 times a day); if there is an increased risk of infection, treatment can be continued in the postoperative period; if there are obvious signs of infection during or after surgery, Amoxiclav is used (parenterally or orally) in the postoperative period. Amoxiclav Quiktab The usual daily dose for adults and children weighing more than 40 kg is 1 tablet of 500 mg/125 mg 2-3 times a day or 1 tablet of 875 mg/125 mg 2 times a day. For the treatment of mild or moderate infections, the usual dosage regimen is 1 tablet 500 mg/125 mg 2 times a day (every 12 hours); for the treatment of severe infections - 1 tablet 500 mg/125 mg 3 times a day (every 8 hours) or 1 tablet 875 mg/125 mg 2 times a day (every 12 hours). The duration of treatment depends on the indications, is determined by the doctor and should not exceed 14 days. The tablets must be dissolved in 1/2 glass of water (at least 100 ml), mixed thoroughly before taking or chewed before swallowing. It is better to take tablets at the beginning of a meal. In patients with renal failure, the excretion of clavulanic acid and amoxicillin is reduced. It is necessary to reduce the dose in accordance with the severity of functional disorders and/or it is necessary to increase the interval between doses. Patients with moderate renal failure (creatinine clearance 0.166–0.5 ml/s) are prescribed 1 tablet 500 mg/125 mg every 12 hours. Patients with severe renal failure (creatinine clearance less than 0.166 ml/s) are prescribed 1 tablet 500 mg/125 mg every 24 hours.
Amoksiklav tablets
Release form, composition and packaging
White or almost white, film-coated tablets, oval, biconvex.
- 1 tab. amoxicillin (in the form of trihydrate) 250 mg;
- clavulanic acid (in the form of potassium clavulanate) 125 mg.
Excipients: colloidal silicon dioxide, crospovidone, croscarmellose sodium, magnesium stearate, talc, microcrystalline cellulose.
Film shell composition: hypromellose, ethylcellulose, diethyl phthalate, macrogol 6000, titanium dioxide. White or almost white, film-coated tablets, oval, biconvex.
- 1 tab. amoxicillin (in the form of trihydrate) 500 mg;
- clavulanic acid (in the form of potassium clavulanate) 125 mg.
Excipients: colloidal silicon dioxide, crospovidone, croscarmellose sodium, magnesium stearate, talc, microcrystalline cellulose.
Film shell composition: hypromellose, ethylcellulose, diethyl phthalate, macrogol 6000, titanium dioxide. Tablets, film-coated, white or almost white, oblong, biconvex, imprinted “AMC” on one side, scored and imprinted “875” and “125” on the other.
- 1 tab. amoxicillin (in trihydrate form) 875 mg;
- clavulanic acid (in the form of potassium clavulanate) 125 mg.
Excipients: colloidal silicon dioxide, crospovidone, croscarmellose sodium, magnesium stearate, talc, microcrystalline cellulose. Film shell composition: hypromellose, ethylcellulose, povidone, triethyl citrate, titanium dioxide, talc.
Clinical and pharmacological group: Broad-spectrum penicillin antibiotic with a beta-lactamase inhibitor.
Pharmacological action Broad-spectrum antibiotic; contains semisynthetic penicillin amoxicillin and β-lactamase inhibitor clavulanic acid. Clavulanic acid provides a stable inactivated complex with these enzymes and ensures the resistance of amoxicillin to the effects of β-lactamases produced by microorganisms. Clavulanic acid, similar in structure to β-lactam antibiotics, has weak intrinsic antibacterial activity. Amoxiclav has a wide spectrum of antibacterial action.
Active against amoxicillin-sensitive strains, including strains producing β-lactamases, incl. aerobic gram-positive bacteria: Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus viridans, Streptococcus bovis, Enterococcus spp., Staphylococcus aureus (except methicillin-resistant strains), Staphylococcus epidermidis (except methicillin-resistant strains), Staphylococcus saprophyticus, Listeria spp.; aerobic gram-negative bacteria: Bordetella pertussis, Brucella spp., Campylobacter jejuni, Escherichia coli, Gardnerella vaginalis, Haemophilus ducreyi, Haemophilus influenzae, Helicobacter pylori, Klebsiella spp., Moraxella catarrhalis, Neisseria gonorrhoeae, Neisseria meningitidis, Pasteurella multocida, Proteus spp. , Salmonella spp., Shigella spp., Vibrio cholerae, Yersinia enterocolitica, Eikenella corrodens; gram-positive anaerobes: Peptococcus spp., Peptostreptococcus spp., Clostridium spp., Actinomyces israelii, Fusobacterium spp., Prevotella spp., gram-negative anaerobes: Bacteroides spp.
Pharmacokinetics
The main pharmacokinetic parameters of amoxicillin and clavulanic acid are similar. Amoxicillin and clavulanic acid in combination do not affect each other.
Suction
After taking the drug orally, both components are well absorbed from the gastrointestinal tract; food intake does not affect the degree of absorption. Cmax in blood plasma is reached 1 hour after taking the drug and is (depending on the dose) for amoxicillin 3-12 μg/ml, for clavulanic acid - about 2 μg/ml.
Distribution
Both components are characterized by a good volume of distribution in body fluids and tissues (lungs, middle ear, pleural and peritoneal fluids, uterus, ovaries). Amoxicillin also penetrates into the synovial fluid, liver, prostate gland, tonsils, muscle tissue, gall bladder, secretions of the paranasal sinuses, saliva, and bronchial secretions. Amoxicillin and clavulanic acid do not penetrate the BBB when the meninges are not inflamed. The active substances penetrate the placental barrier and are excreted in breast milk in trace concentrations. The degree of binding to plasma proteins is low.
Metabolism
Amoxicillin is partially metabolized, clavulanic acid appears to be extensively metabolized. Excretion Amoxicillin is excreted by the kidneys almost unchanged by tubular secretion and glomerular filtration. Clavulanic acid is excreted by glomerular filtration, partly in the form of metabolites. Small amounts may be excreted through the intestines and lungs. T1/2 of amoxicillin and clavulanic acid is 1-1.5 hours.
Pharmacokinetics in special clinical situations
In severe renal failure, T1/2 increases to 7.5 hours for amoxicillin and to 4.5 hours for clavulanic acid. Both components are removed by hemodialysis and minor amounts by peritoneal dialysis.
Indications
Treatment of infectious and inflammatory diseases caused by microorganisms sensitive to the drug:
- infections of the upper respiratory tract and ENT organs (including acute and chronic sinusitis, acute and chronic otitis media, retropharyngeal abscess, tonsillitis, pharyngitis);
- infections of the lower respiratory tract (including acute bronchitis with bacterial superinfection, chronic bronchitis, pneumonia);
- urinary tract infections;
- gynecological infections;
- skin and soft tissue infections, including human and animal bites;
- infections of bone and connective tissue;
- biliary tract infections (cholecystitis, cholangitis);
- odontogenic infections.
Dosage regimen
Adults and children over 12 years of age (or with a body weight >40 kg) with mild or moderate infection are prescribed 1 tablet. (250 mg+125 mg) every 8 hours or 1 tablet. (500 mg + 125 mg) every 12 hours, in case of severe infection and respiratory tract infections - 1 tablet. (500 mg+125 mg) every 8 hours or 1 tablet. (875 mg+125 mg) every 12 hours.
The drug in tablet form is not prescribed to children under 12 years of age (with body weight <40 kg). The maximum daily dose of clavulanic acid (in the form of potassium salt) is 600 mg for adults and 10 mg/kg body weight for children. The maximum daily dose of amoxicillin is 6 g for adults for children . The course of treatment is 5-14 days. The duration of treatment is determined by the attending physician.
Treatment should not continue for more than 14 days without repeated medical examination. For odontogenic infections, 1 tablet is prescribed. (250 mg+125 mg) every 8 hours or 1 tablet. (500 mg + 125 mg) every 12 hours for 5 days. For moderate renal failure (creatinine clearance 10-30 ml/min), 1 tablet is prescribed. (500 mg + 125 mg) every 12 hours, for severe renal failure (creatinine clearance <10 ml/min) - 1 tablet. (500 mg + 125 mg) every 24 hours. For anuria, the interval between doses should be increased to 48 hours or more.
Side effect
From the digestive system: possible loss of appetite, nausea, vomiting, diarrhea; rarely - transient increase in the activity of liver enzymes (ALT, AST), liver dysfunction; in isolated cases - cholestatic jaundice, hepatitis, pseudomembranous colitis. Allergic reactions: erythematous rash, itching, urticaria; rarely - exudative erythema multiforme, angioedema, anaphylactic shock; in isolated cases - exfoliative dermatitis, Stevens-Johnson syndrome.
Other: rarely - development of superinfection (including candidiasis); reversible increase in prothrombin time (when used together with anticoagulants).
Side effects are in most cases mild and transient.
Contraindications
- a history of cholestatic jaundice or liver dysfunction caused by taking amoxicillin/clavulanic acid;
- increased sensitivity to antibiotics of the penicillin group;
- hypersensitivity to amoxicillin or clavulanic acid.
The drug is prescribed with caution to patients with known hypersensitivity to cephalosporin antibiotics, with a history of pseudomembranous colitis, liver failure, and severe renal dysfunction.
Pregnancy and lactation
Amoxiclav can be used during pregnancy if the expected benefit to the mother outweighs the potential risk to the fetus. Amoxicillin and clavulanic acid are excreted in breast milk in small quantities.
Use for liver dysfunction
The drug is prescribed with caution to patients with liver failure.
Use for renal impairment
For moderate renal failure (creatinine clearance 10-30 ml/min), 1 tablet is prescribed. (500+125 mg) every 12 hours, for severe renal failure (creatinine clearance <10 ml/min) - 1 tablet. (500+125 mg) every 24 hours. For anuria, the interval between doses should be increased to 48 hours or more.
special instructions
Due to the fact that an erythematous rash was observed in a large number of patients with infectious mononucleosis and lymphocytic leukemia treated with ampicillin, the use of ampicillin antibiotics for such patients is not recommended.
During a course of treatment, the functions of hematopoiesis, liver and kidneys should be monitored. In patients with severely impaired renal function, adequate adjustment of the dosage regimen or increased intervals between dosing is required. In order to reduce the risk of developing adverse reactions from the gastrointestinal tract, the drug should be taken with meals. Since amoxicillin and clavulanic acid combination tablets of 250 mg + 125 mg and 500 mg + 125 mg contain the same amount of clavulanic acid - 125 mg, then 2 tablets of 250 mg + 125 mg are not equivalent to 1 tablet of 500 mg + 125 mg.
Side effects of the drug Amoxiclav
As a rule, they are mild in severity and pass quickly. From the gastrointestinal tract: diarrhea (4.1%), nausea (3%), vomiting (1.8%) and dyspepsia (1.6%); rarely - anorexia, flatulence, gastritis, stomatitis, glossitis or discoloration of the tongue, enterocolitis. Pseudomembranous colitis, caused by a toxin produced by Clostridium difficile , may develop during or after discontinuation of drug treatment. From the blood system: anemia (including hemolytic anemia), thrombocytopenia, eosinophilia, leukopenia, agranulocytosis. From the nervous system: rarely - headache, dizziness, insomnia, agitation, anxiety, inappropriate behavior, confusion, convulsions, hyperactivity. Hepatobiliary disorders: possible increase in liver function tests, including asymptomatic increases in AST and/or ALT activity, alkaline phosphatase and serum bilirubin levels. Liver dysfunction most often occurs in elderly patients or in patients receiving long-term treatment. Hepatitis and cholestatic jaundice occur rarely. Signs and symptoms usually occur during or immediately after treatment, but in some cases may not appear until several weeks after treatment ends. From the skin: rash, urticaria, angioedema, rarely - erythema multiforme, Stevens-Johnson syndrome, exfoliative dermatitis, toxic epidermal necrolysis. From the urinary system: rarely - interstitial nephritis and hematuria. Other: candidal vaginitis (1%), fever; Long-term use of the drug can cause oral candidiasis.
Special instructions for the use of the drug Amoxiclav
Amoxiclav is prescribed with caution to patients with a history of allergic reactions. There is a possibility of cross-allergy between cephalosporins and penicillin antibiotics, so caution is required when prescribing Amoxiclav to patients who have an allergy to cephalosporins. In case of liver disorders, periodic monitoring of its function is necessary. In 95% of patients with infectious mononucleosis and lymphocytic leukemia, the use of the drug is accompanied by the development of a skin rash, so prescribing Amoxiclav in these cases is not recommended. In patients with severe kidney damage, the dose should be selected individually; the interval between doses of the drug may be increased. When treating with Amoxiclav, it is recommended to consume a significant amount of fluid. Amoxiclav gives false positive results when performing the Benedict test (to determine glucose in urine) and the Coombs test. It is recommended to use glucose tests based on an enzymatic oxidation reaction. There are no data on the teratogenic effect of the active components of Amoxiclav. Amoxiclav can be used during pregnancy according to strict indications.
Instructions for use AMOXIKLAV® (AMOKSIKLAV®)
Before prescribing the drug, a detailed history should be obtained regarding previous hypersensitivity reactions to penicillins, cephalosporins or other β-lactam antibiotics.
Serious (in some cases fatal) hypersensitivity reactions (anaphylactic reactions) to penicillins have been described. The risk of such reactions is highest in patients with a history of hypersensitivity reactions to penicillins. If an allergic reaction occurs, it is necessary to stop treatment with Amoxiclav and begin alternative therapy. For severe hypersensitivity reactions, the patient should be given epinephrine (adrenaline) immediately. Oxygen therapy, intravenous administration of corticosteroids, and airway management, including intubation, may also be required.
Amoxiclav® should not be prescribed if infectious mononucleosis is suspected, since amoxicillin can cause a skin rash in patients with this disease, which makes diagnosing the disease difficult.
It is not recommended to prescribe Amoxiclav® if there is a high risk that the suspected pathogens have reduced susceptibility or are resistant to beta-lactam antibiotics (for example, penicillin-resistant Streptococcus pneumoniae). Seizures may occur in patients with impaired renal function or in patients receiving high doses.
Special caution is required if it is necessary to use the drug Amoxiclav® in patients with severe allergic diseases, bronchial asthma, with diarrhea and/or vomiting.
Amoxiclav® should be prescribed with caution to patients with impaired liver function. Drug-induced liver failure has been reported predominantly in men and elderly patients and may be associated with long-term therapy. Liver dysfunction has been very rarely reported in children. In all groups of patients, symptoms usually begin during or shortly after treatment, but in some cases become apparent only several weeks after treatment has stopped. Symptoms are usually reversible. Liver failure can be severe and, in extremely rare cases, fatal. It is almost always observed in patients with a serious underlying disease or who are concomitantly taking medications that can have undesirable effects on the liver.
During long-term therapy with Amoxiclav, it is recommended to periodically evaluate renal, liver and hematopoietic function. In patients receiving amoxicillin, an increase in prothrombin time is occasionally observed, therefore, with the simultaneous use of Amoxiclav and anticoagulants, it is necessary to regularly monitor the relevant indicators. With this combination, dose adjustment of oral anticoagulants may be required to achieve the desired level of coagulation.
Long-term treatment with Amoxiclav sometimes leads to excessive growth of insensitive microflora.
The appearance of febrile generalized erythema associated with a pustule at the beginning of treatment may be a symptom of acute generalized exanthemal pustulosis. This reaction requires discontinuation of treatment with Amoxiclav and is a contraindication for any subsequent prescriptions of amoxicillin.
In general, amoxicillin is well tolerated and has the low toxicity characteristic of all penicillins.
Antibiotic-induced colitis occurs with almost all antibacterial drugs and can range in severity from mild to life-threatening. Therefore, it is important to recognize this diagnosis in patients with diarrhea during or after taking any antibiotic. In case of colitis caused by an antibiotic, Amoxiclav should be stopped immediately, a doctor should be consulted, and appropriate therapy should be started. Antiperistaltic drugs are contraindicated in this situation.
In patients with reduced diuresis, crystalluria very rarely occurs, mainly during parenteral therapy. During administration of high doses of amoxicillin, it is recommended to take sufficient fluids and maintain adequate diuresis to reduce the likelihood of amoxicillin crystal formation. In patients with a catheter installed in the bladder, it is necessary to regularly monitor its patency.
The presence of clavulanic acid in Amoxiclav may cause nonspecific binding of IgG and albumin to red blood cell membranes, which may lead to false-positive Coombs test results.
When taking Amoxiclav orally, amoxicillin is detected in the urine. High concentrations of amoxicillin give a false-positive reaction to urine glucose when using Benedict's reagent or Fehling's solution. It is recommended to use enzymatic reactions with glucose oxidase.
Cases of positive enzyme immunoassay (ELISA) results for Aspergillus have been observed in patients receiving the drug, who were subsequently determined to be free of Aspergillus infections. Cross-reactions with non-Aspergillus polysaccharides and polyfuranoses have been observed in the Aspergillus ELISA test. Positive test results in patients receiving Amoxiclav® should be interpreted with caution and confirmed by other diagnostic methods.
Impact on the ability to drive vehicles and operate machinery
Undesirable effects may develop (for example, allergic reactions, dizziness, convulsions), potentially affecting the performance of these functions.